Abstract
Methamphetamine, one of the most frequently used illicit drugs worldwide, can induce psychosis in a large fraction of abusers and it is becoming a major problem for the health care institutions. There is some evidence that genetic and epigenetic factors may play roles in methamphetamine psychosis. In this study, we examined methamphetamine-induced epigenetic and expression changes of several key genes involved in psychosis. RNA and DNA extracted from the saliva samples of patients with methamphetamine dependency with and without psychosis as well as control subjects (each group 25) were analyzed for expression and promoter DNA methylation status of DRD1, DRD2, DRD3, DRD4, MB-COMT, GAD1, and AKT1 using qRT-PCR and q-MSP, respectively. We found statistically significant DNA hypomethylation of the promoter regions of DRD3 (P=0.032), DRD4 (P=0.05), MB-COMT (P=0.009), and AKT1 (P=0.0008) associated with increased expression of the corresponding genes in patients with methamphetamine psychosis (P=0.022, P=0.034, P=0.035, P=0.038, respectively), and to a lesser degree in some of the candidate genes in nonpsychotic patients versus the control subjects. In general, methamphetamine dependency is associated with reduced DNA methylation and corresponding increase in expression of several key genes involved in the pathogenesis of psychotic disorders. While these epigenetic changes can be useful diagnostic biomarkers for psychosis in methamphetamine abusers, it is also consistent with the use of methyl rich diet for prevention or suppression of psychosis in these patients. However, this needs to be confirmed in future studies.
Keywords: methamphetamine, DNA methylation, gene expression, saliva, DRD1, DRD2, DRD3, DRD4, MB-COMT, AKT1, GAD1
INTRODUCTION
Methamphetamine is the second most frequently used drug among illicit substances worldwide (United Nations Office on Drugs and Crime [UNODC, 2007, 2012]). Almost 0.7% of the world population (33.8 million people) aged 15–64 years, confirmed the use of an amphetamine derived stimulant in 2010 [UNODC, 2013]. This estimate is rapidly growing along with increasing demand and manufacturing of methamphetamine ([Rawson et al., 2002; UNODC, 2013]). Although, methamphetamine abuse is a global problem, most of the methamphetamine abusers are residents of East/Southeast Asia and North America [UNODC, 2007]. China is the largest methamphetamine marketplace in the world and the rate of methamphetamine abuse has increased in Far East Asia in recent years [Lu et al., 2008]. The annual prevalence rates of methamphetamine abuse in general population of Canada and United States were 0.8% in 2004 and 1.4% in 2006, respectively [Maxwell and Rutkowski, 2008].Methamphetamine has continued to be a widespread illegal substance in the US and a recent study revealed that 12 million people over the age of 12, have used methamphetamine during their lifetime [SAMHSA, 2013].
The history of amphetamine-derived stimulant use, goes back to World War II. At that time, soldiers used these drugs to reduce appetite and fatigue, and to increase energy [Grinspoon and Hedblom, 1975]. In 1940s and 1950s, amphetamine derived stimulants became popular in US and were widely prescribed as medications [Grinspoon and Hedblom, 1975]. The prevalence of methamphetamine use disorders have relatively remained unchanged in the US over the past 5 years. But the rate of admission for methamphetamine related problems in individuals 12 years or older, have been decreasing between 2005 and 2011 [SAMHSA, 2013]. The first national survey of methamphetamine abuse in 2005 showed that its economic burden is approximately $23.4 billion, including hospital based drug treatment, premature death, crimes, and criminal activity policing[Nicosiaetal.,2009].Interestingly, the rate of methamphetamine dependence is relatively equivalent in females compared to males, and it is generally considered as a disorder of the White populace [SAMHSA, 2002–2012].
Manufacturing of methamphetamine is simple, cheap and it is usually produced in small underground laboratories. Methamphetamine is an indirect sympathomimetic agent that compare to amphetamine has faster distribution into the central nervous system (CNS),resulting in a rapid onset of euphoria that is the desired effect for abusing this drug. Increases in monoamines neurotransmission are responsible for the desired effects—wakefulness, higher energy, sense of well-being, and euphoria—as well as the excess of sympathetic tone that mediates many of its adverse health effects [Sulzer etal.,2005;Vearrieretal., 2012]. The amount and the length of time that the drug is used, the method of administration and the purity of substance are among the factors that affect euphoria in abusers, as well its acute or chronic adverse effects. Chronic abuse of metham phetamine could be accompanied with mood disturbances, cardio vascular difficulties, neuro-cognitive problems, and psychotic symptoms [Barr et al., 2006]. Methamphetamine induced psychosis is the most significant adverse effect of this illicit drug which resembles schizophrenia (SCZ). Therefore, it is considered as a pharmacological model of SCZ [Snyder, 1973].
Multiple mechanisms are responsible for increasing the levels of dopamine and other monoamines in cytosol following methamphetamine use. These mechanisms include, an excess release of catecholamines, reversal or inhibition of monoamine transporters, reduced dopamine transports at cell membrane [Sulzer et al., 2005; Barr et al., 2006], inhibition of monoamine oxidase activity [Mantle et al., 1976], increased activity and production of tyrosine hydroxylase, the dopamine synthesizing enzyme [Sulzer et al., 2005]. In addition to dopaminergic system, other neural systems like serotonin, noradrenaline, and glutamate are affected by methamphetamine, as well [Sulzer et al., 2005]. Methamphetamine use can also induce excessive releases of dopamine and glutamate in extracellular space, followed by production of hazardous reactive oxygen and nitrogen species. Due to heightened extracellular glutamate and NMDA receptor activation, Ca2+ leaks into the neurons [Dykens et al., 1987]. The increased intracellular Ca2+ may lead to activation of an enzymatic cascade that increases the production of reactive oxygen and nitrogen species causing neuronal damages [Radi et al., 1991].
Specific genetic variations (i.e., SNPs) of dopaminergic system have been implicated in methamphetamine abuse and/or methamphetamine-induced psychosis. For example, the over active allele (Val) of the Val158Met polymorphism of catechol-O-methyltransferase (COMT),an enzyme involved in the degradation of monoamines is more frequent in subjects with methamphetamine abuse compared to the control subjects [Bousman et al., 2009]. COMT has also been linked to cognition and brain response to amphetamine [Malhotra et al., 2002; Mattay et al., 2003]. Additionally, DRD4, a family member of D2-like receptors that is involved in cognition, emotion and positive reinforcement was correlated to methamphetamine abuse [Missale et al., 1998]. A significant association has also been reported between the 120-bp VNTR and the exon 3 VNTR polymorphisms of DRD4 (as a haplotype) and methamphetamine abuse [Li et al., 2004]. Based on a number of other studies, a link between a genetic locus of dopamine transporter (DAT) and methamphetamine abuse has been reported [Hong et al., 2003]. Although, in two Han Chinese studies, no association has been found between the DAT 3’-VNTR polymorphism and methamphetamine abuse or psychosis [Lott et al., 2005], in a Japanese study, the alleles with fewer repeats of DAT 3’-VNTR were strongly linked to long-term psychosis even after drug abstinence [Ujike et al., 2003]. AKT1 (Protein Kinase B Alpha), PICK1, SLC22A3, and BDNF are among other genes linked to dopaminergic system and involved in addition [Schroeder et al., 2008; Bousman et al., 2009]. It was also shown that a combination of DRD1 receptor agonists and histone deacetylase inhibitors increase the expression of BDNF and tyrosine hydoxylase mediating the enhancement of cocaine induced sensitization and rewards [Schroeder et al., 2008].
The gamma-2 subunit of gamma amino butyric acid (GABA) A receptor (GABRG2) is another gene which was linked to methamphetamine abuse. A cohort Japanese study showed a haplotypic association of GABRG2 with methamphetamine abuse [Nishiyama et al., 2005]. GABRG2 has also been associated with alcoholism and antisocial personality disorder [Loh et al., 2000]. Furthermore, a study of Han Chinese found significant links between multiple haplotypes of the GABAA receptor subunit genes and methamphetamine abuse, specifically strong association was found to females’ methamphetamine abuse [Lin et al., 2003]. Interestingly, other studies also reported a genetic association between the polymorphisms of glutathione S-transferase M1 [Koizumi et al., 2004] and α-synuclein [Kobayashi et al., 2004] genes and methamphetamine abuse only in females.
In addition to genetic variations, epigenetic alterations may cause hazardous effects similar to dysfunctional genetic mutations in psychotic diseases [Abdolmaleky et al., 2011a, 2015a]. For instance, DNA methylation analysis of MB-COMT gene promoter in postmortem brain samples supported that DNA hypomethylation and corresponding increase in the gene expression of MB-COMT has a role in the pathogenesis of bipolar disorder (BD) and SCZ [Abdolmaleky et al., 2006]. Furthermore, we and others have previously shown that a significant fraction of DNA methylation alterations could be observed in various body tissues (reviewed in Abdolmaleky et al. [2015a]). For example, we identified the same epigenetic alterations of MB-COMT, HTR2A, 5-HTT, and DTNBP1 in brain and DNA extracted from the saliva of patients with SCZ or BD, which in part were attenuated by psychiatric drugs [Ghadirivasfi et al., 2011; Nohesara et al., 2011; Abdolmaleky et al., 2014, 2015b]. Therefore, drug-induced epigenetic alteration may be manifested in other tissues that could serve as surrogate to the brain of the living patients.
Here, we used patients’ saliva samples for epigenetic and expression analyses of a number of genes linked to psychotic phenotype and described the identification of epigenetic alterations in methamphetamine-induced psychosis where methamphetamine is presumed to act as an epigenetic modifier. Interestingly, even offsprings of pregnant mice showed hippocampal DNA methylation alterations as the result of in utero methamphetamine exposure [Itzhak et al., 2015].
We investigated promoter DNA methylation of dopaminergic genes, including DRD1, DRD2, DRD3, DRD4, MB-COMT as well as GAD1 (glutamic acid decarboxylase-1) and AKT1 which are known to play significant roles in the pathogenesis of psychotic diseases [Fatemi, 2010; Wockner et al., 2014]. We hypothesized that their epigenetic alterations are predictors of methamphetamine induced psychosis and may have potential diagnostic, preventive and therapeutic applications.
MATERIALS AND METHODS
Samples
For this study, a total of 75 pairs of saliva samples from patients using methamphetamine and individuals without any history of substance abuse and psychosis were collected using Oragene DNA and RNA Saliva Collection Kits (DNAgenotek, Ottawa, Canada). The saliva samples included, 25 sample pairs of DNA and RNA (collected at the same time) from patients using methamphetamine without any psychotic experience,25pairs of samples from patients using methamphetamine with psychosis, and 25 pairs of samples from normal controls matched for age, gender, and other demographics.
According to the guidelines of the local institutional review board and following the approval of the study protocol by the Ethics Committee of Iran University of Medical Sciences (IUMS), in accordance with the international standards that pertains to Human Subjects research (Declaration of Helsinki, and World Medical Association) sample collection was initiated. The study subjects were informed of the purposes of the study and upon their consent they were referred to the Tehran psychiatric Institute for confirmatory diagnostic evaluations by two psychiatrists based on the Structured Clinical Interview for DSM IV-R. Normal control subjects were interviewed as well, and individuals with a history or family history of substance dependency were excluded. Individuals with acute medical conditions (including dental caries and gingivitis representing poor oral hygiene), a past psychiatric history or family history of psychotic diseases were also excluded. Among methamphetamine abusers, only one case was using an antipsychotic drug and few cases were using opioids (two and four in nonpsychotic and psychotic groups, respectively), cannabis (four in each group, non-psychotic and psychotic), and benzodiazepines (one in each group). All of the non-psychotic and 22 of psychotic patients, and two control subjects were smokers. Four patients in each group and two control subjects were female. The collected saliva samples were sent for epigenetic and expression analysis to the laboratory of Cellular and Molecular Research Center, IUMS, without the identifiers.
DNA Extraction and DNA Methylation Analysis
The genomic DNA was extracted from the saliva samples according to the guidelines of the manufacturer (DNAgenotek, Ottawa, Canada). In brief, for DNA extraction 500 out of 2 ml collected samples were incubated in a water bath at 50°C for 1 hr, then 20 ml DNA purifier was added and kept in ice for 10 min. In the next step, the samples were centrifuged at 5,000 rpm for 3min. Then, clear liquid was separated and mixed with 100% alcohol and centrifuged at 13,000 rpm for 5 min and finally, the precipitated DNA was washed with alcohol (70%) and dissolved in buffer provided by the manufacturer and stored in –20°C for the next steps.
For DNA methylation analysis following quality control using agarose gel electrophoresis, 1 μg of DNA was chemically modified with sodium bisulfate using Qiagen bisulfite modification kit (Cat. No. 59104, Hamburg, Germany) to convert unmethylated cytosines to uracil, and finally to thymine. Several primer pairs (Table I) were used for analyzing DNA methylation of the genes promoter regions using quantitative methylation specific PCR (qMSP). Before qMSP, first we conducted MSP analysis using methylated and unmethylated specific primers in separate PCR reactions. The amplified methylated and unmethylated DNA were run in acrylamide gel, stained by ethydium bromide, and illuminated under UV to be sure that the paired primers generate the expected PCR products. Unmethylated placental DNA and in vitro methylated DNA (modified by bisulfite), were used for negative and positive controls for methylation, respectively. Also, blank water was used as template to detect any PCR contamination.
TABLE I.
Primers for DNA Methylation Analyses of the Candidate Genes (M, Methylated; U, Un-Methylated Specific Primers)
| Genes and primer types | Forward (5’-3’) | Reverse (5’-3’) | Ann. tem. °C (size, Bp.) |
|---|---|---|---|
| β-Actin promoter amplification | TTGGGAGGGTAGTTTAGTTGTGGT | CAAAACAAAACACCTTTTACCCTAA | 60 (197) |
|
β-Actin, nested for 2nd round amplification in qMSP analysis |
GGTGGGTTTAGATTTAGGTTGTGTA | CTACCTACTTTTAAAAATAACAATCAC | 60 (125) |
| MB-COMT promoter amplification | GGATTTTTGAGTAAGATTAGATT | CAATATTCCACCCTAAATCTAAAA | 56 (431) |
| MB-COMT MSP M | TATTTTGGTTATCGTCGCGC | AACGAACGCAAACCGTAACG | 56a (142) |
| MB-COMT MSP U | TATTTTGGTTATTGTTGTGT | AACAAACACAAACCATAACA | 56 (142) |
| MB-COMT nested primer for sequencing | GATATTTTTAC(T)GAGGATATT or reverse of MB-COMTpromoter amplification | 56 | |
| DRD1 promoter amplification | ATTAGTTTTGGGAGTGTTTTTTTT | TCTCTCTCAAAACCCCTAAAACTTA | 56 (206) |
| DRD1 MSP M | GATTTTTTTTTTAAACGTATTTCGGC | AAACTTCGCTAAAAACAACGACTCCG | 60 (125) |
| DRD1 MSP U | GATTTTTTTTTTAAATGTATTTTGGT | AAACTTCACTAAAAACAACAACTCCA | 60 (125) |
| DRD2 Promoter Amplification | GGTTTTTGAGTTTTTAAAGGAGAAGAT | ACACTAAAACTAAACAACTCTA | 56 (304) |
| DRD2 MSP M | CGTTTAGGTCGGGGATCGTCG | GACGCCCGAACGCGAAAAACGCG | 67 (118) |
| DRD2 MSP U | TGTTTAGGTTGGGGATTGTTG | AACACCCAAACACAAAAAACACA | 56 (118) |
| DRD2, nested for sequencing | GGATTTAGTTTGTAATTATAGT | – | 60 |
| DRD3 M | GTTAAATAAGAGTTAGTTTTTTTATAATTGTATC | TCCTCTATTTAATCAATATAATAATTCTTCG | 60 (105) |
| DRD3 U | GTTAAATAAGAGTTAGTTTTTTTATAATTGTATT | TCCTCTATTTAATCAATATAATAATTCTTCA | 60 (105) |
| DRD4 promoter amplification | GGTAGAGTT TGAGTTTAGGTT TTTT | AAACCCAATATTTACTCATCTTAA | 56 (341) |
| DRD4 M | CGTTCGAGGGTCGGGAC | GAACAAACCAACATCGCCCGACG | 60 (151) |
| DRD4 U | TGTTTGAGGGTTGGGAT | AAACAAACCAACATCACCCAACA | 60 (151) |
| AKT1 M | GTAGAGCGAGTTTAGAAGCGTC | ACTAAACCGCTAACGAAACCCG | 60 (112) |
| AKT1 U | GTAGAGTGAGTTTAGAAGTGTT | ACTAAACCACTAACAAAACCCA | 60 (112) |
Annealing temperature for qMSP at 62°C.
Notably, MSP analysis of the promoter regions of GAD1 and DRD2 found no evidence for methylation at the investigated CpG sites in DNA extracted from the saliva, neither in patients nor in control subjects. For the other candidate genes, following the observation of unmethylated and methylated PCR products by MSP analysis, the degree of methylation of the promoter region of the candidate genes was measured by qMSP as described in detail elsewhere [Abdolmaleky et al., 2008a; Ghadirivasfi et al., 2011].
Optimizing the Conditions for qMSP Analysis
In order to establish the optimal condition, different concentrations of primers, as well as in vitro methylated DNA and unmethylated placental DNA were used to generate standard curves during pilot studies for each gene of interest. Similar to MSP, for each qMSP analysis we used in vitro methylated DNA and unmethylated placental DNA as standard methylated and unmethylated DNA, respectively. Subsequently, qMSP analysis was performed using SYBR green master mix (Takara, Kusatsu/Shiga, Japan) and gene specific methylated or unmethylated primers (Table I) for the patients and control subjects in duplicate (25μl reaction using Rotor-gene 6000 real-time PCR machine). The amount of methylated and unmethylated PCR products were calculated using ΔΔCT method of quantification, normalized with the PCR product of β-Actin gene promoter amplified with primers designed from a CpG free region as described elsewhere [Abdolmaleky et al., 2011b; Ghadirivasfi et al., 2011].
Notably, DRD3 primer pairs generated single methylated and unmethylated PCR products during MSP as well as melting curve analysis, thus its methylation level was measured using standard qMSP.AKT1 showed a single product in MSP analysis, but there was evidence for the formation of a primer dimer with a melting temperature of 67°C. Therefore, to eliminate the influence of dimer we captured the CT values at72°C,while melting temperature of the target product was 76°C. Finally, the degree of methylation of the promoter regions of DRD1, DRD4, and MB-COMT, which showed primer dimers or nonspecific products were measured using a modified method, amplification of the genes’ promoter regions in multiplex PCR followed by qMSP as described in detail elsewhere [Abdolmaleky et al., 2008a; Ghadirivasfi et al., 2011]. In brief, we PCR amplified a larger fragment of DRD1, DRD4, MB-COMT, and β-Actin promoters flanking the target CpGs in multiplex PCR (25 cycles) using primers designed from CpG free regions (Table I). The PCR products were purified, diluted 300 times and used as template for the qMSP analysis. The ΔCT for methylated product using in vitro methylated DNA was approximately nine cycles less than unmethylated placental DNA (normalized with the CT of β-Actin promoter) indicating that the modified method is highly sensitive. In fact, the amount of false positive PCR product for methylation using unmethylated DNA was <1/500 (2-ΔΔCT = 2−9=0.0019) compared to methylated DNA. Therefore we used this modified method to measure methylation levels of CpGs located in DRD1, DRD4, and MB-COMT promoters regions, normalized with the PCR product of β-Actin promoter amplified with primers designed from a CpG free region in multiples PCR.
RNA Extraction and Gene Expression Analysis
RNA was extracted from the saliva samples according to the manufacturer protocol using the Qiagen RNeasy micro kit (Cat. # 74004, Valencia, CA) as recommended (DNAgenotek, Ottawa, Canada), and stored at –80°C for subsequent cDNA synthesis. RNA quality and integrity was checked using agarose gel electrophoresis to exhibit sharp and clear 28S and 18S rRNA bands with 2:1 ratio. Three samples were excluded due to poor quality and replaced with new saliva samples for both RNA and DNA extraction. Following RNA quantitation using Ultrospec 3000 UV/Visible spectrophotometer, 500 ng of total RNA was used for cDNA synthesis, and quantitative gene expression analysis was performed using SYBR green master mix, primers listed in Table II, and Rotor-gene 6000 machine. The relative expression was calculated by ΔΔCT quantification method normalized with the PCR product of β-Actin as described elsewhere [Abdolmaleky et al., 2011b].
TABLE II.
Primers for Gene Expression (qRT-PCR) Analysis
| Primers | Forward (5’-3’) | Reverse (5’-3’) | Fragment size (BP) |
|---|---|---|---|
| B-Actin | CGAGCACAGAGCCTCGCCTTTGCC | TGTCGACGACGAGCGCGGCGATAT | 100 |
| MB-COMT | CTGCTTTGCTGCCGAGCTCAGAGGAGAC | GCCCAGCAACACAGCTGCCAACAG | 110 |
| DRD1 | CTGCGACGAATAATGCCATAGAGA | ATTGCACTCCTTGGAGATGGAGCC | 104 |
| DRD2 | AAGAAAGCCACTCAGATGCTCG | GGGATGTTGCAGTCACAGTG | 107 |
| DRD3 | TGCTGGTGGCCACCTTGGTGATG | GTACACATCATGACATCCAGGGTG | 118 |
| DRD4 | TCCTGGTGCTGCCGCTCTTCGTCTA | AGGCGGTGCACAGCATGACGTCCAT | 111 |
| AKT1a | GCAGCACGTGTACGAGAAGA | GGTGTCAGTCTCCGACGTG | 140 |
AKT1 primers sequences were taken from the Harvard primer bank.
Note that, to minimize the impacts of experimental variability, samples from each group were randomly assigned during RNA/DNA extraction, cDNA synthesis, and real-time PCR analysis. For expression and quantitative DNA methylation analyses, half of the intermixed samples from each group were run in each session and the duplicates were run in the same day. TheCTvalues of duplicates were averaged for final analysis.
Statistical Analysis
Pair-wise comparisons via the t-test were used for the statistical analyses of the data (both DNA methylation and expression) in patients versus the control subjects (e.g., non-psychotics vs. controls, psychotics vs. controls, and non-psychotics vs. psychotics). The P values of DNA methylation (which determines expression level) were corrected for multiple comparisons. A P-value of ≤ 0.05 was considered as statistically significant.
RESULTS
DNA Hypomethylation of the Promoter Region of Candidate Genes in Methamphetamine-Induced Psychosis
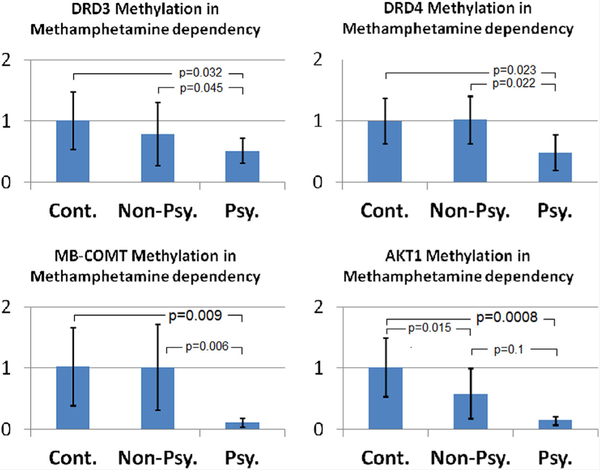
Promoter DNA methylation of the candidate genes that exhibited partial methylation as determined by MSP analysis, including DRD1, DRD3, DRD4, MB-COMT, and AKT1 were re-analyzed using qMSP as described in methods. As shown in Figure 1, we found DNA hypomethylation of the promoter regions of DRD3 (P=0.032), DRD4(P=0.023), MB-COMT(P=0.009), and AKT1 (P=0.0008) genes in patients with methamphetamine-induced psychosis. Additionally, to a lesser extent, there was DNA hypomethylation of AKT1 promoter in methamphetamine dependency without psychosis versus the control subjects (P=0.015). The differences remained significant as P values were corrected for multiple comparisons (P=0.045, 0.045, 0.027, and 0.0032 for DRD3, DRD4, MB-COMT, and AKT1, respectively). The promoter region of DRD1 did not show significant DNA methylation alterations in patients versus the control subjects (data not shown).
FIG. 1.
Relative DNA methylation levels of DRD3, DRD4, MB-COMT, and AKT1 in the saliva samples of patients with methamphetamine dependency without and with psychosis (Non-Psy. and Psy., respectively) compared to control subjects (Cont.). [Color figure can be viewed at wileyonlinelibrary.com].
With respect to GAD1 and DRD2 genes when methylated and unmethylated PCR products were assessed, all of the samples exhibited unmethylated product both in patients and the control subjects. But methylated products were generated only with the in vitro methylated DNA indicating unmethylated promoter of GAD1 and DRD2 genes in both cases and control groups. Bisulfite sequencing of a mixture of DNA from five cases and control subjects also did not show any indication for methylation of these targets. Therefore, we found no evidence for DNA methylation of GAD1 and DRD2 promoter regions in DNA extracted from the saliva samples.
Increased Expression of Genes Showing DNA Hypomethylation in Methamphetamine-Induced Psychosis
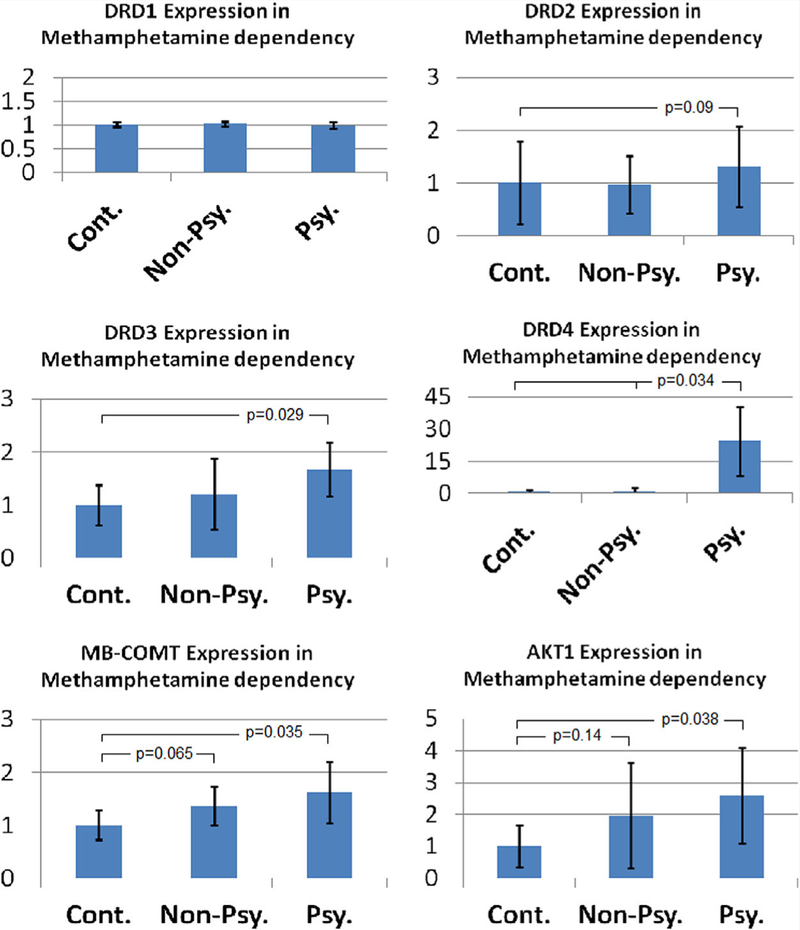
In general, DNA hypomethylation of the candidate genes in methamphetamine-induced psychosis was associated with increased expression of the corresponding gene (Fig. 2). Although, the magnitude of DRD4 expression changes was larger than other genes in psychotic patients versus control subjects (38 times higher, P= 0.034, two tailed t-test) as well as non-psychotic patients with methamphetamine dependency (P = 0.034,twotailed t-test),overall level of DRD4 expression was much less than other genes in cells of the human saliva. Notably, almost 55% of the cases with psychosis exhibited more than 10 times expression of DRD4 compared to the control subjects and/or patients with methamphetamine dependency without psychosis.
FIG. 2.
Relative expression levels of DRD1, DRD2, DRD3, DRD4, MB-COMT, and AKT1 in the saliva samples of patients with metham phetamine dependency without and with psychosis (Non-Psy. and Psy., respectively) compared to control subjects (Cont.). [Color figure can be viewed at wileyonlinelibrary.com].
The expression of DRD3 in psychotic patients was67% more than the control subjects (P= 0.022), however there was no significant change in non-psychotic patients compared to the controls (Fig. 2).
MB-COMT expression was also higher in individuals with methamphetamine-induced psychosis (63%, P = 0.035). In nonpsychotic patients, there was also a trend for higher expression of MB-COMT compared to the control subjects (37%, P = 0.065).
AKT1 expression was higher in methamphetamine-induced psychosis compared to the control subjects as well (2.6 times, P= 0.0385). Although, in non-psychotic patients the expression of AKT1 was almost twice compared to the controls, it was not statistically significant (P= 0.14) due to wide distribution of data (Fig. 2).
With reference to DRD2, although the difference in promoter methylation of DNA extracted from the saliva samples could not be estimated due to low threshold values for detection, in patients with psychosis its expression was almost 20% higher than the control subjects. However, the difference was not statistically significant (P = 0.09, one tailed t-test). Additionally, as expected, DRD1 expression was not different in cases compared to the control subjects (Fig. 2).
DISCUSSION
Environmental stimuli, such as light, temperature, and nutritional elements have been shown to fine-tune gene expression via epigenetic alterations (such as DNA methylation) for adaptation (reviewed in Abdolmaleky et al. [2015a]). In contrast, harsh environmental factors such as traumatic stresses, illicit drugs, and contaminants have been found to induce aberrant DNA methylation leading to psychiatric disorders (reviewed in Abdolmaleky et al. [2011a, 2015a]). Indeed, many studies have shown aberrant promoter DNA methylation of more than two dozen genes in psychotic disorders [Grayson et al., 2005; Abdolmaleky et al., 2006, 2011b; Mill et al., 2008; Dempster et al., 2011; Nohesara et al., 2011; Ikegame et al., 2013; Cheng et al., 2014; Wockner et al., 2014].
In this study, we hypothesized that, methamphetamine as an illicit substance, could modify promoter DNA methylation of D2like receptor genes (i.e., DRD2, DRD3, and DRD4) as well as MB-COMT, GAD1, and AKT1 which are known to be involved in psychosis. Overall, our DNA methylation analyses revealed DNA hypomethylation of the promoter region of candidate genes in methamphetamine dependency, which was greater in individuals with methamphetamine-induced psychosis(Fig. 1). These findings indicate that DNA methylation alterations may occur in genes of dopaminergic system as well as in AKT1 which interacts with this system. Our studies also found that the promoter DNA hypomethylation of these genes (as the result of methamphetamine abuse) exhibit a corresponding increase in the expression of the affected genes (Fig. 2). It is unlikely that smoking or oral hygiene plays significant role in the large differences observed between psychotic patients and control subjects. In fact, with the exception of AKT1, there was no significant difference between non-psychotic and psychotic patients, and almost all of the patients in both groups were smokers and had the same oral hygiene condition. Therefore, non-psychotic patients set a comparison group to rule out the confounding effects of smoking and possible poor oral hygiene.
Consistent with these observations, one study found that the mRNA levels of DNMT2 (DNA methyltransferases-2) to be significantly reduced after methamphetamine injection in rat’s brains [Numachi et al., 2004]. In another study, the same group of researchers uncovered a decreased expression of DNMT1 in the brain of Lewis/N rats, but an increased expression in Fischer 344/N rats [Numachi et al., 2007]. Hence, the observed DNA hypomethylation in the current study might be due to reduced expression of DNMTs. Future studies will be necessary to determine if the expression levels of DNMTs regulate altered promoter DNA methylation in response to methamphetamine intake in humans.
In the analysis of our study samples, we found DNA hypomethylation of MB-COMT gene promoter in individuals with methamphetamine psychosis. While dopamine, as one of the most important neurotransmitters is released as a result of internal/external stimuli and affects different types of dopamine receptors, COMT enzyme (more specifically MB-COMT) degrades dopamine in the synaptic cleft. Thus, DNA hypomethylation of MB-COMT gene promoter, which leads to increased gene expression could enhance dopamine degradation in the synaptic cleft. In contrast to low levels of expression in striatum, COMT has high levels of expression in the prefrontal cortex [Sagud et al., 2010]. Hypodopaminergic activity of the prefrontal cortex causes hypo-activity of the frontal lobe resulting in the impairments of attention, working memory, response inhibition, and executive functions [Apud and Weinberger, 2006]. Therapeutic interventions using COMT inhibitors which increase dopamine levels in this brain region (without significant impact on sub-cortical dopamine levels which is regulated by dopamine transporter) might be a promising therapyin these patients. Interestingly, while MB-COMT promoter DNA hypomethylation was observed in the frontal lobe as well as saliva samples of patients with SCZ and BD [Abdolmaleky et al., 2006; Nohesara et al., 2011], in this study we also found promoter DNA hypomethylation of MB-COMT in psychotic patients. DNA hypomethylation of COMT was also observed in HIV seropositive subjects who used methamphetamine versus other patients [Desplats et al., 2014]. Altogether, these observations suggest that methamphetamine-induced DNA hypomethylation of MB-COMT promoter could play a significant role in its psychotic manifestations.
In our analysis, we also found promoter DNA hypomethylation of DRD3 (a D2-like receptor) associated with an increased gene expression in methamphetamine abusers which was greater in individuals with methamphetamine psychosis. While DRD2 itself showed an unmethylated promoter in the saliva of our study subjects, this gene exhibited a tendency for increased expression in patients with methamphetamine psychosis. Therefore, the increased expression might be due to DNA hypomethylation of other regulatory CpG islands that could be explored in future studies. DRD4 is also enumerated as a subtype of the D2-like receptors inhibiting adenylylcyclase. It has been reported that DRD4 mutations are associated with attention eficit, hyperactivity, and risk taking behavior [Comings et al., 1999; Li et al., 2006]. An increase in the level of DRD4 expression has been previously reported in the frontal cortex of patients with SCZ [Stefanis et al., 1998]. In the current study, the observed DNA hypomethylation of DRD4 in methamphetamine psychosis associated with an increased expression is consistent with the findings reporting increased levels of DRD4 in SCZ. Although, another study reported male specific DNA hypermethylation of interon-1 of DRD4 in the blood cells of SCZ patients, DNA methylation of this region was associated with increased (but not decreased) expression of the DRD4 gene [Cheng et al., 2014].
AKT1 gene encodes AKT1 kinase protein, which has a significant role in signaling pathways related to cellular growth, division, differentiation, and survival [FragaandEsteller,2007]. AKT1 is expressed at high levels in the brain and it has been linked to neural survival and growth, synaptic plasticity, and the functionality of working memory [Pregelj, 2009]. DNA hypomethylation of the promoter region of AKT1 has been reported in a genome-wide methylation analysis of post-mortem brain samples of SCZ patients [Wockner et al., 2014]. Increased expression of AKT1 has also been found in the blood of SCZ patients [Xu et al., 2016]. Hence, our findings indicating methamphetamine-induced promoter DNA hypomethylation associated with increased AKT1 expression is likely linked to the induction of psychosis. Also, it might be considered as a biomarker to evaluate the threshold for psychosis in methamphetamine abusers.
In support of our findings that suggest a strong demethylating activity of methamphetamine, other studies have shown promoter DNA hypomethylation of alpha-synuclein (involved in Parkinson’s disease frequently observed in methamphetamine abusers) associated with increased expression in the substantia nigra of rats exposed to methamphetamine [Jiang et al., 2014]. Interestingly, although some genes of glutamate receptors (e.g., GluA1 and GluA2) exhibited reduced expression following chronic exposure to methamphetamine, they also showed decreased DNA methylation of their promoter regions [Jayanthi et al., 2014]. Notably, other stimulants such as cannabis and nicotine are also known to induce promoter DNA hypomethylation of the vast majority of affected genes [Harlid et al., 2014; Watson et al., 2015] indicating that, in general psycho-stimulants tend to be demethylating agents.
While addictive substances are known to induce epigenetic alterations through DNA methylation as well as histone modifications in prefrontal cortex and nucleus accumbens in animal studies, there is also evidence that these alterations are reversible even in mature neurons [Tian et al., 2012]. For instance, mice exposed to high dosage of cocaine (which has neuro-chemical activities similar to methamphetamine) exhibited DNA hypomethylation in prefrontal cortex which was attenuated by long-term treatment with methionine, a universal methyl donor [Sweatt et al., 2012]. Therefore, considering DNA hypomethylation as the major epigenetic aberration affecting our candidate genes, it is likely that a methyl rich diet may help to attenuate the epigenetic alterations leading to methamphetamine-induced psychosis. Additionally, since epigenetic alterations are involved in modifying neural regulatory pathways following substance abuse (particularly in the prefrontal cortex and nucleus accumbens), these changes could also play a significant role in maintaining substance dependency and/or craving which could be attenuated with a methyl rich diet.
In other branches of medicine, for example oncology, DNA methylation alterations could change cellular functions and generate cancerous cells, and thus certain amounts of folate, vitamin B12, vitamin B6, methionine, selenium, and zinc have been proven to play therapeutic roles [Abdolmaleky et al., 2015c]. Several lines of evidence indicate that these therapeutic approaches might be useful in psychiatric disorders. For example, there is evidence for therapeutic effects of folate in depression, S-Adenosyl methionine (SAM) in bipolar depression, and methyl rich diet in anorexia nervosa (reviewed in Abdolmaleky et al., [2008b]). In the field of substance dependency, at least a recent animal study supports that methyl rich diet supplementation could reduce cocaine-induced c-Fos activation through increased DNA methylation, and attenuated cocaine-seeking behaviors in rats [Wright et al., 2015].
In view of utilizing alterations in DNA methylation for diagnostic, prognostic or therapeutic tests, our findings suggest that a panel of these DNA methylation alterations can be considered as accessible biomarkers in prediction of methamphetamine-induced psychosis or monitoring and follow up of therapeutic response in these patients. Ultimately, these investigations and future studies to include other dopaminergic genes may help to uncover the underlying mechanisms of addictive behavior. Additionally, it may form the basis for preclinical studies, using animal models to help designing novel therapeutics for subsequent clinical trials.
ACKNOWLEDGMENTS
Saliva samples for DNA and RNA extraction were donated by the Tehran Psychiatric Institute, Iran University of Medical Sciences (IUMS). We thank Dr. Reza Alam for his help in editing the revised manuscript and complementary data analysis. This work was primarily supported by grants from the IUMS (90-03-12115089; 90-04-121-16256, 16261 and 16291), and in part by a grant from the NIH (CA165707) to S.T.
Grant sponsor: IUMS; Grant number: 90-03-121-15089; Grant sponsor: NIH; Grant number: CA165707.
Footnotes
Conflict of interest: None.
REFERENCES
- Abdolmaleky HM, Cheng KH, Faraone SV, Wilcox M, Glatt SJ, Gao F, Smith CL, Shafa R, Aeali B, Carnevale J, Pan H, Papageorgis P, Ponte JF, Sivaraman V, Tsuang MT, Thiagalingam S. 2006. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet 15:3132–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolmaleky HM, Smith CL, Zhou JR, Thiagalingam S. 2008a. Epigenetic alterations of the dopaminergic system in major psychiatric disorders. Methods Mol Biol 448:187–212. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Zhou JR, Thiagalingam S, Smith CL. 2008b. Epigenetic and pharmacoepigenomic studies of major psychoses and potentials for therapeutics. Pharmacogenomics 9:1809–1823. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Glatt SJ, Tsuang MT. 2011a. Epigenetics in psychiatry In: Roach HI, Bronner F, Oreffo ROC, editors. Epigenetic aspects of chronic diseases. London, UK: Springer; pp 163–174. [Google Scholar]
- Abdolmaleky HM, Yaqubi S, Papageorgis P, Lambert AW, Ozturk S, Sivaraman V, Thiagalingam S. 2011b. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr Res 129:183–190. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Nohesara S, Ghadirivasfi M, Lambert AW, Ahmadkhaniha H, Ozturk S, Wong CK, Shafa R, Mostafavi A, Thiagalingam S. 2014. DNA hypermethylation of serotonin transporter gene promoter in drug naive patients with schizophrenia. Schizophr Res 152:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolmaleky HM, Zhou JR, Thiagalingam S. 2015a. An update on the epigenetics of psychotic diseases and autism. Epigenomics 7:427–449. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Pajouhanfar S, Faghankhani M, Joghataei MT, Mostafavi A, Thiagalingam S. 2015b. Antipsychotic drugs attenuate aberrant DNA methylation of DTNBP1 (dysbindin) promoter in saliva and post-mortem brain of patients with schizophrenia and Psychotic bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 168: 687–696. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Eskandari MR, Zhou JR. 2015c. Dietary and environmental influences on the genomic and epigenomic codes in cancer In: Thiagalingam S, editor. Systems biology of cancer. Cambridge UK: Cambridge University Press; pp 154–168. [Google Scholar]
- Apud JA, Weinberger DR. 2006. Pharmacogenetic tools for the developmentoftarget-orientedcognitive-enhancingdrugs.NeuroRx3:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. 2006. The need for speed: An update on methamphetamine addiction. J Psychiatry Neurosci 31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Glatt SJ, Everall IP, Tsuang MT. 2009. Genetic association studies of methamphetamine use disorders: A systematic review and synthesis. Am J Med Genet B Neuropsychiatr Genet 150B(1):1025–1049. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wang Y, Zhou K, Wang L, Li J, Zhuang Q, Xu X, Xu L, Zhang K, Dai D, Zheng R, Li G, Zhang A, Gao S, Duan S. Male-specific association between dopamine receptor D4 gene methylation and schizophrenia. PLoS ONE 9(2):e89128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Gonzalez N, Wu S, Gade R, Muhleman D, Saucier G, Johnson P, Verde R, Rosenthal RJ, Lesieur HR, Rugle LJ, Miller WB, MacMurray JP. 1999. Studies of the 48 bp repeat polymorphism of the DRD4 gene in impulsive, compulsive, addictive behaviors: Tourette syndrome, ADHD, pathological gambling, and substance abuse. Am J Med Genet 88:358–368. [DOI] [PubMed] [Google Scholar]
- Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F, Kalidindi S, Picchioni M, Kravariti E, Toulopoulou T, Murray RM, Mill J. 2011. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet 20:4786–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Cronin P, Gianella S, Woods S, Letendre S, Smith D, Masliah E, Grant I. 2014. Epigenetic alterations in the brain associated with HIV-1 infection and methamphetamine dependence. PLoS ONE 9: e102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens JA, Stern A, Trenkner El. 1987. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous toreperfusion tissue injury. J Neurochem 49:1222–1228. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. 2010. Co-occurrence of neurodevelopmental genes in etiopathogenesis of autism and schizophrenia. Schizophr Res 118:303–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Esteller M. 2007. Epigenetics and aging: The targets and the marks. Trends Genet 23:413–418. [DOI] [PubMed] [Google Scholar]
- Ghadirivasfi M, Nohesara S, Ahmadkhaniha HR, Eskandari MR, Mostafavi S, Thiagalingam S, Abdolmaleky HM. 2011. Hypomethylation of the serotonin receptor type-2A Gene (HTR2A) at T102C polymorphic site in DNA derived from the saliva of patients with schizophrenia and bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 156:536–545. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. 2005. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA 102:9341–9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon L, Hedblom P. 1975. The speed culture: Amphetamine use and abuse in America. Cambridge, MA: Harvard University Press. [Google Scholar]
- Harlid S, Xu Z, Panduri V, Sandler DP, Taylor JA. 2014. CpG sites associated with cigarette smoking: Analysis of epigenome-wide data from the sister study. Environ Health Perspect 122:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CJ, Cheng CY, Shu LR, Yang CY, Tsai SJ. 2003. Association study of the dopamine and serotonin transporter genetic polymorphisms and methamphetamine abuse in Chinese males. J Neural Transm 110:345–351. [DOI] [PubMed] [Google Scholar]
- Ikegame T, Bundo M, Sunaga F, Asai T, Nishimura F, Yoshikawa A, Kawamura Y, Hibino H, Tochigi M, Kakiuchi C, Sasaki T, Kato T, Kasai K, Iwamoto K. 2013. DNA methylation analysis of BDNF gene promoters in peripheral blood cells of schizophrenia patients. Neurosci Res 77:208–214. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ergui I, Young J. 2015. Long-term parental methamphetamine exposure of mice influences behavior and hippocampal DNA methylation of the offspring. Mol Psychiatry 20:232–239. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, Cadet JL. 2014. Methamphetamine down regulates sriatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry 76:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Li J, Zhang Z, Wang H, Wang Z. 2014. Epigenetic upregulation of alpha-synuclein in the rats exposed to methamphetamine. Eur J Pharmacol 15:243–248. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ide S, Hasegawa J, Ujike H, Sekine Y, Ozaki N, Inada T, Harano M, Komiyama T, Yamada M, Iyo M, Shen HW, Ikeda K, Sora I. 2004. Study of association between alpha-synuclein gene polymorphism and methamphetamine psychosis/dependence. Ann NY Acad Sci 1025:325–334. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Hashimoto K, Kumakiri C, Shimizu E, Sekine Y, Ozaki N, Inada T, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Takei N, Iyo M. 2004. Association between the glutathione S-transferase M1 gene deletion and female methamphetamine abusers. Am J Med Genet B Neuropsychiatr Genet 126:43–45. [DOI] [PubMed] [Google Scholar]
- Li T, Chen CK, Hu X, Ball D, Lin SK, Chen W, Sham PC, Loh el-W, Murray RM, Collier DA. 2004. Association analysis of the DRD4 and COMT genes in methamphetamine abuse. Am J Med Genet B Neuropsychiatr Genet 129B:120–124. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. 2006. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet 15:2276–2284. [DOI] [PubMed] [Google Scholar]
- Lin SK, Chen CK, Ball D, Liu HC, Loh EW. 2003. Gender-specific contribution of the GABA(A) subunit genes on 5q33 in methamphetamine use disorder. Pharmacogenomics J 3:349–355. [DOI] [PubMed] [Google Scholar]
- Loh EW, Higuchi S, Matsushita S, Murray R, Chen CK, Ball D. 2000. Association analysis of the GABA(A) receptor subunit genes cluster on 5q33–34 and alcohol dependence in a Japanese population. Mol Psychiatry 5:301–307. [DOI] [PubMed] [Google Scholar]
- Lott DC, Kim SJ, Cook EH Jr, de Wi H. 2005. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuro-psychopharmacology 30:602–609. [DOI] [PubMed] [Google Scholar]
- Lu L, Fang Y, Wang X. 2008Drug abuse in China: Past, present and future. Cell Mol Neurobiol 28:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. 2002. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry 159:652–654. [DOI] [PubMed] [Google Scholar]
- Mantle TJ, Tipton K, Garrett NJ. 1976. Inhibition of monoamine oxidase by amphetamine and related compounds. Biochem Pharmacol 25:2073–2077. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. 2003. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA 100:6186–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JC, Rutkowski BA. 2008. The prevalence of methamphetamine and amphetamine abuse in north America: A review of the indicators 1992–2007. Drug Alcohol Rev 27:229–235. [DOI] [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. 2008. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet 82:696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. 1998. Dopamine receptors: From structure to function. Physiol Rev 78:189–225. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. 2009. The economic cost of methamphetamine use in the United States. Santa Monica CA, USA: RAND Corporation. [Google Scholar]
- Nishiyama T, Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Sekine Y, Iyo M, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Inada T, Furukawa T, Ozaki N. 2005. Haplotype association between GABAA receptor gamma2 subunit gene (GABRG2) and methamphetamine use disorder. Pharmacogenomics J 5:89–95. [DOI] [PubMed] [Google Scholar]
- Nohesara S, Ghadirivasfi M, Mostafavi S, Eskandari MR, Ahmadkhaniha H, Thiagalingam S, Abdolmaleky HM. 2011. DNA hypomethylation of MB-COMT promoter in the DNA derived from saliva in schizophrenia and bipolar disorder. J Psychiatr Res 45:1432–1438. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Yoshida S, Yamashita M, Fujiyama K, Naka M, Matsuoka H, Sato M, Sora I. 2004. Psychostimulant alters expression of DNA methyltransferase mRNA in therat brain. Ann NY Acad Sci 1025:102–109. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Shen H, Yoshida S, Fujiyama K, Toda S, Matsuoka H, Sora I, Sato M. 2007. Methamphetamine alters expression of DNA methyltransferase 1 mRNA in rat brain. Neurosci Lett 414:213–217. [DOI] [PubMed] [Google Scholar]
- Pregelj P. 2009. Neurobiological aspects of psychosis and gender. Psychiatr Danub 21:128–131. [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. 1991. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266:4244–4250. [PubMed] [Google Scholar]
- Rawson RA, Anglin MD, Ling W. 2002. Will the methamphetamine problem goaway? J Addict Dis 21:5–19. [DOI] [PubMed] [Google Scholar]
- Sagud M, Mück-Seler D, Mihaljević c-Peles A, Vuksan-Cusa B, Zivković M, Jakovljević M, Pivac N. 2010. Catechol-O-methyl transferase and schizophrenia. Psychiatr Danub 22:270–274. [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. 2008. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology 33:2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH. 1973. Amphetamine psychosis: A “model” schizophrenia mediated by catecholamines. Am J Psychiatry 130:61–67. [DOI] [PubMed] [Google Scholar]
- (SAMHSA); Substance Abuse and Mental Health Services Administration. (2002–2012). Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13–4795. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- (SAMHSA); Substance Abuse and Mental Health Services Administration. (2013). Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14–4863. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Stefanis NC, Bresnick JN, Kerwin RW, Schofield WN, McAllister G. 1998. Elevation of D4 dopamine receptor mRNA in postmortem schizophrenic brain. Brain Res Mol Brain Res 53:112–119. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. 2005. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol 75:406–433. [DOI] [PubMed] [Google Scholar]
- Sweatt D, Meaney MJ, Nestler EJ, Akbarian S. 2012. Epigenetic regulation in the nervous system: Basic mechanisms and clinical impact. London, UK: Academic Press. [Google Scholar]
- Tian W, Zhao M, Li M, Song T, Zhang M, Quan L, Li S, Sun ZS. 2012. Reversal of cocaine-conditioned place preference through methyl supplementationinmice:AlteringglobalDNAmethylationintheprefrontal cortex. PLoS ONE 7:e33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Harano M, Inada T, Yamada M, Komiyama T, Sekine Y, Sora I, Iyo M, Katsu T, Nomura A, Nakata K, Ozaki N. 2003. Nine-or fewer repeat alleles in VNTR polymorphism of the dopamine transporter gene is a strong risk factor for prolonged methamphetamine psychosis. Pharmacogenomics J 3:242–247. [DOI] [PubMed] [Google Scholar]
- UNODC (United Nations Office on Drugs and Crime). 2007. World drug report 2007. Vienna: United Nations. [Google Scholar]
- UNODC (United Nations Office on Drugs and Crime). 2012. World Drug Report 2012. Vienna: United Nations. [Google Scholar]
- UNODC (United Nations Office on Drugs and Crime). 2013. World Drug Report 2013. United Nations, Vienna. [Google Scholar]
- Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA. 2012. Methamphetamine: History, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis Mon 58:38–89. [DOI] [PubMed] [Google Scholar]
- Watson CT, Szutorisz H, Garg P, Martin Q, Landry JA, Sharp AJ, Hurd YL. 2015. Genome-wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross-generational effects of adolescent THC exposure. Neuropsychopharmacology 40:2993–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wockner LF, Noble EP, Lawford BR, Young RM, Morris CP, Whitehall VL, Voisey J. 2014. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl Psychiatry 4:e339. PMID: 24399042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, Mercer R, Feng J, Dietz DM, Lobo MK, Nestler EJ, Kabbaj M. 2015. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci 35:8948–8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yao Shugart Y, Wang G, Cheng Z, Jin C, Zhang K, Wang J, Yu H, Yue W, Zhang F, Zhang D. 2016. Altered expression of mRNA profiles in blood of early-onset schizophrenia. Sci Rep 6:16767. [DOI] [PMC free article] [PubMed] [Google Scholar]