Abstract
Background:
Breast cancer incidence in sub-Saharan Africa (SSA) is increasing, and SSA has the highest age-standardized breast cancer mortality rate worldwide. However, high-quality breast cancer data are limited in SSA.
Material and Methods:
We examined breast cancer patient and tumor characteristics among women in Lilongwe, Malawi and evaluated risk factor associations with patient outcomes. We consecutively enrolled 100 women ≥18 years with newly diagnosed, pathologically confirmed breast cancer into a prospective longitudinal cohort with systematically assessed demographic data, HIV status, and clinical characteristics. Tumor subtypes were further determined by immunohistochemistry, overall survival (OS) was estimated using Kaplan-Meier methods, and hazards ratios (HR) were calculated by Cox proportional hazard analyses.
Results:
Of the 100 participants, median age was 49 years, 19 were HIV-positive, and 75 presented with late stage (III/IV) disease. HER2-enriched and triple negative/basal-like subtypes represented 17% and 25% tumors, respectively. One-year OS for the cohort was 74% (95% CI, 62%−83%). Multivariable analyses revealed mortality was associated with HIV (HR, 5.15; 95% CI, 1.58–16.76; p=0.006), stage IV disease (HR, 8.86; 95% CI, 1.07–73.25; p=0.043), and HER2-enriched (HR, 7.46; 95% CI, 1.21–46.07; p=0.031) and triple-negative subtypes (HR, 7.80; 95% CI, 1.39–43.69; p=0.020).
Conclusions:
Late stage presentation, HER2-enriched and triple-negative subtypes, and HIV coinfection were overrepresented in our cohort relative to resource-rich settings and were associated with mortality. These findings highlight robust opportunities for population- and patient-level interventions across the entire cascade of care to improve breast cancer outcomes in low-income countries in SSA.
Keywords: breast cancer, global health, sub-Saharan Africa
Introduction
Breast cancer is the most common malignancy among women worldwide, with rising incidence and mortality in low- and middle-income countries (LMICs) [1]. As a region, sub-Saharan Africa (SSA) has one of the highest age-standardized breast cancer mortality rate globally (17 per 100,000) despite lower incidence than high-income countries (HICs) including the United States, Australia, and the United Kingdom (33, 85, 94 and 94 per 100,000, respectively [2, 3]. This high mortality-to-incidence ratio is multifaceted and attributable to limited early detection and surveillance, advanced stage at diagnosis, and limited access to standard treatment modalities including targeted therapies [4–7].
In Malawi, a LMIC in southeastern SSA with a population of ~18 million and GDP per capita of $338, breast cancer is the fourth most commonly diagnosed cancer among women [8–10]. Of the ~18 million residents of Malawi, ~1 million reside in the 403 km3 area of the capital, Lilongwe, located in the central region which primarily consists of Chewa (71%), Tumbuka (3.2%), and Lomwe (3.1%) tribes [11]. Though breast cancer represents approximately 4–7% of new female cancer diagnoses in Malawi, this incidence is predicted to increase due to population aging, ‘Westernization’ of lifestyles, declining morbidity and mortality related to infectious diseases such as HIV, and corresponding rise of non-communicable diseases [8, 10, 12–15]. Given high HIV prevalence in SSA, HIV is a relatively common comorbidity among women with breast cancer in the region [16]. Breast cancer is a non-AIDS-defining cancer, and HIV infection has previously been associated with increased mortality among women with breast cancer [17], as well as advanced stage [18, 19]. Other studies, however, have not observed these associations [20, 21].
Despite significant recent advancements in cancer clinical research capacity in Malawi [22], high-quality data on breast cancer are limited. These constraints are partially attributed to the severe healthcare worker shortage, particular for cancer services, with only two Malawian clinical oncologists and four Malawi pathologists currently living and working in the country. Additionally, prior research has been limited to cross-sectional or retrospective studies and has lacked detailed clinical and pathologic characterization [23]. Thus, little is known about patient risk factors, tumor histological subtypes, patterns of treatment response, and long-term clinical outcomes. Without high-quality data in these domains, breast cancer control efforts are severely constrained.
We report initial results from a prospective cohort of consecutively enrolled Malawian women with newly diagnosed, pathologically confirmed breast cancer at Kamuzu Central Hospital (KCH), one of two national teaching hospitals, and provides cancer services for approximately half the country, or a catchment area of ~9 million people. In the absence of rigorous, prospective, longitudinal breast cancer data from SSA, we describe clinical presentation, tumor immunophenotypes, treatment, and outcomes among women in our cohort. Our overall study aim was to generate foundational data for optimizing breast cancer management in Malawi and throughout SSA.
Methods
Patient Enrollment, Pathology, and Clinical Care
We consecutively enrolled 100 women aged 18 years or older with newly diagnosed, pathologically confirmed breast cancer in a prospective longitudinal cohort study between December 2016 and October 2018. The study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill and the Malawi National Health Science Research Committee, and all participants provided written informed consent at the time of enrollment.
Women with clinically suspected breast cancer underwent core needle biopsy. There is no routine breast cancer screening in Malawi currently, and essentially all women therefore presented with clinically symptomatic disease as defined by at least one self-reported physical breast abnormality consistent with breast cancer. Tumor specimens were assessed in the KCH Pathology Laboratory by a dedicated Malawian study pathologist (T.T.). Tumor grade was determined using the modified Bloom-Richardson system [24]. All specimens were evaluated for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67 antigen by immunohistochemistry and scored according to the College of American Pathologist guidelines [25, 26]. Antibodies targeting ER (NCL-L-ER-6F11; Novocastra, Buffalo Grove, IL, USA), PR (NCL-L-PGR-312; Novocastra, Buffalo Grove, IL, USA), and HER2 (ACA342B; Biocare Medical, Pacheco, CA, USA) were used for receptor detection. ER and PR positivity were defined as ≥1% of tumor cell nuclei being immunoreactive [25]. HER2 positivity (3+) was defined as >30% of tumor cell membranes exhibiting uniform intense staining, HER2 negativity (0 and 1+ ) was defined as no staining or weak, and partial membrane staining in any proportion of tumor cells [26]. In situ hybridization (ISH) was not available to adjudicate equivocal cases (2+). Following pathologic confirmation of invasive breast cancer, demographic and risk factor data were obtained through patient interviews and medical record review. HIV status was confirmed at the time of study enrollment, along with HIV viral load and CD4 count assessment for women who were HIV-positive. Patients with HIV were initiated or maintained on antiretroviral therapy throughout the study in collaboration with HIV clinicians, according to Malawi national guidelines. Breast cancer staging was performed using physical exam, chest radiography, abdominal ultrasonography, and plain radiography as clinically indicated. Other imaging studies including computed tomography were applied selectively, due to limited public sector availability and high cost. Magnetic resonance imaging and nuclear medicine scanning were not available at KCH.
Participants received treatment according to KCH institutional standards of care, which are modeled after the National Comprehensive Cancer Network Harmonized Guidelines for SSA [27]. Women with locally advanced tumors but without evidence of distant metastases were typically offered neoadjuvant chemotherapy with a goal of achieving adequate tumor shrinkage to facilitate surgical resection. Women with operable tumors, before or after chemotherapy, underwent modified radical mastectomy. Adjuvant and neoadjuvant chemotherapy typically included four to six 21-day cycles of doxorubicin plus cyclophosphamide followed by a single-agent taxane (paclitaxel or docetaxel) for three 21-day cycles. Palliative-intent chemotherapy generally included single-agent paclitaxel or doxorubicin given in 21-day cycles. Tamoxifen was administered to ER/PR+ women. No women received HER2-targeted treatments, which are not available in the Malawi public sector.
Statistical Analyses
Cohort characteristics were summarized using simple descriptive statistics. Categorical data was analyzed using Chi-squared or Fisher’s exact tests and were summarized using frequencies and percentages as appropriate. Continuous variables were summarized using median values with absolute ranges. Differences between groups were evaluated using the nonparametric Mann-Whitney test as indicated. P-values were 2-sided, and a p-value < 0.05 was considered statistically significant. Kaplan-Meier curves were used to estimate overall survival (OS) and the log-rank test was used to assess differences in survival between groups. Follow-up time was calculated from enrollment until death, loss to follow-up (LTFU), or administrative censoring on January 15, 2019. Adjusted and unadjusted Cox proportional hazards regressions were used to estimate hazard ratios (HR) and 95% confidence intervals (CIs) to determine factors associated with mortality. For our multivariable cox regression model, we selected variables based on a p-value <0.2 from the univariable analysis or high clinical significance. Our multivariable analysis included age (continuous), HIV status (reference was negative status), stage (reference was stage II), and molecular subtype (reference was luminal A). Given a high HIV infection rate in our cohort, we also explored differences in patient characteristics and outcomes stratified by HIV status. To evaluate potential bias in OS estimates resulting from LTFU, additional sensitivity analyses were performed assuming all LTFU patients died at the time of their last known contact (worst case scenario). All analyses were conducted using STATA version 15.1 (STATA, College Station, TX, USA) and GraphPad Prism version 8.0 (GraphPad, San Diego, CA, USA).
Results
Patient Characteristics
Between December 2016 and October 2018, 100 of 101 eligible women with newly diagnosed breast cancer were enrolled in the cohort (Table 1), with only one woman with pathologically confirmed breast cancer at KCH declining to be enrolled. The median age was 49 years (range 21–80 years). HIV status was determined for 99 (99%) of patients in which 19 (19%) were HIV-positive—4 (4%) were newly diagnosed with HIV at the time of enrollment and 15 (15%) had a prior HIV diagnosis and were enrolled a median of 3.1 years (range, 0.1–9.9 years) after their initial HIV diagnosis. Among the 19 HIV-positive patients, 13 had CD4 count and HIV viral load measurements performed at enrollment, with a median CD4 count of 466 cells/μL (range 101–737 cells/μL) and 10 (77%) with an HIV viral load <1000 copies/mL (Table 1). Two patients had AIDS at the time of enrollment as defined as CD4 count <200 cells/μL. Seventy-eight patients presented with a palpable mass >5cm, and 28 had clinically evident tumor ulceration. Of 91 formally staged patients, 48 (53%) presented with stage III, and 27 (30%) presented with stage IV. No patients presented with stage I. Most women had T4 tumors (n=64, 68%), and an even higher proportion had at least N1 clinical lymph node involvement (n=77, 87%).
Table 1.
Patient Characteristics among Newly Diagnosed Women with Breast Cancer in Lilongwe, Malawi, 2016–2018
| Characteristic | n |
|---|---|
| Total | 100 |
| Sociodemographic Factors | |
| Age, years | 49.2 (21–80) a |
| Resides outside of Lilongwe | 82 |
| Did not complete primary school | 43 |
| Earthen floor | 45 |
| Non-tap water source | 38 |
| Medical History and Risk Factors | |
| Family history of cancer | 17 |
| Breast cancer | 4 |
| Performed prior self-breast exam(s) in past year, self-reported | 59 |
| Age at menarche, years | 15 (10–19) a |
| Number of pregnancies | 6 (0–15) a |
| Ever breastfed | 94 |
| Used contraception | 51 |
| Combined oral contraception | 17 |
| Prior alcohol use | 19 |
| Prior tobacco use | 8 |
| Clinical Exam | |
| ECOG b performance score | |
| Score ≤ 2 | 98 |
| Score > 2 | 2 |
| HIV-Status | |
| Positive | 19 |
| CD4 count, cell/µL | 466 (101–737 ) a,c |
| HIV RNA <1000 copies/mL | 10 |
| Negative | 88 |
| Unknown | 1 |
| Palpable mass | |
| >5cm | 78 |
| ≤5cm | 20 |
| Size not recorded | 2 |
| Tumor ulceration | |
| Present | 28 |
| Absent | 70 |
| Not recorded | 2 |
| Clinical tumor size classification | |
| ≤ T3 | 29 |
| T4 (Direct extension to chest wall and/or skin) | 64 |
| Not recorded | 7 |
| Clinical lymph node involvement | |
| N0 | 12 |
| ≥N1 | 77 |
| Not recorded | 11 |
| Tumor Staging | |
| Stage I | 0 |
| Stage II | 16 |
| Stage III | 48 |
| Stage IV | 27 |
| Not recorded | 9 |
median, (range);
Eastern Cooperative Oncology Group;
n=13
Tumor Characteristics
Histologic characterization of biopsies from all 100 women revealed 35 grade II tumors and 37 grade III tumors (Table 2). Immunophenotyping revealed 49 tumors to be ER+, 40 PR+, and 24 HER2+. The Ki67 proliferation index was high in the cohort overall, evidenced by 49 women with a Ki67 staining scored of >15%. Based on immunophenotype, tumor samples were further classified into anticipated molecular subtypes based on the St. Gallen International Expert Consensus with the following results: 23 luminal A (ER/PR+/HER2−), 27 luminal B (ER/PR+/HER2+ or ER/PR+/HER2−/Ki67>15%), 17 HER2-enriched (ER/PR−/HER2+), 25 triple negative/basal-like (ER/PR−/HER2−), and 8 unclassifiable tumors (Table 2) [28].
Table 2:
Tumor Characteristics among Newly Diagnosed Women with Breast Cancer in Lilongwe, Malawi, 2016–2018.
| Characteristic | n |
|---|---|
| Total | 100 |
| Histological Type | |
| Invasive Ductal | 91 |
| Invasive Lobular | 2 |
| Mucinous/Colloid | 4 |
| Papillary | 1 |
| Carcinoma NOS | 2 |
| Pathologic Grade | |
| I | 25 |
| II | 35 |
| III | 37 |
| Unable to grade | 3 |
| Receptor and Ki67 Status by Immunohistochemistry | |
| Estrogen receptor-positive (ER+) | 49 |
| Unable to assess | 3 |
| Progesterone receptor-positive (PR+) | 40 |
| Unable to assess | 4 |
| HER2-positive (HER2+) | 24 |
| Unequivocal | 2 |
| Unable to assess | 6 |
| Ki67 ≥15% | 49 |
| Unable to assess | 5 |
| Molecular Subtype by Immunohistochemistry | |
| Luminal A | 23 |
| Luminal B | 27 |
| HER2-enriched | 17 |
| Triple-negative | 25 |
| Unable to classify | 8 |
ER=Estrogen receptor; PR=Progesterone receptor; HR+=ER+ or PR+;
Luminal A= HR+/HER2−; Luminal B= HR+/HER2+ or HR+/HER2− /Ki67>15%; HER2-enriched= HR-, HER2+; Triple negative=HR−/HER− [21].
Treatment
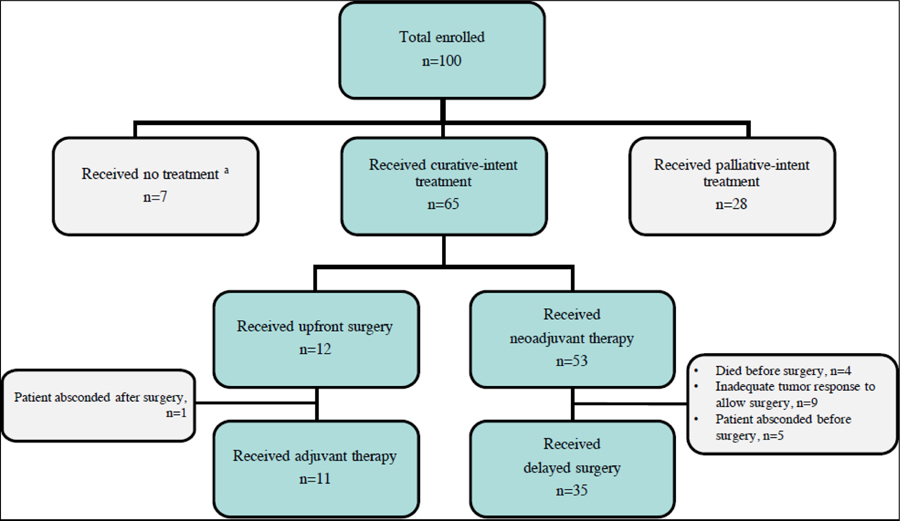
Treatment for enrolled patients is summarized in Figure 1. Women initially underwent either upfront curative- or palliative-intent treatment based on stage at presentation and tumor characteristics. Twenty-eight women immediately began palliative-intent treatment including one patient who received palliative surgery, most of whom presented with stage IV disease. Of 72 women eligible for curative-intent treatment, four patients died before receiving treatment, three elected not to receive any cancer treatment despite counseling, and 65 received treatment. Among 65 women who received initial curative-intent treatment, 12 received upfront surgical intervention. Fifty-three women were eligible for possible delayed surgical intervention after neoadjuvant treatment with adequate tumor downsizing, 35 of whom underwent surgery (Figure 1).
Figure 1: Treatment Overview among Newly Diagnosed Women with Breast Cancer in Lilongwe, Malawi, 2016–2018.
a Died prior to cancer treatment, n=4; Declined any cancer treatment, n=3.
Outcomes and Cancer-Related Risk Factors for Mortality
As of January 15, 2019, vital status was known for 84 of the 100 women enrolled in the cohort. Median OS was 20.2 months, and 1-year OS was 74% (95% CI, 62–83%). Sensitivity analysis incorporating worst case scenario for LTFU patients resulted in median OS 16.5 months and 1-year OS was 67% (95% CI, 55–76%) (Figure 2A). Advanced stage was associated with decreased OS (Figure 2B; p<0.001), such that one-year OS for stage II was 93% (95% CI, 61–99), for stage III was 85% (95% CI, 68–93), and for stage IV was 43% (95% CI, 22–63). Larger tumor size and lymph node involvement were also associated with worse OS (Figure 2C&D; p=0.018, p=0.027). Worst case scenario sensitivity analyses revealed similar associations with mortality for stage, tumor size, and clinical lymph node involvement.
Figure 2: Overall survival (OS) among newly diagnosed women with breast cancer in Lilongwe, Malawi, 2016–2018, by clinical and pathological characteristics.
(A) OS of the Malawi breast cancer cohort with sensitivity analysis demonstrating best and worst case scenarios; (B) OS by clinical stage; (C) OS by tumor size; (D) OS by clinical node involvement; (E) OS by histological grade; (F) OS by molecular subtype.
Tumor characteristics had mixed associations with mortality. There was no detected relationship between histologic grade and mortality (p=0.26), but immunophenotypically assigned molecular subtype had a significant relationship with OS (Figure 2E&F; p=0.015). One-year OS among patients with luminal A breast cancer was 100%, for luminal B was 74% (95% CI, 50%−88%), for HER2-enriched was 60% (95% CI, 22%−84%), and for triple negative was 57% (95% CI, 30%−75%).
Cox regression analyses were used to determine whether specific factors were associated with increased mortality (Table 3). Our multivariable analysis included age, HIV status, stage, and molecular subtype (Table 3). Advanced stage was associated with mortality (HR for stage IV vs. stage II, 8.86; 95% CI, 1.07–73.25; p=0.043). Compared to patients with luminal A tumors, mortality was also associated with having a HER2-enriched (HR, 7.46; 95% CI, 1.21–46.07; p=0.031), and triple-negative (HR, 7.80; 95% CI, 1.39–43.69; p=0.020) tumor immunophenotype.
Table 3:
Hazard Ratios for Mortality among Newly Diagnosed Women with Breast Cancer in Lilongwe, Malawi, 2016–2018.
| Variable | Unadjusted HR [95% CI] | p-value | Adjusted HR [95% CI] | p-value |
|---|---|---|---|---|
| Age | 1.00 [0.98–1.04] | 0.520 | 1.00 [0.96–1.04] | 0.850 |
| HIV+ | 1.46 [0.61–3.48] | 0.401 | 5.15 [1.58–16.76] | 0.006 |
| Stage | ||||
| II | 1 (ref) | 1 (ref) | ||
| III | 3.11 [0.40–24.47] | 0.279 | 1.39 [0.16–11.79] | 0.761 |
| IV | 10.89 [1.44–82.50] | 0.021 | 8.86 [1.07–73.25] | 0.043 |
| Molecular Subtype | ||||
| Luminal A | 1 (ref) | 1 (ref) | ||
| Luminal B | 3.83 [0.78–18.78] | 0.098 | 2.55 [0.47–13.70] | 0.276 |
| HER2-enriched | 10.17 [1.94–53.48] | 0.006 | 7.46 [1.21–46.07] | 0.031 |
| Triple-negative | 6.91 [1.42–33.61] | 0.017 | 7.80 [1.39–43.69] | 0.020 |
The adjusted model includes all variables listed above.
Impact of HIV
In adjusted analyses, we also found HIV status was associated with increased mortality (Table 3; HR, 5.15; 95% CI, 1.58–16.76; p=0.006), although this was not observed in unadjusted analyses. Given HIV infection in 19% of our cohort, reflecting high seroprevalence overall in Malawi, we explored differences in patient characteristics and outcomes stratified by HIV status (Table 4). The median age of HIV-positive and HIV-negative women was similar, and the distribution of tumor stage and molecular subtypes were also comparable between both groups. Furthermore, HIV-positive women had a 1-year OS of 71% (95% CI, 43%−87%), and HIV-negative women had a 1-year OS of 73% (95% CI, 58%−84%) (Figure 3A; p=0.40), although exploratory analyses stratified by the presence of distance metastases at enrollment suggested possible adverse effects of HIV particularly among the subgroup of women with distant metastases (Figure 3B; p<0.001). Since immunosurveillence plays a role in breast cancer progression [29], we further evaluated whether a low CD4 count (<500 cells/μL) within our HIV-positive patients was associated with distant metastases, yet we did not observed a significant association (Fisher’s exact p=0.075).
Table 4:
Baseline Characteristics by HIV Status among Newly Diagnosed Women with Breast Cancer in Lilongwe, Malawi, 2016–2018.
| Total (n=99) | HIV-negative (n=80) | HIV-positive (n=19) | p-value |
|---|---|---|---|
| Age, years | 51 (27–65) a | 48 (21–80) a | 0.79 b |
| Stage | 0.89 c | ||
| I | 0 | 0 | |
| II | 13 | 3 | |
| III | 39 | 9 | |
| IV | 20 | 6 | |
| Not recorded | 8 | 1 | |
| Molecular subtype | 0.51c | ||
| Luminal A | 18 | 5 | |
| Luminal B | 21 | 6 | |
| HER2-enriched | 14 | 2 | |
| Triple-negative | 22 | 3 | |
| Unable to classify | 5 | 3 | |
Median (range)
Mann Whitney test
Chi-square test
Figure 3. Overall survival (OS) among newly diagnosed women with breast cancer in Lilongwe, Malawi, 2016–2018, by HIV status.
(A) OS by HIV status; (B) OS by HIV status and M1 disease.
Discussion
Breast cancer is the most common malignancy among women worldwide and the fourth most common in Malawi [1, 8, 10]. As the first prospective breast cancer cohort in Malawi, and one of the first such cohorts with detailed clinical and pathologic characterization from SSA, we sought to provide robust baseline data highlighting unique features of breast cancer presentation, treatment, and outcomes from a low-income country in the region.
Similar to other breast cancer studies in SSA, women in our cohort presented at a younger age and with more advanced disease compared to HICs. Recent efforts in Malawi have sought to increase breast cancer awareness and promote early detection through use of clinical breast exams [23, 30]. However, we found continued presentation by most women at KCH with bulky tumors often having associated ulceration, indicating a critical need for continued community education, screening, and early detection efforts to identify breast cancers at earlier stages. This is particularly important in light of the significant, albeit expected, associations between mortality and clinical stage, tumor size, and lymph node involvement in our cohort, and the high 1-year OS for the minority of women diagnosed with stage II tumors, even with locally available public sector treatment in Malawi. Optimal strategies for breast cancer screening and early detection in SSA remain uncertain, and much more additional work is needed in this area to identify the most culturally acceptable, programmatically scalable, and economically efficient strategies.
In addition to advanced disease stage and young age at the time of diagnosis, women in SSA are more frequently diagnosed with biologically aggressive tumors like HER2-enriched or TNBC [31, 32]. Our study presents the first description of immunophenotypically defined molecular subtypes from Malawi, and are also among the first such data from SSA. To date, many breast receptor profiling studies in SSA have described women primarily from West and East Africa, with few reports focusing on Central and Southern Africa [33, 34]. Reflecting marked population and environmental differences across SSA, these studies have reported marked variation in ER positivity (ranging from 14–70%), PR positivity, and HER2 positivity [34].
In our cohort, 23% of women had luminal A subtype, in contrast to HICs where luminal A accounts for 59–73% of breast cancer cases [35–37]. This is of particular significance since luminal A is a less aggressive subtype, can often be treated with endocrine therapy without chemotherapy, and has a high survival probability. Additionally, 17% of women had HER2-enriched tumors and 25% of women had triple-negative breast cancer, which again differs from subtype distributions in HICs, but is similar to distributions among women of African descent in HICs and highlights previously described racial differences in breast cancer biology [37, 38].
In addition to late diagnosis and aggressive tumor biology, our results also highlight potential adverse effects of limited treatment resources in the Malawi public health sector. Currently, the only targeted therapy available for breast cancer in the Malawi public sector is tamoxifen. Trastuzumab is not available, similar to many SSA countries, despite its proven efficacy for HER2-positive breast cancers in HICs and recent efficacy demonstration for a trastuzumab biosimilar [39, 40]. Although the HER2-enriched subtype typically accounts for only 6% of cases in HICs [36], 17% of women in our cohort presented with the subtype, similar to findings from other studies in the region [41–43]. Moreover, those who presented with HER2-enriched breast cancer among our group experienced a high mortality rate, underscoring a clear opportunity to improve outcomes by applying a proven treatment to this selected subgroup of patients. Although the addition of trastuzumab to current systemic therapies would likely improve outcomes for many HER2-enriched breast cancer patients, increasing access to this targeted therapy will require multilateral engagement by patient advocacy groups, development agencies, pharmaceutical companies, and policymakers, analogous to such efforts for HIV.
Although breast cancer is not a malignancy with known HIV association, 19% of our patients were HIV-positive, compared with a 13% HIV-infection prevalence among age-matched women in Malawi [44]. In HICs, HIV-positive individuals have lower breast cancer incidence compared with HIV-negative populations [45]. However, our findings are consistent with recent reports from Botswana and South Africa, which found 31% and 18% prevalence of HIV among women with breast cancer, respectively, in both instances higher than national HIV prevalence in the general population [42, 46]. As in Botswana and South Africa, HIV-infected women in our cohort also had typically well controlled HIV with high CD4 counts. With increasing HIV treatment access, HIV-infected populations in SSA are experiencing reduced HIV-associated morbidity and mortality, and life expectancies are becoming more similar to those in HICs [47]. As in HICs, this will likely lead to increased risk of non-AIDS-associated comorbidities compared with untreated HIV-infected populations. While further investigations are needed to determine possible epidemiologic links between breast cancer and HIV, the high prevalence of HIV in SSA and improving life expectancy for people living with HIV suggest that many women in the region with breast cancer will also be HIV-positive. Thus, elucidating potential effects of HIV on clinical presentations, tumor biology, treatment intensity and tolerance, and outcomes is an important regional research priority. This is especially important since our data and other regional reports suggest these women may have inferior outcomes, and data from HICs have reported possibly increased treatment-related infectious complications among HIV-positive women with breast cancer [48].
The primary strengths of our study include its prospective, longitudinal design, with standardized clinical and histologic evaluation of enrolled patients, including detailed tumor immunophenotyping. Women were consecutively enrolled and received routine care in the public sector of a national teaching hospital without major exclusions. LTFU rates were relatively low. Limitations include study conduct at a single institution with relatively small sample size, and the lack of detailed data regarding cause of death and treatment-related complications among enrolled women. Additionally, there is intrinsic referral bias among women seen at a tertiary care hospital, who may not represent women with breast cancer in community settings, especially rural areas.
In conclusion, among prospectively enrolled women with breast cancer in Malawi, we found young age, advanced disease, aggressive tumor biology, frequent HIV coinfection, and poor outcomes, relative to HICs. Advanced stage, tumor immunophenotype, and HIV coinfection were associated with mortality. These findings highlight major opportunities to intervene across the entire breast cancer control spectrum, from earlier detection to improved access to proven treatments to enhanced HIV co-management. It is likely only through such multifaceted, comprehensive efforts, that the overall goal of reducing morbidity and mortality from breast cancer in SSA can be achieved.
Acknowledgments
We are grateful to patients, families, staff, and leadership at Kamuzu Central Hospital and UNC Project-Malawi for their support of this project.
Financial Support:
UJMT Fogarty Global Health Fellowship Program: D43TW009340;
Lineberger Comprehensive Cancer Center: P30CA016086
UNC Breast Cancer Specialized Program of Research Excellence: P50CA058223
Malawi Cancer Consortium and Regional Center of Excellence for NCDs: U54CA190152, P20CA210285
Medical Education Partnership Initiative: R24TW008927;
UNC Center for AIDS Research: P30AI050410.
Footnotes
There are no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends - An update. Cancer Epidemiol. Biomarkers Prev [DOI] [PubMed] [Google Scholar]
- 2.Azubuike SO, Muirhead C, Hayes L, McNally R (2018) Rising global burden of breast cancer: The case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: A review. World J. Surg. Oncol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4.Der EM, Gyasi RK, Tettey Y, et al. (2015) Triple-negative breast cancer in Ghanaian women: The Korle Bu Teaching Hospital experience. Breast J 10.1111/tbj.12527 [DOI] [PubMed] [Google Scholar]
- 5.McKenzie F, Zietsman A, Galukande M, et al. (2018) Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer – disparities in outcomes (ABC-DO) study. Int J Cancer 10.1002/ijc.31187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukong KE, Ogunbolude Y, Kamdem JP (2017) Breast cancer in Africa: prevalence, treatment options, herbal medicines, and socioeconomic determinants. Breast Cancer Res. Treat [DOI] [PubMed] [Google Scholar]
- 7.Kohler RE, Gopal S, Miller AR, et al. (2017) A framework for improving early detection of breast cancer in sub-Saharan Africa: A qualitative study of help-seeking behaviors among Malawian women. Patient Educ Couns 10.1016/j.pec.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Msyamboza KP, Dzamalala C, Mdokwe C, et al. (2012) Burden of cancer in Malawi; Common types, incidence and trends: National population-based cancer registry. BMC Res Notes 10.1186/1756-0500-5-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The World Bank Group (2019) Malawi GDP per capita (US$), 2017. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2017&locations=LR-BI-CF-MW&start=2014&view=chart%0A. Accessed 3 Jun 2019
- 10.Chasimpha SJD, Parkin DM, Masamba L, Dzamalala CP (2017) Three-year cancer incidence in Blantyre, Malawi (2008–2010). Int J cancer 141:694–700. 10.1002/ijc.30777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Statistical Offce; Malawi Government (2018) 2018 Malawi population and housing census Main report [Google Scholar]
- 12.UNAIDS (2019) HIV Prevalence, Malawi, 2017. http://aidsinfo.unaids.org/. Accessed 3 Jun 2019
- 13.Hontelez JAC, de Vlas SJ, Baltussen R, et al. (2012) The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS 26 Suppl 1:S19–S30. 10.1097/QAD.0b013e3283558526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliver NT, Chiao EY (2017) Malignancies in women with HIV infection. Curr Opin HIV AIDS 12:69–76. 10.1097/COH.0000000000000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chirkut S (2019) Breast cancer, human immunodeficiency virus and highly active antiretroviral treatment; implications for a high-rate seropositive region. Oncol Rev 13:376 10.4081/oncol.2019.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grover S, Martei YM, Puri P, et al. (2017) Breast Cancer and HIV in Sub-Saharan Africa: A Complex Relationship. J Glob Oncol 1–11. 10.1200/JGO.2016.006585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coghill AE, Shiels MS, Suneja G, Engels EA (2015) Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol 33:2376–2383. 10.1200/JCO.2014.59.5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantanowitz L, Sen S, Crisi GM, et al. (2011) Spectrum of breast disease encountered in HIV-positive patients at a community teaching hospital. Breast. 10.1016/j.breast.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 19.Pantanowitz L, Connolly JL (2002) Pathology of the breast associated with HIV/AIDS. Breast J 10.1046/j.1524-4741.2002.08409.x [DOI] [PubMed] [Google Scholar]
- 20.Sarhan M, Depaz HA, Oluwole SFD (2010) Breast cancer in women with human immunodeficiency virus infection: Pathological, clinical, and prognostic implications. J Women’s Heal 10.1089/jwh.2010.2026 [DOI] [PubMed] [Google Scholar]
- 21.Coghill AE, Newcomb PA, Madeleine MM, et al. (2013) Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS 27:2933–2942. 10.1097/01.aids.0000433236.55937.cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal S, Krysiak R, Liomba NG, et al. (2013) Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One 8:e70361–e70361. 10.1371/journal.pone.0070361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohler RE, Miller AR, Gutnik L, et al. (2017) Experiences and perceptions regarding clinical breast exam screening by trained laywomen in Malawi. Cancer Causes Control. 10.1007/s10552-016-0844-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ELSTON CW, ELLIS IO (1991) pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 25.Hammond MEH, Hayes DF, Dowsett M, et al. (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 134:907–922. 10.1043/1543-2165-134.6.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff AC, Hammond MEH, Schwartz JN, et al. (2006) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J Clin Oncol 25:118–145. 10.1200/JCO.2006.09.2775 [DOI] [PubMed] [Google Scholar]
- 27.NCCN (2017) NCCN Harmonized Guidelines for Sub-Saharan Africa. NCCN.org 2.2017:
- 28.Goldhirsch A, Winer EP, Coates AS, et al. (2013) Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroemer G, Senovilla L, Galluzzi L, et al. (2015) Natural and therapy-induced immunosurveillance in breast cancer. Nat Med 21:1128–1138. 10.1038/nm.3944 [DOI] [PubMed] [Google Scholar]
- 30.Gutnik L, Msosa V, Moses A, et al. (2016) Uptake and Performance of Clinical Breast Exam Screening Program by Trained Laywomen in Malawi. J Glob Oncol 2:45s–45s. 10.1200/JGO.2016.004051 [DOI] [Google Scholar]
- 31.Huo D, Ikpatt F, Khramtsov A, et al. (2009) Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 27:4515–4521. 10.1200/JCO.2008.19.6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galukande M, Wabinga H, Mirembe F, et al. (2014) Molecular breast cancer subtypes prevalence in an indigenous Sub Saharan African population. Pan Afr Med J 17:249 10.11604/pamj.2014.17.249.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adeloye D, Sowunmi OY, Jacobs W, et al. (2018) Estimating the incidence of breast cancer in Africa: a systematic review and meta-analysis. J Glob Health 8:10419 10.7189/jogh.08.010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eng A, McCormack V, dos-Santos-Silva I (2014) Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS Med 11:e1001720–e1001720. 10.1371/journal.pmed.1001720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fallahpour S, Navaneelan T, De P, Borgo A (2017) Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. C open 5:E734–E739. 10.9778/cmajo.20170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blows FM, Driver KE, Schmidt MK, et al. (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7:e1000279–e1000279. 10.1371/journal.pmed.1000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howlader N, Altekruse SF, Li CI, et al. (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiagge E, Oppong JK, Bensenhaver J, et al. (2016) Breast Cancer and African Ancestry: Lessons Learned at the 10-Year Anniversary of the Ghana-Michigan Research Partnership and International Breast Registry. J Glob Oncol 2:302–310. 10.1200/JGO.2015.002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rugo HS, Barve A, Waller CF, et al. (2017) Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: A randomized clinical trial. JAMA - J Am Med Assoc 10.1001/jama.2016.18305 [DOI] [PubMed] [Google Scholar]
- 40.Pivot X, Bondarenko I, Nowecki Z, et al. (2018) A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: Final safety, immunogenicity and survival results. Eur J Cancer. 10.1016/j.ejca.2018.01.072 [DOI] [PubMed] [Google Scholar]
- 41.Ohene-Yeboah M, Adjei E (2012) Breast cancer in Kumasi, Ghana. Ghana Med J 46:8–13 [PMC free article] [PubMed] [Google Scholar]
- 42.Dickens C, Joffe M, Jacobson J, et al. (2014) Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J cancer 135:2173–2182. 10.1002/ijc.28861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormack VA, Joffe M, van den Berg E, et al. (2013) Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res 15:R84–R84. 10.1186/bcr3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ministry of Health (2016) Summary sheet preliminary findings: the Malawi population-based HIV impact assessment
- 45.Coghill AE, Engels EA, Schymura MJ, et al. (2018) Risk of Breast, Prostate, and Colorectal Cancer Diagnoses Among HIV-Infected Individuals in the United States. J Natl Cancer Inst 110:959–966. 10.1093/jnci/djy010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadigh K, Hodgeman R, Tapela N, et al. (2019) HIV is associated with decreased breast cancer survival: a prospsctive cohort study. In: Conference on Retroviruses and Opportunistic Viruses. Seattle, p Abstract 16 [Google Scholar]
- 47.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. (2015) Cancer incidence following expansion of HIV treatment in Botswana. PLoS One. 10.1371/journal.pone.0135602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochoa RE, Kyriakopoulos C, Hurley J (2012) Outcomes of 47 patients with human immunodeficiency virus infection treated for breast cancer: A 20-year experience. J Clin Oncol 30:1071 10.1200/jco.2012.30.15_suppl.1071 [DOI] [Google Scholar]