Abstract
Dietary restriction (DR) is the most successful and widespread means of extending organismal lifespan. However, the evolutionary basis of life extension under DR remains uncertain. The traditional evolutionary explanation is that when organisms experience DR, they allocate endogenous resources to survival and postpone reproduction until conditions improve. However, this life-extension strategy should be maladaptive if DR continues for multiple generations due to trade-offs between longevity and reproduction. To test this prediction, we subjected the budding yeast Saccharomyces cerevisiae to 1800 generations of evolution on restricted versus non-restricted diets. Adaptation to a non-restricted diet improved reproductive fitness by 57%, but provided a much smaller (14%) advantage on a restricted diet. By contrast, adaptation to DR resulted in an approximately 35% increase in reproductive fitness on both restricted and non-restricted diets. Importantly, the life-extending effect of DR did not decrease following long-term evolution on the restricted diet. Thus, contrary to theoretical expectations, we found no evidence that the life-extending DR response became maladaptive during multigenerational DR. Together, our results suggest that the DR response has a low cost and that this phenomenon may have evolved as part of a generalist strategy that extends beyond the benefits of postponing reproduction.
Keywords: adaptation, yeast, experimental evolution, longevity, nutrition
1. Introduction
Dietary restriction (DR) occurs when an organism experiences a reduction in energy consumption without any vitamin or mineral deficiencies [1]. It is the most consistent intervention known to extend lifespan, effective in taxa from single-celled organisms to primates [1–6]. The conserved molecular mechanisms and genetic pathways by which DR promotes longevity have been well described [5,7]. Yet, the evolutionary basis of the phenotypic plasticity that extends longevity in response to DR is not well understood [8] despite its important implications for topics ranging from modern medicine to life-history theory [5,8–11].
The longstanding evolutionary explanation of the DR response is based on the disposable soma theory of ageing [10,12–16]. Disposable soma theory describes a resource allocation trade-off between somatic and reproductive functions. An organism can increase longevity by investing resources in the repair of somatic cell damage that accrues over time. Alternatively, resources can be allocated to the germline, which increases reproductive ability. Thus, lifespan could theoretically be extended by increasing allocation to the soma, for example in response to DR, although this should incur a trade-off manifested as decreased reproduction.
Despite the trade-off, life extension afforded by DR is thought to be adaptive under certain conditions, for example, in environments with fluctuating resource supply [10,12–15]. Specifically, the postponed reproduction hypothesis states that surviving periods of resource limitation via extended lifespan is beneficial because it allows individuals to reproduce later when conditions are more favourable [13–15]. If this hypothesis is correct, then organisms must outlive the duration of the poor conditions in order for extended lifespan and postponed reproduction to yield increased fitness. Otherwise, if resource limitation continues for multiple generations, there would be no pay-off and the longevity-extending DR response should become maladaptive [10,17]. Therefore, multigenerational DR should favour individuals that cease to respond to DR with extended lifespan at the cost of allocation to reproduction.
Here, we tested predictions about longevity and reproductive fitness in response to multigenerational DR by conducting long-term experimental evolution trials with the budding yeast, Saccharomyces cerevisiae. Budding yeast is a model organism in the study of ageing, due to its easily measured lifespan and the fact that many mechanisms and genetic pathways involved in yeast ageing are shared across eukaryotic taxa [5,7,8,18]. Although originally developed to describe ageing in animals, the disposable soma theory readily applies to single-celled organisms such as S. cerevisiae that exhibit mother–offspring reproductive asymmetry (e.g. by budding) [19–21]. The allocation between longevity and reproduction, and adaptive responses to DR, are highly relevant in yeast life histories, because feast-and-famine cycles are typical for microorganisms in nature, including S. cerevisiae [22,23]. To test the postponed reproduction hypothesis, we selected for reproductive fitness for 1800 generations. We measured life expectancy of evolved populations to assay for the expected diminishment of the DR response due to its predicted cost. We also measured evolved reproductive fitness to further investigate the effect of DR on adaptation.
2. Material and methods
(a). Evolution experiment
We used haploid (mating type MATa) S. cerevisiae of strain background W303, commonly used in ageing research [24–26]. The experimental evolution was conducted using non-restricted (NR: 20 g l−1 d-glucose) and restricted (DR: 5 g l−1 d-glucose) YPD medium (electronic supplementary material, table S1). The 75% glucose reduction induces DR in S. cerevisiae [2,26]. We propagated five replicate lineages per treatment in 10 ml of the medium by 1% v/v daily serial transfer for 275 days (>1800 generations; see electronic supplementary material) in a shaking incubator (200 RPM) at 30°C. Because ageing-associated mortality is negligible until the fourth day [18,26], this regime selects for reproductive fitness and does not select for longevity.
(b). Measurement of longevity
We measured longevity using a chronological lifespan (CLS) assay. This assay quantifies survivorship by plating aliquots of post-stationary phase yeast cultures over time. We grew yeast cultures under NR (20 g l−1 d-glucose) or DR (5 g l−1 d-glucose) conditions using SDC medium (electronic supplementary material, table S2) which greatly reduced variation in lifespan measurements compared to YPD [18]. Replicate aliquots from each cryopreserved evolved population were grown to stationary phase in NR or DR for 3 days. Mortality was assumed negligible during this period [18]. To quantify the abundance of viable individuals, we counted colony-forming units after spread-plating dilutions onto NR plates and incubating them for 3 days at 30°C. We sampled each culture in this fashion every 2 days until survivorship fell below 1% (NR: 16 days; DR: 38 days). We then quantified longevity by calculating the life expectancy (e0) from the life table derived from the CLS assay [27]: , where l0, l1, … lω, are the proportions of individuals surviving to timepoints 0, 1, … , tfinal [27]. We chose this metric because it is effective for comparing expected longevity between populations with the same life history and magnitude of lifespan [28].
(c). Measurement of reproductive fitness
We measured reproductive fitness by competing each evolved population against a common third-party strain in a competitive growth assay that integrated reproductive fitness over a 24-h period [29]. We used strain YDL185 W from the green fluorescent protein (GFP) clone collection (Invitrogen), which expresses Vma1p-GFP fusion protein [30], as the third-party yeast strain. We used the 488 nm laser of a Novocyte flow cytometer (ACEA Biosciences) to differentiate between GFP-expressing and non-GFP cells, and monitored changes in abundance of the two cell types over 24 h. Relative reproductive fitness (W) versus the third party was calculated as
where N0 represents an initial abundance of the focal strain, N24 is the abundance of the focal strain after 24 h, and NG24 and NG0 are final and initial abundances of the Vma1p-GFP third-party strain [29]. We then expressed the reproductive performance of evolved lines on each diet relative to the W303 ancestor. To do this, we relativized W to the reproductive fitness of ancestor strain: , where WW303 is reproductive fitness of the W303 ancestor, calculated as before.
(d). Statistical analyses
We used two-way ANOVA on replicate evolved populations to test for main effects and interaction of evolution diet (NR versus DR) and assay diets (NR versus DR) on longevity and reproductive fitness. These two models allowed us to evaluate yeast performance under both ‘home' (e.g. DR-evolved lines on DR diet) and ‘away' (e.g. DR-evolved lines on NR diet) conditions. We used post-hoc Tukey's HSD tests for pairwise comparisons. In addition, to test the hypothesis that life expectancy decreased relative to the ancestor, we compared the life expectancy of evolved replicate lines to the ancestral value on each diet using one-sample t-tests. All statistical analyses were conducted in R [31].
3. Results
(a). Longevity
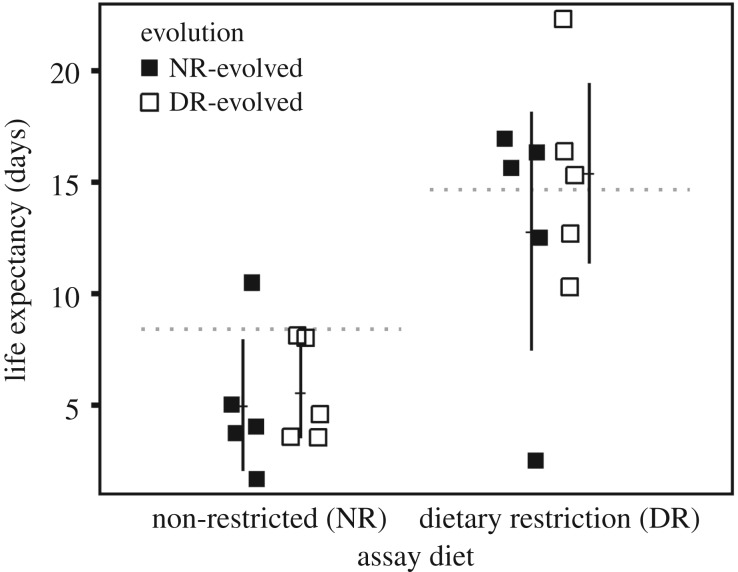
As expected, lifespan was longer when yeast were maintained under DR, confirming the DR phenomenon was successfully induced in our experiments. Prior to evolution, life expectancy of ancestral W303 was 75% higher on DR than NR diet (8.4 ± 0.8 days and 14.7 ± 2.5 days, respectively; t13 = 2.80, p = 0.008). Among evolved lines, life expectancy was also extended on DR relative to NR, suggesting the DR response was retained during evolution (two-way ANOVA F1,16 = 21.4, p = 2.83 × 10−4) (figure 1). In contrast with predictions from the postponed reproduction hypothesis, life expectancy of DR-evolved lines was not shorter than the ancestor on DR (one-sample t4 = 0.36, p = 0.632). On an NR diet, life expectancy among evolved lines was marginally decreased relative to the ancestor (DR-evolved: one-sample t4 = −2.73, p = 0.053; NR-evolved: one-sample t4 = −2.30, p = 0.083). In addition, contrary to predictions, there was no significant difference in life expectancy between the evolved lines (two-way ANOVA F1,16 = 0.699, p = 0.415).
Figure 1.
Life expectancy of non-restricted (NR)-evolved and dietary restriction (DR)-evolved budding yeast populations. The life-extending DR response was observed in evolved lines, which exhibited significantly longer life expectancy on DR. There were marginally significant decreases in life expectancy on NR relative to the ancestor (left dashed line). There were no decreases in life expectancy on DR relative to the ancestor (right dashed line). Life expectancy did not differ between the evolution treatments. Error bars: ± 2 standard errors of the mean (s.e.m.).
(b). Reproduction
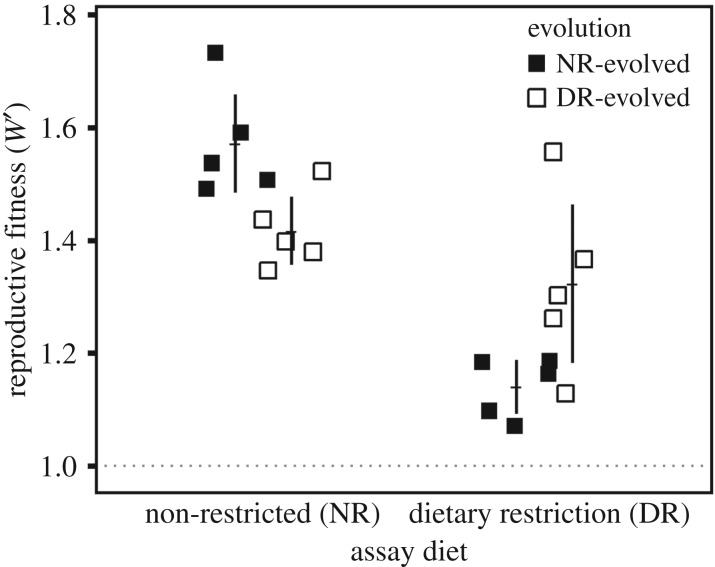
Evolved lines exhibited higher reproductive fitness on their ‘home' evolutionary diets than on their ‘away' evolutionary diets (two-way ANOVA F1,16 = 13.74, p = 0.002) (figure 2). However, post-hoc Tukey's HSD tests showed that the magnitude of this effect was not equal between evolution treatments. Specifically, assayed on home diets, lines evolved on NR exhibited 19% higher reproductive fitness than did DR-evolved lines (Padj = 0.007). In addition, when assayed on the away diets, reproductive fitness was 24% higher for the DR-evolved lines assayed on NR diet than for NR-evolved lines assayed on DR (Padj = 0.003). The reproductive fitness of the DR lines was the same whether they were assayed on the home or away diet (Padj = 0.488), while the reproductive fitness of the NR-evolved lines was 27% lower on their away diet (Padj = 2.80 × 10−5).
Figure 2.
Reproductive fitness of evolved budding yeast lines relative to ancestral reproductive fitness (dashed line). Reproductive fitness increased across all environments. Non-restricted (NR)-evolved lines attained higher reproductive fitness on their home diet than dietary restriction (DR)-evolved lines did on theirs. Meanwhile, lines evolved on DR performed better on their away diet than did NR-evolved lines. In fact, the reproductive fitness of DR-evolved lines was not significantly different on the away diet versus the home diet. Meanwhile, NR-evolved lines exhibited poor performance on their away diet. Errors bars: ± 2 s.e.m.
4. Discussion
Results from our long-term evolution experiment with budding yeast do not support the postponed reproduction hypothesis for the evolution of the life-extending response to DR. Specifically, the DR response was not diminished in yeast populations that evolved under long-term DR, and there was no difference in life expectancy between the DR-evolved and NR-evolved populations regardless of assay condition (figure 1). Together, these findings suggest that the DR response was not maladaptive when the organisms were challenged by resource limitation for multiple generations. Instead, the evolutionary retention of the DR response suggests it may be adaptive irrespective of a pay-off from postponed reproduction.
We also found that home diet reproductive fitness of the NR-evolved populations was higher than that of DR-evolved populations. This finding too is contrary to the postponed reproduction hypothesis. According to the postponed reproduction hypothesis, the DR response is the optimum phenotype [32] in fluctuating resource environments [10,13,14]. In a constant environment, however, the optimum phenotype is one with limited longevity in order to attain high reproduction. This means that in our laboratory evolution with constant diet, and no selection for longevity, the long-lived DR phenotype would be farther away from the optimum than the shorter-lived NR phenotype. Because of the increased distance to the optimum phenotype, reproductive fitness is predicted to increase by a greater amount during evolution on DR [32]. However, we observed the opposite, suggesting the DR response phenotype was not farther from the optimum. Future experiments should investigate whether fluctuating diets select for enhanced DR response as predicted by the theory.
Evidence from other systems has also cast doubt on the universality of the postponed reproduction hypothesis. For example, in experimental evolution trials with Drosophila melanogaster, in contrast with predictions and similar to our results, the longevity of males did not decrease during evolution on DR [17]. Meanwhile, the longevity of D. melanogaster females decreased, without a correlated increase in reproductive fitness, after multigenerational DR [33]. While the latter result differs from our finding that life expectancy was not reduced by DR evolution, it similarly fails to match the predictions of longevity–reproduction trade-offs from disposable soma theory. Separately, diet fluctuations within the lifespans of D. melanogaster individuals demonstrated no advantage to postponed reproduction [34]. Specifically, there was high mortality and no increase in fecundity when flies were switched from restricted to NR diet, suggesting the absence of postponed pay-off [34]. Thus, our study is consistent with observations made in a separate model system and suggests that other mechanisms can contribute to the evolution of extended lifespan under DR.
One potential explanation for the evolutionary retention of the DR response in our study is that evolution on DR confers other advantages. For example, the results suggest that maintaining populations on a restricted diet led to the evolution of better generalists. Specifically, DR-evolved lines had higher reproductive fitness on the away diet than did NR-evolved lines on the away diet. Moreover, the reproductive fitness of DR-evolved lines was not significantly lower on the away diet than the home diet (figure 2). Thus, adaptation to dietary stress resulted in the evolution of generalists. Interestingly, this bears similarity to findings which have shown that generalist microbial species tend to be better adapted to a different type of stress, namely desiccation [35]. Our study's DR-evolved generalists also align with experimental evolution trials that used D. melanogaster, where DR-evolved lines exhibited high reproductive fitness on all assay diets tested [17].
Together, our results show that the DR response did not become maladaptive after multigenerational DR. This suggests that the DR response may be adaptive by means other than postponed reproduction, perhaps having evolved as part of a more general environment-responsive adaptation than response to nutrition status alone [8]. Second, we observed the low costs of adaptation to DR. DR-evolved lines exhibited improved reproductive fitness on DR and NR, a better generalist strategy than NR-evolved lines, and no trade-off in the ability to extend lifespan via DR. The low costs of DR specialization in our study suggest that phenotypic plasticity in response to nutrition status may not have evolved solely due to constraints of resource allocation [8].
Supplementary Material
Acknowledgements
We thank Soni Lacefield for providing ancestor yeast strains and Farrah Bashey-Visser for critical feedback and suggestions.
Data accessibility
Data and code used in this manuscript can be found on GitHub (https://github.com/LennonLab/dietary-restriction) and Dryad (https://doi.org/10.5061.dryad.dncjsxkvq) [36].
Authors' contributions
R.Z.M. and J.T.L. conceived the study; R.Z.M., E.V.S., and K.L.M. conducted the laboratory experiments and collected the data; R.Z.M. analysed the data; R.Z.M. and J.T.L. wrote the manuscript. All authors gave final approval for the publication and agree to be held accountable for its content.
Competing interests
We declare we have no competing interests
Funding
Financial support was provided by the National Science Foundation (1442246, J.T.L.), US Army Research Office (W911NF-14-1-0411, J.T.L.), and National Aeronautics and Space Administration (80NSSC20K0618, J.T.L.).
References
- 1.Fontana L, Partridge L. 2015. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106-118. ( 10.1016/j.cell.2015.02.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin S-J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P-A, Culotta VC, Fink GR, Guarente L. 2002. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344-348. ( 10.1038/nature00829) [DOI] [PubMed] [Google Scholar]
- 3.Mair W, Dillin A. 2008. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 77, 727-754. ( 10.1146/annurev.biochem.77.061206.171059) [DOI] [PubMed] [Google Scholar]
- 4.Mattison JA, et al. 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14063 ( 10.1038/ncomms14063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gems D, Partridge L. 2013. Genetics of longevity in model organisms: debates and paradigm shifts. Annu. Rev. Physiol. 75, 621-644. ( 10.1146/annurev-physiol-030212-183712) [DOI] [PubMed] [Google Scholar]
- 6.Pifferi F, et al. 2018. Caloric restriction increases lifespan but affects brain integrity in grey mouse lemur primates. Commun. Biol. 1, 30 ( 10.1038/s42003-018-0024-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapahi P, Kaeberlein M, Hansen M. 2017. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev. 39, 3-14. ( 10.1016/j.arr.2016.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan JC, Froy H, Walling CA, Moatt JP, Nussey DH. 2019. Dietary restriction and insulin-like signalling pathways as adaptive plasticity: a synthesis and re-evaluation. Funct. Ecol., 34 107-128. ( 10.1111/1365-2435.13418) [DOI] [Google Scholar]
- 9.Adler MI, Bonduriansky R. 2014. Why do the well-fed appear to die young? A new evolutionary hypothesis for the effect of dietary restriction on lifespan. Bioessays 36, 439-450. ( 10.1002/bies.201300165) [DOI] [PubMed] [Google Scholar]
- 10.Kirkwood TBL, Shanley DP. 2005. Food restriction, evolution and ageing. Mech. Ageing Dev. 126, 1011-1016. ( 10.1016/j.mad.2005.03.021) [DOI] [PubMed] [Google Scholar]
- 11.Shanley DP, Kirkwood TBL. 2006. Caloric restriction does not enhance longevity in all species and is unlikely to do so in humans. Biogerontology 7, 165-168. ( 10.1007/s10522-006-9006-1) [DOI] [PubMed] [Google Scholar]
- 12.Harrison DE, Archer JR. 1989. Natural selection for extended longevity from food restriction. Growth Dev. Aging GDA 53, 3. [PubMed] [Google Scholar]
- 13.Holliday R. 1989. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays 10, 125-127. ( 10.1002/bies.950100408) [DOI] [PubMed] [Google Scholar]
- 14.Masoro EJ, Austad SN. 1996. The evolution of the antiaging action of dietary restriction: a hypothesis. J. Gerontol. A. Biol. Sci. Med. Sci. 51, B387-B391. ( 10.1093/gerona/51A.6.B387) [DOI] [PubMed] [Google Scholar]
- 15.Shanley DP, Kirkwood TBL. 2000. Calorie restriction and aging: a life-history analysis. Evolution 54, 740 ( 10.1554/0014-3820(2000)054[0740:CRAAAL]2.3.CO;2) [DOI] [PubMed] [Google Scholar]
- 16.Kirkwood TBL, Holliday R.. 1979. The evolution of ageing and longevity. Proc. R. Soc. Lond. B 205, 531-546. ( 10.1098/rspb.1979.0083) [DOI] [PubMed] [Google Scholar]
- 17.Zajitschek F, Zajitschek SRK, Canton C, Georgolopoulos G, Friberg U, Maklakov AA. 2016. Evolution under dietary restriction increases male reproductive performance without survival cost. Proc. R. Soc. B 283, 20152726 ( 10.1098/rspb.2015.2726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabrizio P, Longo VD. 2007. The chronological life span of Saccharomyces cerevisiae. In Biological aging (ed. Tollefsbol TO.), pp. 89-95. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood TBL. 2005. Understanding the odd science of aging. Cell 120, 437-447. ( 10.1016/j.cell.2005.01.027) [DOI] [PubMed] [Google Scholar]
- 20.Baig UI, Bhadbhade BJ, Watve MG. 2014. Evolution of aging and death: what insights bacteria can provide. Q. Rev. Biol. 89, 209-233. ( 10.1086/677572) [DOI] [PubMed] [Google Scholar]
- 21.Moger-Reischer RZ, Lennon JT. 2019. Microbial ageing and longevity. Nat. Rev. Microbiol. 17, 679-690. ( 10.1038/s41579-019-0253-y) [DOI] [PubMed] [Google Scholar]
- 22.Lennon JT, Jones SE. 2011. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 9, 119-130. ( 10.1038/nrmicro2504) [DOI] [PubMed] [Google Scholar]
- 23.Liti G. 2015. The fascinating and secret wild life of the budding yeast S. cerevisiae. eLife 4, e05835 ( 10.7554/eLife.05835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ocampo A, Liu J, Schroeder EA, Shadel GS, Barrientos A. 2012. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 16, 55-67. ( 10.1016/j.cmet.2012.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ralser M, Kuhl H, Ralser M, Werber M, Lehrach H, Breitenbach M, Timmermann B. 2012. The Saccharomyces cerevisiae W303-K6001 cross-platform genome sequence: insights into ancestry and physiology of a laboratory mutt. Open Biol. 2, 120093 ( 10.1098/rsob.120093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. 2008. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 4, e13 ( 10.1371/journal.pgen.0040013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey JR. 2001. Insect biodemography. Annu. Rev. Entomol. 46, 79-110. ( 10.1146/annurev.ento.46.1.79) [DOI] [PubMed] [Google Scholar]
- 28.Baudisch A. 2011. The pace and shape of ageing. Meth. Ecol. Evol. 2, 375-382. ( 10.1111/j.2041-210X.2010.00087.x) [DOI] [Google Scholar]
- 29.Wiser MJ, Lenski RE. 2015. A comparison of methods to measure fitness in Escherichia coli. PLoS ONE 10, e0126210 ( 10.1371/journal.pone.0126210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huh W-K, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. 2003. Global analysis of protein localization in budding yeast. Nature 425, 686-691. ( 10.1038/nature02026) [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 32.Orr HA. 2005. The genetic theory of adaptation: a brief history. Nat. Rev. Genet. 6, 119-127. ( 10.1038/nrg1523) [DOI] [PubMed] [Google Scholar]
- 33.Zajitschek F, Georgolopoulos G, Vourlou A, Ericsson M, Zajitschek SRK, Friberg U, Maklakov AA. 2019. Evolution under dietary restriction decouples survival from fecundity in Drosophila melanogaster Females. J. Gerontol. Ser. A 74, 1542-1548. ( 10.1093/gerona/gly070) [DOI] [PubMed] [Google Scholar]
- 34.McCracken AW, Adams G, Hartshorne L, Tatar M, Simons MJP. 2020. The hidden costs of dietary restriction: implications for its evolutionary and mechanistic origins. Sci. Adv. 6, eaay3047 ( 10.1126/sciadv.aay3047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lennon JT, Aanderud ZT, Lehmkuhl BK, Schoolmaster DR. 2012. Mapping the niche space of soil microorganisms using taxonomy and traits. Ecology 93, 1867-1879. ( 10.1890/11-1745.1) [DOI] [PubMed] [Google Scholar]
- 36.Moger-Reischer RZ, Snider EV, McKenzie KL, Lennon JT. 2020. Data from: Low costs of adaptation to dietary restriction Dryad Digital Repository. ( 10.5061/dryad.dncjsxkvq) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Moger-Reischer RZ, Snider EV, McKenzie KL, Lennon JT. 2020. Data from: Low costs of adaptation to dietary restriction Dryad Digital Repository. ( 10.5061/dryad.dncjsxkvq) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and code used in this manuscript can be found on GitHub (https://github.com/LennonLab/dietary-restriction) and Dryad (https://doi.org/10.5061.dryad.dncjsxkvq) [36].