Abstract
Dinosaur migration patterns are very difficult to determine, often relying solely on the geographical distribution of fossils. Unfortunately, it is generally not possible to determine if a fossil taxon's geographical distribution is the result of migration or simply a wide distribution. Whereas some attempts have been made to use isotopic systems to determine migratory patterns in dinosaurs, these methods have yet to achieve wider usage in the study of dinosaur ecology. Here, we have used strontium isotope ratios from fossil enamel to reconstruct the movements of an individual hadrosaur from Dinosaur Provincial Park in Alberta, Canada. Results from this study are consistent with a range or migratory pattern between Dinosaur Provincial Park and a contemporaneous locality in the South Saskatchewan River area, Alberta, Canada. This represents a minimum distance of approximately 80 km, which is consistent with migrations seen in modern elephants. These results suggest the continent-wide distribution of some hadrosaur species in the Late Cretaceous of North America is not the result of extremely long-range migratory behaviours.
Keywords: hadrosaur, migration, strontium isotopes, geochemistry, Late Cretaceous
1. Introduction
Since it was first suggested by von Huene [1], the idea of dinosaur migration has been the subject of much debate. Traditional ideas of migration were centred around the distribution of fossil taxa which, in many cases, span areas of thousands of kilometres [1,2]. This approach is flawed however, as some species may have had a wide species distribution unrelated to migration, such as the modern cougar (Puma concolor), which is found from the Yukon of Canada to the southern tip of Argentina and Chile [3]. The debate about possible dinosaur migration achieved new prominence with the discovery of dinosaur trackways at polar latitudes [4], which have since been corroborated by skeletal dinosaur finds in Antarctica [5,6], Alaska [7], New Zealand [8] and Russia [9]. These high latitude faunas raised questions surrounding the ability of dinosaurs to survive polar winters, resulting in competing hypotheses of migration versus overwintering [2,7]. This has led to debate about the biomechanical ability of dinosaurs to migrate, metabolism, endothermy and bone structure [2,7,9–12]. Most recent work suggests that dinosaurs did possess the necessary adaptations to overwinter in the much milder Cretaceous climate [2,7,8,9,11,12]; however, this does not preclude the possibility that some dinosaurs may have migrated within both polar and more temperate regions [2,7].
One approach to this problem is to use the concept of isoscapes, which can be described as spatially driven changes in the availability of different isotopes over broad geographical areas (or landscapes) [13]. In recent years, some workers have attempted to measure migration using stable isotopes of carbon and oxygen preserved in the enamel of dinosaur teeth [10,14]. By contrast, our study uses the ratio between the radiogenic strontium 87 isotope and the stable strontium 86 isotope (87Sr/86Sr). Unlike carbon and oxygen isotope ratios, which vary depending on a variety of environmental conditions [10,14], strontium isotope ratios in the enamel of terrestrial vertebrates are generally reflective of diet and freshwater consumption, as any mass fractionation that has occurred is normalized according to an exponential mass fractionation law during analysis [15]. Strontium isotope ratios in animals and plants reflect values in nearby freshwater sources [15,16] and soils [17], which are primarily derived from bedrock weathering [17]. As a result, strontium isotope signatures can vary between nearby river basins, as documented in modern Canadian river basins [18], and can therefore be used to infer the presence of an individual organism in a specific geographical region. While strontium has been used to study food web patterns in fossil materials for more than half a century [19], it was not until 1985 when strontium isotopes began to be used in human migration studies [20]. Since then, strontium isotope ratios have been increasingly used in migration studies in archaeology and palaeontology [17,21–24] including habitat preference studies of Mesozoic aquatic reptiles [25,26], but applications of this methodology to terrestrial animals outside the Quaternary are largely absent.
For this study, we applied a strontium isotope approach to investigate migratory behaviours in hadrosaurs from the Late Cretaceous of Alberta, Canada. Hadrosaurs were widespread during the Late Cretaceous of North America, with some genera ranging from Mexico to Alaska [2], and the genus Edmontosaurus confirmed to range over 2600 km [2]. Hadrosaurs are therefore considered as one of the more likely dinosaur groups to undertake long-distance migrations. They are also known to have rapidly grown new teeth (every 6–12 months, with replacement rates of approx. 2 months [27]), allowing an individual to possess teeth formed in different localities, with each tooth reflecting the 87Sr/86Sr value of the locality in which it formed. These factors make hadrosaurs an ideal target for dinosaur migration studies.
To determine if strontium isotope data in hadrosaur teeth indicate migratory behaviour, it was necessary to compare the results against those from presumed non-migratory taxa. These taxa included freshwater fish, crocodiles and small theropods. These non-migratory taxa were used to estimate the range of strontium isotope ratios that were present in a given locality during the Late Cretaceous (figure 1).
Figure 1.
Palaeogeographic reconstruction of Western Canada 75 Ma, with the current modern map area indicated on the inset (a). This diagram depicts three separate hypothetical ancient river basins, each with a corresponding strontium isotope signature as signified by the colours purple, green and pink. These isotopic signatures are hypothesized to be recorded in newly formed teeth in a hadrosaur, as depicted in (b,c). Strontium isotope ratios (means shown in brackets) from each studied locality (DPP, Dinosaur Provincial Park; SSR, South Saskatchewan River; BR, Battle River; MRA, Milk River area) were determined by analysing enamel from vertebrates confined to a single river, including freshwater rayfish teeth (d), gar-pike scales (e) and crocodile teeth (f), which were then compared with animals with greater potential range such as carnivorous and herbivorous dinosaurs (g,h, respectively). The dashed red circle depicts the simplest solution for the range of the individual hadrosaur in this study as supported by the data.
2. Material and methods
Samples of microvertebrate material from a variety of approximately time-equivalent localities were obtained from the Royal Tyrrell Museum, including samples from Dinosaur Provincial Park, the South Saskatchewan River, Milk River and Battle River areas. Most material was from the Dinosaur Park Formation (DPF), with the exception of some samples from the Dinosaur Provincial Park and Milk River areas. Additional samples from the Dinosaur Provincial Park area were obtained through field collections. It was important to minimize the effects of possible changes in the environmental strontium isotope content that can occur over geological time, so fossil localities were chosen for their approximate time equivalency (see electronic supplementary material, for geological details).
Specimens analysed in this study represent a variety of taxa, including hadrosaurs, small theropods, crocodilians, Myledaphus (freshwater guitarfish) and gar. Most isotope samples were collected from the enamel or enameloid of fossil teeth or scales, as dentine is more susceptible to diagenetic alteration [21]; however, some dentine samples were also collected to evaluate the possibility of diagenetic overprinting in the enamel samples (see table 1 for a complete summary). Enamel samples from 17 teeth from an individual hadrosaur specimen from the DPF in Dinosaur Provincial Park were also examined, along with a few dentine samples. Finally, two teeth and a bone fragment were analysed for strontium concentrations along with a modern deer tooth, using an isotope dilution. This was done as a further means of assessing possible diagenetic contamination. Details on how samples were prepared and analysed can be found in the electronic supplementary material.
Table 1.
Summary of all collected data from enamel/enameloid sources. The analytical uncertainty is 20 ppm at k = 2. Locations are indicated by the same abbreviations as in figure 1. Formations are indicated by the same abbreviations as in the text. Samples numbers J-1 to J-17 are from an individual hadrosaur, while all other samples are from isolated elements.
| sample | 87Sr/86Sr | taxon | location | formation |
|---|---|---|---|---|
| T1 | 0.706673 | theropod | DPP | DPF |
| T2 | 0.706593 | theropod | DPP | DPF |
| T3 | 0.706679 | theropod | DPP | DPF |
| C1 | 0.706711 | crocodile | DPP | DPF |
| C2 | 0.706643 | crocodile | DPP | DPF |
| C3 | 0.706692 | crocodile | DPP | DPF |
| C4 | 0.706648 | crocodile | DPP | DPF |
| M1 | 0.706620 | Myledaphus | DPP | DPF |
| M2 | 0.706651 | Myledaphus | DPP | DPF |
| M3 | 0.706651 | Myledaphus | DPP | DPF |
| M4 | 0.706761 | Myledaphus | DPP | DPF |
| G1 | 0.706865 | gar | DPP | DPF |
| G2 | 0.706716 | gar | DPP | DPF |
| G4 | 0.706707 | gar | DPP | DPF |
| 146-1 | 0.706643 | crocodile | DPP | DPF |
| 146-2 | 0.706676 | crocodile | DPP | DPF |
| 146-3 | 0.706706 | crocodile | DPP | DPF |
| 146-4 | 0.706592 | crocodile | DPP | DPF |
| 146-6 | 0.706515 | gar | DPP | DPF |
| 146-7 | 0.706696 | gar | DPP | DPF |
| H1 | 0.706818 | hadrosaur | DPP | DPF |
| H2 | 0.706672 | hadrosaur | DPP | DPF |
| H3 | 0.706278 | hadrosaur | DPP | DPF |
| H4 | 0.706959 | hadrosaur | DPP | DPF |
| J-1 | 0.706739 | hadrosaur | DPP | DPF |
| J-2 | 0.706901 | hadrosaur | DPP | DPF |
| J-3 | 0.706861 | hadrosaur | DPP | DPF |
| J-4 | 0.706923 | hadrosaur | DPP | DPF |
| J-5 | 0.706813 | hadrosaur | DPP | DPF |
| J-6 | 0.7068 | hadrosaur | DPP | DPF |
| J-7 | 0.706885 | hadrosaur | DPP | DPF |
| J-8 | 0.706975 | hadrosaur | DPP | DPF |
| J-9 | 0.706759 | hadrosaur | DPP | DPF |
| J-10 | 0.706932 | hadrosaur | DPP | DPF |
| J-11 | 0.707061 | hadrosaur | DPP | DPF |
| J-12 | 0.707059 | hadrosaur | DPP | DPF |
| J-13 | 0.70694 | hadrosaur | DPP | DPF |
| J-14 | 0.706917 | hadrosaur | DPP | DPF |
| J15 | 0.706936 | hadrosaur | DPP | DPF |
| J-16 | 0.706855 | hadrosaur | DPP | DPF |
| J-17 | 0.707044 | hadrosaur | DPP | DPF |
| 100-1 | 0.707293 | Myledaphus | DPP | OMF |
| 100-2 | 0.707227 | Myledaphus | DPP | OMF |
| 100-3 | 0.707366 | Myledaphus | DPP | OMF |
| 100-4 | 0.707246 | Myledaphus | DPP | OMF |
| 100-5 | 0.707752 | crocodile | DPP | OMF |
| 100-6 | 0.707558 | gar | DPP | OMF |
| 100-7 | 0.707293 | theropod | DPP | OMF |
| Br-1.1 | 0.706373 | Myledaphus | BR | DPF |
| Br-1.2 | 0.706109 | Myledaphus | BR | DPF |
| Br-1.3 | 0.706383 | Myledaphus | BR | DPF |
| Br-1.4 | 0.706605 | Myledaphus | BR | DPF |
| Br-2 | 0.706356 | crocodile | BR | DPF |
| Br-3 | 0.706409 | crocodile | BR | DPF |
| Br-4.1 | 0.706148 | Myledaphus | BR | DPF |
| Br-4.2 | 0.706176 | Myledaphus | BR | DPF |
| Br-4.3 | 0.706224 | Myledaphus | BR | DPF |
| Br-5.1 | 0.706438 | crocodile | BR | DPF |
| Br-5.2 | 0.706751 | crocodile | BR | DPF |
| HAS-1 | 0.708269 | theropod | MRA | OMF |
| HAS-2 | 0.708347 | theropod | MRA | OMF |
| HAS-3 | 0.708323 | gar | MRA | OMF |
| HAS-4 | 0.708289 | gar | MRA | OMF |
| HAS-5 | 0.708254 | gar | MRA | OMF |
| SSR-1 | 0.707399 | Myledaphus | SSR | DPF |
| SSR-2 | 0.707306 | Myledaphus | SSR | DPF |
| SSR-3 | 0.707183 | Myledaphus | SSR | DPF |
| SSR-4 | 0.707279 | crocodile | SSR | DPF |
| SSR-5 | 0.707321 | crocodile | SSR | DPF |
| SSR-6 | 0.707053 | theropod | SSR | DPF |
| SSR-7 | 0.706923 | crocodile | SSR | DPF |
| SSR-8 | 0.706931 | crocodile | SSR | DPF |
| SSR-10 | 0.706991 | crocodile | SSR | DPF |
| SSR-12 | 0.707041 | Myledaphus | SSR | DPF |
| SSR-13 | 0.707108 | crocodile | SSR | DPF |
| SSR-14 | 0.707506 | Myledaphus | SSR | DPF |
| SSR-15 | 0.707275 | Myledaphus | SSR | DPF |
3. Results and discussion
Chemical information, including strontium isotope ratios, is at risk of being altered after burial, primarily through contact with post-burial fluids. For this study, isotope data were primarily acquired from enamel, owing to that tissue's much higher resistance to post-burial alterations. Steps were also taken to reduce or eliminate any residual diagenetic strontium. We also performed a Sr concentration analysis on two hadrosaur teeth to check for diagenetic signals (see electronic supplementary material, figure S1). This analysis showed possible enrichment in total strontium in all tissues, with much higher values in dentine.
Five dentine samples were collected from two hadrosaur teeth and one fish scale at DPP and were found to preserve near identical Sr isotope ratios (electronic supplementary material, figure S2). This lack of variability suggests a single diagenetic event, which overprinted the original ratios in these fossils. By contrast, enamel samples maintain a significant variance, with enamel and dentine samples from the same teeth preserving divergent isotope ratios. While enamel Sr concentrations may be elevated, this greater variability in isotope ratios suggests that enamel does preserve at least some of the original biogenic strontium signature.
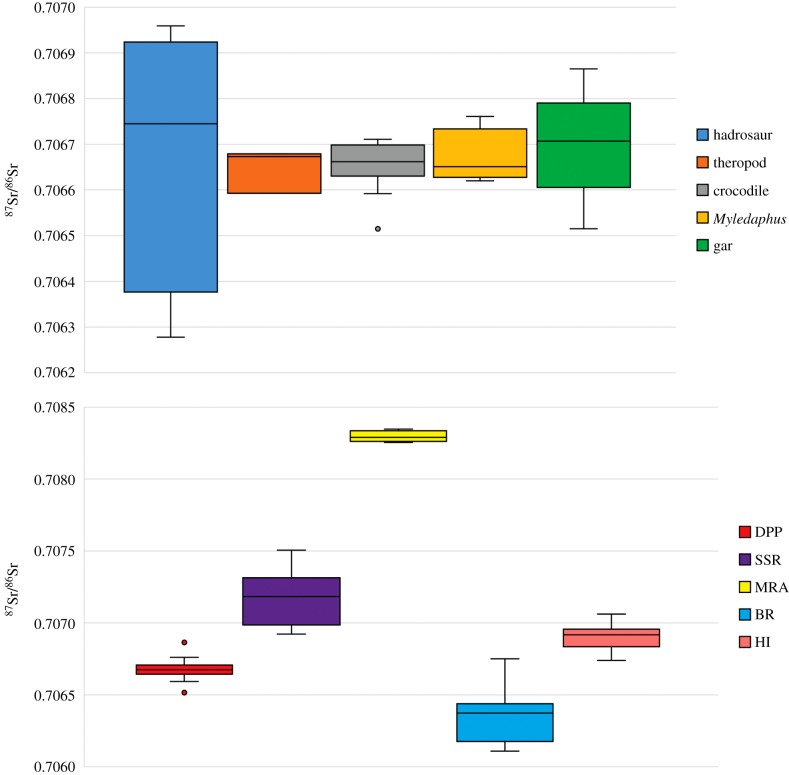
The results are summarized in table 1 and figure 2. Material from the DPF at Dinosaur Provincial Park show a relatively narrow range of 87Sr/86Sr ratios with the only exception being the hadrosaur specimens, which show a much wider range of values (figure 2), a result consistent with hadrosaurs being more mobile. Other taxa in this study are interpreted as non-migratory, owing to the narrower range of strontium isotope values and their general biology (smaller size, mobility tied to waterways, etc.). Presumed non-migratory taxa from other localities produced distinctive ranges of Sr isotope ratios; the SSR locality preserved slightly more radiogenic Sr ratios while the MRA locality preserved much more radiogenic ratios, and the BR locality preserved a significantly lower radiogenic range. To these results, we compared the Sr isotope ratio range preserved in the enamel of the individual hadrosaur from DPP. The jaw presents an overall range of values that overlaps with non-migratory values found in both the DPP and SSR localities, with a mean value close to the midpoint between the mean values at DPP and SSR. A Tukey statistical test confirmed the individual preserved a unique signature from these localities (electronic supplementary material, figure S3). In total, these data support a limited hadrosaur migration distance or large home range between the Dinosaur Provincial Park and South Saskatchewan River areas while discounting any migratory link to the Milk River area.
Figure 2.
Summary of isotope data collected in this study. The upper chart shows the data ranges for different taxa collected from the Dinosaur Park Formation at Dinosaur Provincial Park, while the lower chart contains data from presumed non-migratory taxa from a variety of approximately co-eval localities alongside data collected from the hadrosaur individual (HI) from the DPF at Dinosaur Provincial Park.
The total distance between the Dinosaur Provincial Park and South Saskatchewan River localities is approximately 80 km, a distance that is relatively comparable to migrations covered by modern elephants in Africa [28] or the home ranges of elephants in Borneo [29], which are among the best modern analogues for hadrosaurs on the basis of similarities in mass and presumed diet. There is little palaeo-environmental difference between these two localities; both are located in the Dinosaur Park Formation, which represents a coastal floodplain environment dominated by meandering river systems [30]. The only difference between the localities is the relative proximity to the coastline (with the South Saskatchewan River locality nearer the coast). Using the current results, it is not yet possible to determine if this is a seasonal migration pattern, or simply represents the natural range of the individual. Given the environmental similarities, it is unlikely that seasonal weather or food shortages would be the impetus for such a migration; however, we cannot rule out migratory behaviour for the purposes of breeding.
Given the abundance of hadrosaur fossils collected in the DPF [31,32], biological productivity rates must have been quite high all year to support a large population of resident hadrosaurs. While most other herbivores found in the DPF consumed very low browse, hadrosaurs were capable of consuming higher elevation plants, being able to reach vegetation as high as 5 m [33,34]. This ability to exploit different vegetation sources may have been key to the success of hadrosaurs in the area, allowing them to avoid large-scale migrations.
It has previously been suggested that arctic hadrosaurs migrated hundreds or thousands of kilometres every year in a fashion similar to modern caribou [7,35,36]. The results of this study do not support this model, and corroborate a non-migratory model based on biomechanical grounds [12]. Our data show no overlap between the hadrosaur individual from Dinosaur Provincial Park and the southern Milk River locality or more northern Battle River locality, suggesting no significant southerly or northerly migration. While we cannot rule out larger migrations to the west because of the absence of other locality data, the simplest solution supported by the data is that of a smaller migratory path or large home range in southern Alberta, well short of the thousands of kilometres that would have been necessary for dinosaurs on the North Slope of Alaska to reach the milder winter conditions that would have been found in Alberta during the Late Cretaceous. This is further supported by strontium isotope data from a previous study on stratigraphically younger hadrosaurs from the Prince Creek Formation of Alaska, in which values between 0.7081 and 0.7092 were obtained [37]. While we should be cautious in ascribing limited migratory behaviour to all species of hadrosaurs found in all regions of North America, our conclusions are in agreement with recent work suggesting dinosaurs were limited in their migrations and likely overwintered in the ancient arctic [2,8,10,11,12]. Our conclusions are also in agreement with those of Fricke et al. [10], who used oxygen and carbon isotopes to evaluate migratory behaviours (or lack thereof) in hadrosaurs and concluded that large continent-scale migrations were unlikely.
The use of strontium isotopes in this study shows considerable utility in determining migratory and ranging behaviours in extinct dinosaurs. While data from a larger number of localities could better constrain this movement, we are confident that our method suggests a minimum 80 km range for hadrosaurs living in southern Alberta during the Late Cretaceous. This method could be extended to other possibly migratory taxa, particularly ceratopsians, which are interpreted to have travelled in very large herds during this time [38,39]. Using strontium isotopes, we may be able to quantify migratory behaviours, thereby gaining a better understanding of the ecology of extinct terrestrial organisms and how they moved through their respective environments.
Supplementary Material
Acknowledgements
We acknowledge Dr Don Brinkman (Royal Tyrrell Museum) for his assistance in acquiring suitable fossil materials for this study from the museum collections, as well as the Royal Tyrrell Museum for loaning materials. We also acknowledge Kerri Miller for her help with isotope analysis procedures.
Ethics
All fossils collected from the field for this study were obtained under the collection permit 17-154, as required under Alberta Provincial Law. Information on storage facility and accession numbers for all fossils in this study can be found in the electronic supplementary material.
Data accessibility
All data used in this study can be accessed via the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.x95x69pf0 [40].
Authors' contributions
D.F.T., C.M.H. and J.S.A. contributed to the design of the study. All field and laboratory work was performed by D.F.T. Data analysis and initial drafting of the manuscript and figures were performed by D.F.T., while significant revising was performed by J.S.A. and C.M.H. All authors approved the final version of the manuscript, and agree to be held accountable for the contents of the final version.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a Natural Sciences Research and Engineering Council of Canada grant (no. RT730228), and by a student grant from the Dinosaur Research Institute.
References
- 1.Von Huene F. 1928. Lebensbild des SaurischierVorkommens in obersten Keuper von Trossingen in Würtemberg [Pictures of the life of the Saurischia present in the upper-most Keuper of Trossingen in Württemberg]. Palaeobiologica 1, 103–116. [In German.] [Google Scholar]
- 2.Bell PR, Snively E. 2008. Polar dinosaurs on parade: a review of dinosaur migration. Alcheringa 32, 271–284. ( 10.1080/03115510802096101) [DOI] [Google Scholar]
- 3.Culver M, Johnson WE, Pecon-Slattery J, O'Brien SJ. 2000. Genomic ancestry of the American puma (Puma concolor). J. Heredity 91, 186–197. ( 10.1093/jhered/91.3.186) [DOI] [PubMed] [Google Scholar]
- 4.de Lapparent AF. 1962. Footprints of dinosaurs in the Lower Cretaceous of Vestspitsbergen-Svalbard. Norsk Polarinstitutt Arbok 1960, 14–21. [Google Scholar]
- 5.Case JA, Martin JE, Chaney DS, Reguero M, Marenssi SA, Santillana SM, Woodburne MO. 2000. The first duck-billed dinosaur (family Hadrosauridae) from Antarctica. J. Vertebr. Paleontol. 20, 612–614. ( 10.1671/0272-4634(2000)020[0612:TFDBDF]2.0.CO;2) [DOI] [Google Scholar]
- 6.Hammer WR, Hickerson WJ. 1994. A crested theropod dinosaur from Antarctica. Science 264, 828–830. ( 10.1126/science.264.5160.828) [DOI] [PubMed] [Google Scholar]
- 7.Parrish JM, Parrish JT, Hutchison JH, Spicer RA. 1987. Late Cretaceous vertebrate fossils from the North Slope of Alaska and implications for dinosaur ecology. Palaios 2, 377–389. ( 10.2307/3514763) [DOI] [Google Scholar]
- 8.Molnar RE, Wiffen J. 1994. A Late Cretaceous polar dinosaur fauna from New Zealand. Cretaceous Res. 15, 689–706. ( 10.1006/cres.1994.1038) [DOI] [Google Scholar]
- 9.Godefroit P, Golovneva L, Shchepetov S, Garcia G, Alekseev P. 2009. The last polar dinosaurs: high diversity of latest Cretaceous arctic dinosaurs in Russia. Naturwissenschaften 96, 495–501. ( 10.1007/s00114-008-0499-0) [DOI] [PubMed] [Google Scholar]
- 10.Fricke HC, Rogers RR, Gates TA. 2009. Hadrosaurid migration: inferences based on stable isotope comparisons among Late Cretaceous dinosaur localities. Paleobiology 35, 270–288. ( 10.1666/08025.1) [DOI] [Google Scholar]
- 11.Chinsamy A, Thomas DB, Tumarkin-Deratzian AR, Fiorillo AR. 2012. Hadrosaurs were perennial polar residents. Anat. Rec. 295, 610–614. ( 10.1002/ar.22428) [DOI] [PubMed] [Google Scholar]
- 12.Fiorillo AR, Gangloff RA. 2001. The caribou migration model for arctic hadrosaurs (Dinosauria: Ornithischia): a reassessment . Hist. Biol. 15, 323–334. ( 10.1080/0891296021000037327) [DOI] [Google Scholar]
- 13.West JB, Sobek A, Ehleringer JR. 2008. A simplified GIS approach to modelling global leaf water isoscapes. PLoS ONE 3, e2447 ( 10.1371/journal.pone.0002447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fricke HC, Hencecroth J, Hoerner ME. 2011. Lowland–upland migration of sauropod dinosaurs during the Late Jurassic epoch. Nature 480, 513–515. ( 10.1038/nature10570) [DOI] [PubMed] [Google Scholar]
- 15.Bentley RA. 2006. Strontium isotopes from the earth to the archaeological skeleton: a review. J. Archaeol. Method Theory 13, 135–187. ( 10.1007/s10816-006-9009-x) [DOI] [Google Scholar]
- 16.Blum JD, Taliaferro EH, Weisse MT, Holmes RT. 2000. Changes in Sr/Ca, Ba/Ca and 87Sr/86Sr ratios between trophic levels in two forest ecosystems in the northeastern U.S.A. Biogeochemistry 49, 87–101. ( 10.1023/A:1006390707989) [DOI] [Google Scholar]
- 17.Hoppe KA, Koch PL, Carlson RW, Webb SD. 1999. Tracking mammoths and mastodons: reconstruction of migratory behaviour using strontium isotope ratios. Geology 27, 439–442. () [DOI] [Google Scholar]
- 18.Wadleigh MA, Veizer J. 1985. Strontium and its isotopes in Canadian rivers: fluxes and global implications. Geochem. Cosmochem. Acta 49, 1727–1736. ( 10.1016/0016-7037(85)90143-7) [DOI] [Google Scholar]
- 19.Toots H, Voorhies MR. 1965. Strontium in fossil bones and the reconstruction of food chains. Science 149, 854–855. ( 10.1126/science.149.3686.854) [DOI] [PubMed] [Google Scholar]
- 20.Ericson JE. 1985. Strontium isotope characterization in the study of prehistoric human ecology. J. Hum. Evol. 14, 503–514. ( 10.1016/S0047-2484(85)80029-4) [DOI] [Google Scholar]
- 21.Hoppe KA, Koch PL, Furutani TT. 2003. Assessing the preservation of biogenic strontium in fossil bones and tooth enamel. Int. J. Osteoarchaeol. 13, 20–28. ( 10.1002/oa.663) [DOI] [Google Scholar]
- 22.Müller W, Fricke H, Halliday AN, McCulloch MT, Wartho J. 2003. Origin and migration of the Alpine Iceman. Science 302, 862–865. ( 10.1126/science.1089837) [DOI] [PubMed] [Google Scholar]
- 23.Price TD, Knipper C, Grupe G, Smrcka V. 2004. Strontium isotopes and prehistoric human migration: the Bell Beaker Period in Central Europe. Eur. J. Archaeol. 7, 9–40. ( 10.1177/1461957104047992) [DOI] [Google Scholar]
- 24.Szostek K, Madrzyk K, Cienkosz-Stepanczak B. 2015. Strontium isotopes as an indicator of human migration—easy questions, difficult answers. Anthropol. Rev. 78, 133–156. ( 10.1515/anre-2015-0010) [DOI] [Google Scholar]
- 25.Martin JE, et al. 2015. Strontium isotopes and the long-term residency of thalattosuchians in the freshwater environment. Paleobiology 42, 143–156. ( 10.1017/pab.2015.42) [DOI] [Google Scholar]
- 26.Kocsis L, Osi A, Vennemann TCN. 2009. Geochemical study of vertebrate fossils from the Upper Cretaceous (Santonian) Csehbanya Formation (Hungary): evidence for a freshwater habitat of mosasaurs and pycnodont fish. Palaeogeogr. Palaeoclimatol. Palaeoecol. 280, 532–542. ( 10.1016/j.palaeo.2009.07.009) [DOI] [Google Scholar]
- 27.Erikson GM. 1996. Incremental lines of Von Ebner in dinosaurs and the assessment of tooth replacement rates using growth line counts. Proc. Natl Acad. Sci. USA 93, 14 623–14 627. ( 10.1073/pnas.93.25.14623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purdon A, Mole MM, Chase MJ, van Aarde RJ.. 2018. Partial migration in savanna elephant populations distributed across southern Africa. Scient. Rep. 8, 11331 ( 10.1038/s41598-018-29724-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfred R, Ahmad AH, Payne J, Williams C, Ambu LN, How PM, Goosens B. 2012. Home range and ranging behaviour of Bornean elephant (Elephas maximus borneensis) females. PLoS ONE 7, e31400 ( 10.1371/journal.pone.0031400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberth DA, Hamblin AP. 1993. Tectonic, stratigraphic, and sedimentological significance of a regional discontinuity in the upper Judith River Group (Belly River wedge) of southern Alberta, Saskatchewan, and northern Montana. Can. J. Earth Sci. 30, 174–200. ( 10.1139/e93-016) [DOI] [Google Scholar]
- 31.Brinkman DB, Ryan MJ, Eberth DA. 1998. Paleogeographic and stratigraphic distribution of ceratopsids in the Upper Judith River Group of Western Canada. Palaios 13, 160–169. ( 10.2307/3515487) [DOI] [Google Scholar]
- 32.Brinkman DB. 1990. Paleoecology of the Judith River Formation (Campanian) of Dinosaur Provincial Park Alberta, Canada: evidence from vertebrate microfossil localities. Palaeogeogr. Palaeoclimatol. Palaeoecol. 78, 37–54. ( 10.1016/0031-0182(90)90203-J) [DOI] [Google Scholar]
- 33.Mallon JC, Evans DC, Ryan MJ, Anderson JS. 2013. Feeding height stratification among the herbivorous dinosaurs from the Dinosaur Provincial Park Formation (Upper Campanian) of Alberta, Canada. BMC Ecol. 13, 14 ( 10.1186/1472-6785-13-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallon JC. 2019. Competition structured a Late Cretaceous megaherbivorous dinosaur assemblage. Scient. Rep. 9, 15447 ( 10.1038/s41598-019-51709-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul GS. 1988. Physiological, migratorial, climatological, geophysical, survival, and evolutionary implications of Cretaceous polar dinosaurs. J. Paleontol. 64, 640–652. [Google Scholar]
- 36.Currie PJ. 1989. Long-distance dinosaurs. Nat. Hist. Mag. 89, 60–65. [Google Scholar]
- 37.Mori H. 2014. Osteology, relationships and paleoecology of a new arctic hadrosaurid (Dinosauria: Ornithopoda) from the Prince Creek Formation of northern Alaska, pp. 297–319. PhD thesis, University of Alaska Fairbanks. [Google Scholar]
- 38.Ryan MJ, Russell AP, Eberth DA, Currie PJ. 2001. The taphonomy of a Centrosaurus (Ornithischia: Ceratopsidae) bone bed from the Dinosaur Park Formation (Upper Campagnian), Alberta, Canada, with comments on cranial ontogeny. Palaios 16, 482–506. () [DOI] [Google Scholar]
- 39.Chiba K, Ryan MJ, Braman DR, Eberth DA, Scott EE, Brown CM, Kobayashi Y, Evans DC. 2015. Taphonomy of a monodominant Centrosaurus apertus (Dinosauria: Ceratopsia) bonebed from the upper Oldman Formation of southeastern Alberta. Palaios 30, 655–667. ( 10.2110/palo.2014.084) [DOI] [Google Scholar]
- 40.Terrill DF, Henderson CM, Anderson JS. 2020. Data from: New applications of strontium isotopes reveals evidence of limited migratory behaviour in Late Cretaceous hadrosaurs Dryad Digital Repository. ( 10.5061/dryad.x95x69pf0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Terrill DF, Henderson CM, Anderson JS. 2020. Data from: New applications of strontium isotopes reveals evidence of limited migratory behaviour in Late Cretaceous hadrosaurs Dryad Digital Repository. ( 10.5061/dryad.x95x69pf0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data used in this study can be accessed via the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.x95x69pf0 [40].