Abstract
A number of epidermal proteins are closely related to skin barrier function, the abnormalities of which can lead to specific skin diseases. These proteins must be quantified to further investigate the changes in the skin barrier between healthy and disease states. However, the non-invasive and proteome-wide quantification of skin proteins without any labelling steps remains a challenge. In this study, 3M medical adhesive tapes were used to obtain skin samples from volunteers. Proteins were extracted from fresh skin samples and digested with trypsin. Each tryptic peptide was analysed in three replicates using liquid chromatography with tandem mass spectrometry analysis and label-free quantification. The data were searched against the Human Universal Protein Resource (UniProt) to match with known proteins. Using this method, 1,157 skin proteins recorded in the UniProt were quantified. A total of 50 identical proteins were identified in the three replicate analyses of all samples with no significant differences in abundance. The results provided an objective metric for further study of skin ageing and various skin diseases. Specifically, the non-invasive proteome-wide method used in this study can be applied to future studies of skin diseases related to barrier destruction by monitoring the changes in the levels of epidermal proteins.
Keywords: skin barrier, skin protein, proteome, mass spectrometry, label-free quantification
Introduction
The stratum corneum (SC) is the outermost layer of the epidermis and is required for barrier function to retain moisture in the skin and protect the skin from external invasion (1,2). If the barrier integrity or function is disrupted, water in the dermis and epidermis will be lost, the balance of the skin microecology will be disrupted (3), and hostile factors from the external environment will easily invade the skin, leading to skin ageing and the occurrence of various skin diseases (1,2,4).
Numerous functions of the skin barrier, including structural, regulatory, hygroscopic and signalling functions, rely on epidermal proteins (5,6). Keratins, cytoskeletal proteins of the skin, are responsible for constituting stable keratin cytoskeletons that maintain stable intercellular adhesion and cell rigidity (6,7). Filaggrin (FLG) and FLG2 are critical for bundling keratins to form dense keratin filaments and attain flat squames in the cornified layer (8). Caspase-14 is involved in processing FLG precursors to produce natural moisturizing factors (NMFs) (9). During cornification, loricrin (LOR), S100 proteins, involucrin, the late cornified envelope (CE) family of proteins and hornerin (HRNR) are cross-linked by transglutaminase (TGM) to form a strong, indissoluble CE, which functions as a barrier for the skin against the external environment (2,8). At sites of attachment to the hemidesmosome and desmosome, the junction plakoglobin (JUP), plectin, desmoplakin (DSP), desmoglein1 (DSG1), desmocollin1 (DSC1) and corneodesmosin (CDSN) proteins are responsible for maintaining the mechanical integrity of the epidermal barrier by binding the intermediate filaments (IFs) to the cornified cells (10–13). Abnormalities of these skin barrier-related proteins can lead to disruptions in skin barrier function and have been verified to be associated with multiple skin diseases, including atopic dermatitis (AD) (1,2), ichthyosis (8) and bullous disease (14).
The present study describes a non-invasive, quantitative method to analyse epidermal proteins in healthy individuals using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, in combination with the most convenient sampling method: tape stripping. LC-MS/MS is a high-throughput quantitative technique that can be used to analyse large numbers of proteins in human tissues (15). In the present study, LC-MS/MS analysis was performed on an EASY 1200 nanoflow liquid chromatography (EASY-nLC™ 1200) instrument coupled to a Q Exactive™ HF-X mass spectrometer. Q Exactive HF-X is the most recently developed MS instrument in the benchtop Orbitrap series, with a novel peak selection algorithm and a bright ion source that can efficiently capture MS/MS data above 40 Hz at a resolution of 7500 (16). MaxLFQ was also used, a novel intensity determination and normalization procedure for label-free quantification based on the intensities of the precursor ion signals. In addition, the label-free quantification method was coupled with accurate quantitative standard statistical methods to quantify thousands of proteins without any labelling steps, and reduce undesirable biases associated with the additional steps (17).
Materials and methods
Study participants
Skin samples were collected from two healthy Chinese females (aged 25–35 years with a mean age of 29 years) from Anhui Medical University on September 2, 2017. The study was performed between September 2017 and January 2018. Individuals whose history indicated tape allergies, skin diseases or other systemic diseases involving the skin were excluded from the study. No topical emollient or other cosmetics were used 24 h before the experiment. This study was conducted in accordance with the recommendations of the Medical Ethics Committee of Anhui Medical University, and written informed consent was obtained from all included subjects.
Materials
Sodium dodecyl sulfate (SDS), sodium phosphate, 67% ethanol, dithiothreitol, iodoacetamide, ammonium bicarbonate, trifluoroacetic acid (TFA; ≥99.0% purity), and mass acetonitrile (ACN; Sigma-Aldrich; Merck KGaA). Sequencing-grade modified trypsin was obtained from Promega Corporation. 3M™ Empore™ C18 47 mm extraction discs, Model 2215, Pierce™ C18 tips, a 10 µl bed, a Thermomixer unit (MS-100), CentriVap, rotor, cold trap, vacuum pump, glass bottle, translucent solvent-absorbing trap, ammonia-absorbing trap stuffing and vacuum tube kit (Thermo Fisher Scientific, Inc.). A 10 K 1.5 ml ultrafiltration device (flat base) was obtained from Pall Corporation. The Q Exactive HF-X instrument and the EASY-nLC 1200 system were obtained from Thermo Fisher Scientific, Inc. Unless stated otherwise, all materials were purchased from Thermo Fisher Scientific, Inc.
Sample preparation
The volar forearm skin was cleaned by gentle swabbing with a sterile cotton ball, after which 3M medical adhesive tape was used to remove the skin layers from the volar forearm area. All skin samples were sequentially collected from the same site. The procedure was conducted by the same technician on all volunteers during the study to minimize variations.
Protein extraction, digestion and clean up
Each tape was transferred (adhesive side towards the centre) to a sterile 15 ml plastic tube containing a 2% SDS solution in 0.1 mol/l sodium phosphate (pH 7.8; buffer A). The tubes were incubated at room temperature for 2 days. Then, cells that were dissociated from the tape accumulated at the bottom of the tubes. Afterwards, the cells were removed by pipetting, rinsed twice with 600 µl of buffer A solution, centrifuged at 8,000 × g for 3 min at 4°C, and then resuspended in 300 µl of buffer A solution. Next, 15.8 µl of dithiothreitol was mixed into the cell suspension. After incubation at 37°C for 2 h, 35 µl of iodoacetamide was added. After incubation at 25°C in the dark for 40 min, 900 µl of 67% ethanol was added to precipitate the proteins, and then the protein pellets were washed twice with 67% ethanol. After centrifugation at 10,000 × g for 5 min at 4°C, the protein was digested at 37°C in 300 µl of ammonium bicarbonate/10% ACN by adding 20 mg of trypsin every 24 h until the enzymatic digestion was completed at ~72 h. The digest was centrifuged at 8,000 × g for 2 min at 4°C, 10% TFA was added to the supernatant, and the solution was dried by centrifuging at 8,000 × g for 20 min at 4°C. Then, the residue was re-dissolved, desalted and concentrated to obtain the peptide mixture.
LC-MS/MS analysis
The resulting peptide mixture was subjected to LC-MS/MS analysis on an EASY-nLC 1200 system coupled to a Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific, Inc.); each peptide was analysed via LC-MS/MS in three replicates. The samples were loaded onto a C18 µ-precolumn and capillary analytical C18 column at a flow rate of 0.3 µl/min with a pressure of 860 bar (12,470 psi). The gradient of 80% ACN/ 0.2% TFA increased from 5 to 100% over a period of 60 min. Eluted peptides were analysed using the Q Exactive HF-X mass spectrometer with a nanoelectrospray ionization source and calibrated using tuned instrument control software. The mass spectrometer was operated in positive ionization. The tuned spray voltage was set to 2 kV, the maximum spray current was set to 50, the S-Lens RF level was set to 60, and the capillary temperature was 275°C. In full MS scans, the resolution was set to 60,000 at a range of 350–1500 m/z, and the AGC target was 3e6 with a maximum IT of 20 ms. In subsequent scans, the fragments were analysed at a resolution of 15,000, and the AGC target was set to 5e4 with an IT of 45 msec. An isolation window of 1.6 m/z and one microscan were used to collect suitable tandem mass spectra; the scan range was set to 200–2,000 m/z. The AGC target value for fragment spectra was set to 1e3, and the intensity threshold was maintained at 2.2e4.
Data analysis
The raw data generated from the LC-MS/MS analysis and protein identification and quantification were processed using Proteome Discoverer™ software 2.2 (Thermo Fisher Scientific, Inc.). The data were searched against the Human Universal Protein Resource (UniProt; version June 2017) (18) to match with known proteins. MaxQuant v1.5.8.3 software (19) was used to conduct the label-free database search with the uniqueness degree of ‘razor plus unique peptides’. The MaxLFQ algorithm (17) was adopted to obtain the final results for each sample, and the LFQ intensities were determined as the full peak volume. To obtain the minimal overall proteome variation, the protein quantities were determined through a global normalization procedure after summing up the intensities with normalization factors as free variables (17).
Results
In the present study, three replicates of each peptide were analysed by LC-MS/MS and the MaxLFQ algorithm. Using this method, 1,157 proteins included in UniProt were quantified. A total of 50 skin barrier-related proteins were detected in all skin samples with no significant difference in LFQ intensities. After the peptides were subjected to LC-MS/MS analysis, the protein peaks were obtained (Figs. 1–4). The observed abundances of the proteins are depicted as log2 median LFQ intensities, which are the median LFQ intensity values of the three replicates of each sample (Table I). These proteins were then divided into 12 groups according to their specific properties (Table I).
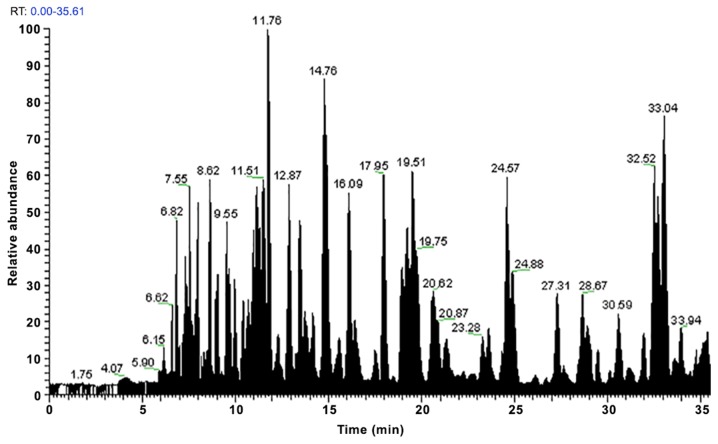
Figure 1.
Peak results from the first analysis of the three replicates of sample 1.
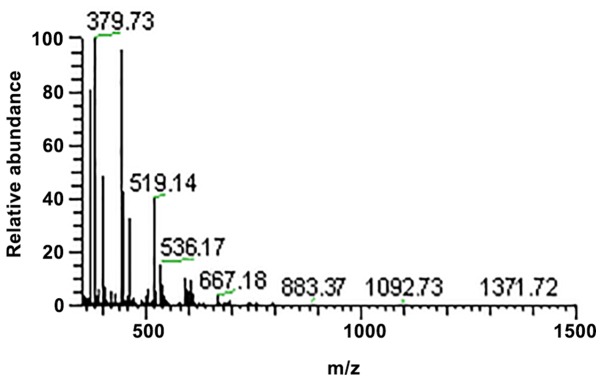
Figure 4.
Tandem mass spectrometry spectra of sample 2.
Table I.
Log2 median LFO intensities of 50 skin-barrier-related proteins detected in the normal forearm skin.
| LFQ intensities (medians) | ||||
|---|---|---|---|---|
| Majority protein IDs | Protein names | Gene names | Sample 1 | Sample 2 |
| Keratins | ||||
| P13645 | Keratin, type I cytoskeletal 10 | KRT10 | 36.95459 | 36.93528 |
| P02533 | Keratin, type I cytoskeletal 14 | KRT14 | 31.31441 | 31.5455 |
| P08779 | Keratin, type I cytoskeletal 16 | KRT16 | 27.96714 | 28.47433 |
| Q04695 | Keratin, type I cytoskeletal 17 | KRT17 | 31.9048 | 32.24141 |
| P35527 | Keratin, type I cytoskeletal 9 | KRT9 | 32.05689 | 32.9607 |
| Q9C075 | Keratin, type I cytoskeletal 23 | KRT23 | 26.70504 | 26.67815 |
| Q15323 | Keratin, type I cuticular Ha1 | KRT31 | 27.02739 | 26.21795 |
| Q14525 | Keratin, type I cuticular Ha3-II | KRT33B | 24.78502 | 24.83408 |
| O76011 | Keratin, type I cuticular Ha4 | KRT34 | 23.76223 | 24.07802 |
| P04264 | Keratin, type II cytoskeletal 1 | KRT1 | 36.64541 | 36.80581 |
| P35908 | Keratin, type II cytoskeletal 2 epidermal | KRT2 | 37.13996 | 36.82632 |
| P13647 | Keratin, type II cytoskeletal 5 | KRT5 | 31.75963 | 32.1306 |
| P02538 | Keratin, type II cytoskeletal 6A | KRT6A | 26.15018 | 26.35316 |
| P04259 | Keratin, type II cytoskeletal 6B | KRT6B | 29.9709 | 30.36661 |
| Q7Z794 | Keratin, type II cytoskeletal 1b | KRT77 | 30.76232 | 30.73876 |
| Q8N1N4 | Keratin, type II cytoskeletal 78 | KRT78 | 29.05447 | 28.91809 |
| Q6KB66 | Keratin, type II cytoskeletal 80 | KRT80 | 28.45615 | 28.4225 |
| O43790 | Keratin, type II cuticular Hb6 | KRT86 | 27.69677 | 26.73221 |
| CE constituents | ||||
| Q5D862 | Filaggrin | FLG | 29.40947 | 29.98427 |
| P20930 | Filaggrin-2 | FLG2 | 24.93454 | 27.0202 |
| P23490 | Loricrin | LOR | 26.81913 | 27.16087 |
| Q86YZ3 | Hornerin | HRNR | 27.98104 | 28.781 |
| Q5T750 | Skin-specific protein 32 | XP32 | 29.76257 | 29.93143 |
| CE processing enzymes | ||||
| P22735 | Transglutaminase 1 | TGM1 | 27.61105 | 26.87912 |
| Q08188 | Transglutaminase 3 | TGM3 | 27.36745 | 27.56856 |
| O75342 | Arachidonate 12-lipoxygenase, 12R-type | ALOX12B | 27.79241 | 27.72338 |
| Q9BYJ1 | Hydroperoxide isomerase ALOXE3 | ALOXE3 | 25.4928 | 25.24318 |
| Calcium binding proteins | ||||
| Q9HCY8 | Protein S100-A14 | S100A14 | 25.99766 | 25.62522 |
| Q96FQ6 | Protein S100-A16 | S100A16 | 25.70376 | 25.82092 |
| Enzymes contributing to | ||||
| NMFs generation | ||||
| P31944 | Caspase-14 | CASP14 | 25.40636 | 25.44815 |
| Q13867 | Bleomycin hydrolase | BLMH | 25.61336 | 25.63619 |
| P05089 | Arginase-1 | ARG1 | 25.52211 | 25.33279 |
| P42357 | Histidine ammonia-lyase | HAL | 26.91957 | 24.17463 |
| Protease inhibitors | ||||
| Q96P63 | Serpin B12 | SERPINB12 | 27.14705 | 26.9812 |
| A8K2U0 | α-2-macroglobulin-like protein 1 | A2ML1 | 25.62439 | 24.16 |
| Cornedesmosome | ||||
| constituents | ||||
| P14923 | Junction plakoglobin | JUP | 24.98385 | 25.92189 |
| Q15149 | Plectin | PLEC | 23.68239 | 22.36937 |
| P15924 | Desmoplakin | DSP | 26.95593 | 27.4182 |
| Q02413 | Desmoglein-1 | DSG1 | 29.14523 | 29.43571 |
| Q08554 | Desmocollin-1 | DSC1 | 27.99108 | 28.31514 |
| Q15517 | Corneodesmosin | CDSN | 25.82845 | 25.97169 |
| Annexin | ||||
| P07355;A6NMY6 | Annexin A2 | ANXA2 | 27.51893 | 27.104 |
| Substance metabolism | ||||
| proteins/enzymes | ||||
| P04406 | Glyceraldehyde-3-phosphate | GAPDH | 25.89973 | 24.53011 |
| dehydrogenase | ||||
| P25311 | Zinc-α-2-glycoprotein | AZGP1 | 25.35336 | 25.00945 |
| Signal transduction | ||||
| proteins/enzymes | ||||
| Q96QA5 | Gasdermin-A | GSDMA | 25.79793 | 26.26551 |
| Q5T749 | Keratinocyte proline-rich protein | KPRP | 30.88103 | 30.98854 |
| Q16610 | Extracellular matrix protein 1 | ECM1 | 24.89957 | 24.52139 |
| Proteins involved in | ||||
| REDOX reactions | ||||
| P10599 | Thioredoxin | TXN | 26.88925 | 26.86361 |
| P04040 | Catalase | CAT | 25.53703 | 25.25785 |
| Actin binding proteins | ||||
| Q09666 | Neuroblast differentiation-associated protein AHNAK | AHNAK | 24.43657 | 24.1404 |
CE, cornified envelope; NMFs, natural moisturizing factors.
Keratins
A total of 18 keratins were detected in the volar forearm skin: K9, K10, K14, K16, K17, K23, K31, K33B, K34 (type I), K1, K2, K5, K6A, K6B, K77, K78, K80 and K86 (type II). The LFQ intensities of K1, K2 and K10 were relatively higher than those of the other keratins (Table I).
CE constituents and processing enzymes
The proteins that constitute the CE, including FLG, FLG2, LOR, HRNR and XP32, were detected in this study. Four enzymes related to CE processing were also detected, including TGM1, TGM3, arachidonate 12-lipoxygenase (ALOX12B) and the hydroperoxide isomerase ALOXE3 (ALOXE3).
Calcium-binding proteins
In this study, the expression of two calcium-binding proteins was identified (S100A14 and S100A16).
Enzymes contributing to NMF generation
The expression of bleomycin hydrolase, caspase-14, arginase1 and histidine ammonia lyase was detected; these contribute to the generation of NMFs.
Protease inhibitors
Two protease inhibitors (serpin B12 and α-2-macroglobulin-like protein 1) were observed in the SC obtained from the volunteers.
Proteins related to SC cohesion
In the present study, 6 proteins were detected, plectin, JUP, DSP, DSG1, DSC1 and CDSN, that are responsible for reinforcing the cohesion of the SC.
Annexin
Annexin A2 was detected in this study.
Substance metabolism regulator
The expression of GAPDH and zinc-α-2-glycoprotein (ZAG), which participate in regulating substance metabolism, were also detected.
Signal transduction proteins
Gasdermin-A, keratinocyte proline-rich protein (KPRP) and extracellular matrix protein 1 (ECM1) were identified in this study.
Antioxidant related proteins/enzymes
The expression of thioredoxin and catalase was observed, these are both related to antioxidant activity.
Actin-binding protein
The expression of the neuroblast differentiation-associated protein AHNAK (AHNAK) was also detected.
Discussion
As a barrier between the external and internal environment of the human body, the major function of our epidermis is to protect us from the hostile external invasion, such as ultraviolet radiation (UVR) and air pollutants (4,20,21). The main cell type in the epidermis is the keratinocyte, which expresses cytoskeletal proteins called keratins. The keratin family is a primary subclass of the IF superfamily, contains 54 proteins and is subdivided into 28 type I (K9-K40) and 26 type II (K1-K8 and K71-K86) IF proteins. Numerous members of the keratin family are widely expressed in the epithelium, so alterations in these keratins are predicted to be the underlying causes of several epithelial diseases (22–24). The expression of 9 type I and 9 type II keratins were detected in the volar forearm skin (Table I). These proteins have various functions, including stabilizing cytoskeletal elements, modulating cellular metabolism, regulating cellular differentiation and proliferation, as well as mediating inflammatory pathways (5–7,25). The keratin pair K5/K14 is expressed in the basal layer, and mutations in K5 or K14 cause 75% of the basal subtypes of epidermolysis bullosa simplex (EBS) (14,26,27) and a rare keratin disease known as Dowling-Degos disease, as well as its variant named Galli-Galli disease (14,28). In the initial stage of terminal differentiation, the keratin pair K1/K10 replaces the K5/K14 pair in the suprabasal layer. One of the earliest proteins expressed during cornification, the K1/K10 pair is a significant scaffold protein that sequentially directs the deposition and cross-linking of CE proteins (7,24). If the epidermal barrier is disrupted, the K1/K10 pair is consumed to repair the injured tissue (29,30). In addition, mutations in K1/K10 lead to ichthyosis, epidermolytic hyperkeratosis and epidermolytic palmoplantar keratoderma (14,23). The hyperproliferation-related keratins, K6a, K6b, K16 and K17, which have specialized roles in the inflammatory response and wound healing, are biomarkers of psoriasis (31,32) and cancers (33,34). When the epidermis is injured, nuclear factor erythroid-derived 2-related factor 2 promotes the proliferation of human keratinocytes by upregulating the expression of K6, K16 and K17 (31,32). The expression of K9 was also detected, which plays important roles in the response to stress and contributes to enhancing mechanical strength (35).
In the SC, FLG and keratins interact with each other to form a dense keratin fibre tract that serves as a strong supportive scaffold for the construction of the CE, which is the basis of the defensive epidermal barrier (8). In the present study, the expression of FLG and FLG2 was detected (Table I). FLG bundles keratin IFs to attain a flattened shape of the cell during the terminal differentiation of the epidermis and plays an important role in SC hydration. The dysfunction of FLG can lead to AD (1,2,8,36) and ichthyosis vulgaris (37). FLG2 is essential for maintaining intercellular adhesion in the cornified layers and provides proper integrity and mechanical strength to the SC. Mutations in FLG2 can cause peeling skin syndrome type A, which is characterized by a superficial detachment of the epidermal cornified layers (38). The expression of caspase-14 and bleomycin hydrolase (Table I), which can hydrolyse FLG monomers into free amino acids in the cornified cell, was also detected. Then, NMFs, an important component required for SC hydration, are produced. The high concentration of these hydrolysed amino acids helps to retain the moisture and elasticity of the cornified layer. Therefore, abnormalities in caspase-14 and bleomycin hydrolase may affect the decomposition of FLG in the SC, leading to reduced levels of NMFs and the subsequent loss of barrier moisture function (8,9,36). This study also detected two other enzymes, arginase-1 and histidine ammonia lyase (Table I), which also contribute to the production of NMFs, urea and urocanic acid (36).
The CE is further reinforced by a series of structural proteins, including LOR, S100 proteins and HRNR, which are cross-linked by TGMs. LOR, a major component of the CE, constitutes 70% of the total protein mass (8). The expression of LOR was detected in the SC of healthy subjects. Mutations in LOR may disrupt keratinocyte differentiation, affect the formation and maturation of CE, and aggravate skin barrier disorders, such as LOR keratoderma, palmoplantar keratoderma and psoriasis (39). S100 proteins belong to the calcium-binding protein family and are characterized by their EF-hand calcium-binding domains, which play an important role in keratinocyte differentiation and function as antimicrobial peptides in the epidermis that protect the skin from microbial invasion (40). Researchers have found that the expression level of S100A in skin lesions of psoriasis patients is higher than that of healthy individuals and is directly proportional to the severity of psoriasis (41). In the present study, the expression of HRNR was detected in the volar forearm skin. HRNR, FLG and FLG-2 share amino acid homology outside the S100 domain and thus have similar structures and numerous common functions in barrier formation and maintenance. Similar to FLG expression, HRNR expression was reduced in the epidermis of patients with AD, which might contribute to the impaired epidermal barrier in patients with AD (42,43).
In the present study, the expression of TGM1, TGM3 and two lipoxygenases (ALOX12B and ALOXE3) were detected in the healthy epidermis (Table I). TGMs provide a direct link between the proteins and lipid components of the CE and esterify ceramides to increase the hydrophobicity of the CE. Therefore, the dysfunction of TGM1 may result in an inappropriate protein scaffold for deposition of the lipid barrier. TGM3 is expressed in terminally differentiated keratinocytes and is involved in the cornification process (8). Notably, ALOX12B and ALOXE3 oxidize the linoleoyl moiety in omega-hydroxy ceramides, which are crucial for producing the intermolecular organization necessary to maintain skin barrier function (44).
Some proteins responsible for SC cohesion were also identified, including JUP, plectin, DSP, DSG1, DSC1 and CDSN (Table I). As a major component of the desmosome, JUP is responsible for cell-cell adhesion (45). Plectin plays a vital role in binding to IFs and protecting cells from osmotic stress (46). At sites of attachment to the hemidesmosome, the keratin filaments of basal keratinocytes are less tightly bundled in the absence of plectin, which may result in the occurrence of EBS (10,46,47). DSP contributes to maintaining the mechanical integrity of the epidermal barrier by linking the IFs to the plasma membrane at sites of attachment to the desmosome. The absence of DSP in the epidermis can lead to the collapse of the keratin cytoskeleton and weaken the intercellular adhesion (11). By analogy to DSP, DSG1, DSC1 and CDSN are cross-linked to the CE to form corneodesmosomes, which bind to cornified cells and further reinforce the barrier function (12). The lack of DSG1 can cause pemphigus vulgaris, indicating important roles for DSG1 in maintaining intercellular adhesion and epidermal integrity (13).
Serpin B12, a member of the intracellular serine protease inhibitors, was highly expressed in the normal forearm skin in this study. It plays a cytoprotective role in skin barrier function and has been shown to be a therapeutic target for multiple disease processes (48). Another wide-range protease inhibitor, α-2-macroglobulin-like protein 1, may be implicated in maintaining the homeostasis of the epidermal barrier as well as the immune defence process (49). As such, α-2-macroglobulin-like protein 1 is considered a target antigen of paraneoplastic pemphigus (50).
In this study, the expression of annexin A2 was observed, this protein functions in regulating fibroblast proliferation and migration. It is hypothesized that Annexin A2 is involved in skin keloid formation and may be a potential target for therapeutic interventions of keloid lesions (51).
The expression of GAPDH and ZAG was also detected; these are involved in substance metabolism. GAPDH not only participants in glycolytic metabolism but also plays a critical role in apoptosis and multiple cellular functions (52). Recently, research has shown that GAPDH may be a prognostic marker and therapeutic target for patients with cutaneous melanoma (53). As an adipokine, ZAG can modulate lipid metabolism, immune responses and skin barrier function in AD, and is expected to be a biomarker and therapeutic target of AD (54).
ECM1 consists of a signal peptide and four functional domains, suggesting that this protein has signal transduction functions (55). Another two signal transduction proteins, Gasdermin-A and KRRP, were also detected in this study. Gasdermin-A has multiple functions, including forming pores in the cell membrane, transmitting inflammatory signals and inducing apoptosis and inflammation (56). The definite function of KRRP is not yet fully understood. Recently, Suga et al (57) found that a decrease in KPRP can lead to dysfunction of the skin barrier in AD, which revealed that KPRP may enhance the immune responses in AD and could be a new target for therapeutic interventions of AD.
In this study, the expression of thioredoxin and catalase were also identified, which are involved in the antioxidant system. The skin is constantly exposed to oxidative stress generated by external factors including UVR, which may lead to premature senescence (4). Thioredoxin plays a pivotal role in antioxidant and antiapoptotic defence in the human epidermis (58,59). Catalase is one of the most important antioxidant enzymes in the process of skin ageing, and its decrease can lead to accelerated ageing of human skin (60). The expression of AHNAK was also detected, which is downregulated in melanoma cells and may be a prognostic marker of melanoma (61).
In summary, the present study explored a non-invasive and proteome-wide method for the quantification of skin proteins. These results provided an objective metric for future studies on monitoring changes in skin protein levels in the context of skin ageing and skin diseases. Compared to the commonly invasive skin biopsy and traditional methods of protein identification, the tape stripping method combined with LC-MS/MS analysis and the MaxLFQ algorithm used in the present study has the advantages of simple application, no surgical trauma, no extra labelling steps, no requirement of a reference standard, high throughput, high sensitivity and high accuracy. These properties may allow this approach to be used in future studies of skin diseases related to barrier destruction by monitoring changes in the levels of epidermal proteins, which may provide insight into the diagnosis, prognosis and development of new targets for therapeutic intervention of these skin diseases in the future. As a methodological exploration, the present study still had some limitations. First, the small sample size was not representative enough, requiring more detailed studies in the future. Second, there were inevitably errors in the process of sample collection and processing, although everything was done to minimize the effects of human factors.
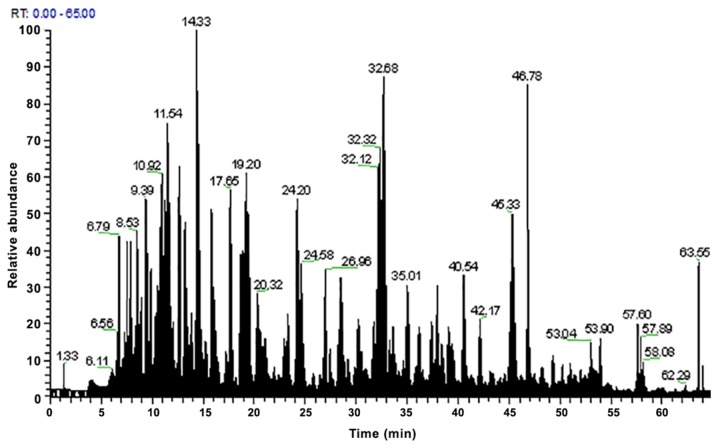
Figure 2.
Peak results from the second analysis of the three replicates of sample 2.
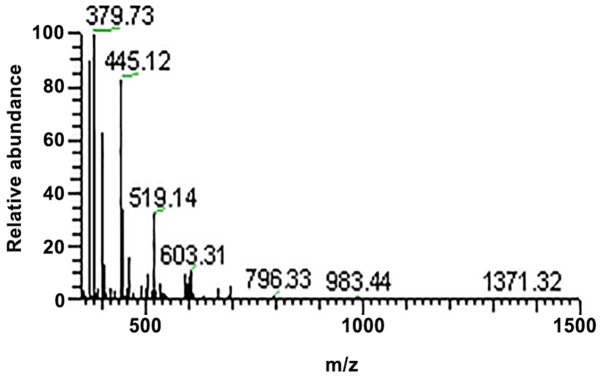
Figure 3.
Tandem mass spectrometry spectra of sample 1.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the Science & Technology Action Plans for the Prevention and Treatment of Major Diseases sponsored by National Health and Family Planning Commission of the People's Republic of China (grant no. 2017ZX-01E-002).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
All authors participated in the design and interpretation of the studies and review of the paper. JZ performed the experiments. ML and JZ performed the data analysis. ML, YW and CX interpreted and collected the data. JM, SX and XW performed the experiments and revised the manuscript. SY, JG and XZ designed and guided the study. The paper was written by ML. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was conducted in accordance with the recommendations of the Medical Ethics Committee of Anhui Medical University (reference no. PJ2016-03-02), and written informed consent was obtained from all included subjects.
Patient consent for publication
Consent for publication was obtained from all of the patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Egawa G, Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J Allergy Clin Immunol. 2016;138:350–358.e1. doi: 10.1016/j.jaci.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai W, Huang Y, Zhang X, Fei W, Chang Y, Cheng S, Zhou Y, Gao J, Tang X, Zhang X, Yang S. Profile of the skin microbiota in a healthy Chinese population. J Dermatol. 2018;45:1289–1300. doi: 10.1111/1346-8138.14594. [DOI] [PubMed] [Google Scholar]
- 4.Bocheva G, Slominski RM, Slominski AT. Neuroendocrine aspects of skin aging. Int J Mol Sci. 2019;20:E2798. doi: 10.3390/ijms20112798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–1597. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- 6.Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Homberg M, Magin TM. Beyond expectations: Novel insights into epidermal keratin function and regulation. Int Rev Cell Mol Biol. 2014;311:265–306. doi: 10.1016/B978-0-12-800179-0.00007-6. [DOI] [PubMed] [Google Scholar]
- 8.Candi E, Schmidt R, Melino G. The cornified envelope: A model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 9.Hvid M, Johansen C, Deleuran B, Kemp K, Deleuran M, Vestergaard C. Regulation of caspase 14 expression in keratinocytes by inflammatory cytokines-a possible link between reduced skin barrier function and inflammation. Exp Dermatol. 2011;20:633–636. doi: 10.1111/j.1600-0625.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 10.Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol. 2006;174:557–568. doi: 10.1083/jcb.200605172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3:1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- 12.Kitajima Y. Regulation and impairments of dynamic desmosome and corneodesmosome remodeling. Eur J Dermatol. 2013 Apr 30; doi: 10.1684/ejd.2013.1976. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13.Koga H, Tsuruta D, Ohyama B, Ishii N, Hamada T, Ohata C, Furumura M, Hashimoto T. Desmoglein 3, its pathogenecity and a possibility for therapeutic target in pemphigus vulgaris. Expert Opin Ther Targets. 2013;17:293–306. doi: 10.1517/14728222.2013.744823. [DOI] [PubMed] [Google Scholar]
- 14.Szeverenyi I, Cassidy AJ, Chung CW, et al. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum Mutat. 2008;29:351–360. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, et al. Proteogenomic characterization of human colon and rectal cancer. Nature. 2014;513:382–387. doi: 10.1038/nature13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelstrup CD, Bekker-Jensen DB, Arrey TN, Hogrebe A, Harder A, Olsen JV. Performance evaluation of the Q exactive HF-X for shotgun proteomics. J Proteome Res. 2018;17:727–738. doi: 10.1021/acs.jproteome.7b00602. [DOI] [PubMed] [Google Scholar]
- 17.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 20.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: Regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212(v, vii):1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018;159:1992–2007. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan X, Hobbs RP, Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol. 2013;25:47–56. doi: 10.1016/j.ceb.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toivola DM, Boor P, Alam C, Strnad P. Keratins in health and disease. Curr Opin Cell Biol. 2015;32:73–81. doi: 10.1016/j.ceb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123–153. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- 25.Arul S, Dayalan H, Jegadeesan M, Damodharan P. Induction of differentiation in psoriatic keratinocytes by propylthiouracil and fructose. BBA Clin. 2016;6:82–86. doi: 10.1016/j.bbacli.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolling MC, Lemmink HH, Jansen GH, Jonkman MF. Mutations in KRT5 and KRT14 cause epidermolysis bullosa simplex in 75% of the patients. Br J Dermatol. 2011;164:637–644. doi: 10.1111/j.1365-2133.2010.10146.x. [DOI] [PubMed] [Google Scholar]
- 27.Haines RL, Lane EB. Keratins and disease at a glance. J Cell Sci. 2012;125:3923–3928. doi: 10.1242/jcs.099655. [DOI] [PubMed] [Google Scholar]
- 28.Hanneken S, Rütten A, Pasternack SM, Eigelshoven S, El Shabrawi-Caelen L, Wenzel J, Braun-Falco M, Ruzicka T, Nöthen MM, Kruse R, Betz RC. Systematic mutation screening of KRT5 supports the hypothesis that Galli-Galli disease is a variant of Dowling-Degos disease. Br J Dermatol. 2010;163:197–200. doi: 10.1111/j.1365-2133.2010.09741.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Wong P. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 30.Reichelt J, Magin TM. Hyperproliferation, induction of c-Myc and 14-3-3 sigma, but no cell fragility in keratin-10-null mice. J Cell Sci. 2002;115:2639–2650. doi: 10.1242/jcs.115.13.2639. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Fan X, Cui T, Dang E, Wang G. Nrf2 promotes keratinocyte proliferation in psoriasis through up-regulation of Keratin 6, Keratin 16 and Keratin 17. J Invest Dermatol. 2017;137:2168–2176. doi: 10.1016/j.jid.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Jin L, Wang G. Keratin 17: A critical player in the pathogenesis of psoriasis. Med Res Rev. 2014;34:438–454. doi: 10.1002/med.21291. [DOI] [PubMed] [Google Scholar]
- 33.Stoler A, Duvic M, Fuchs E. Unusual patterns of keratin expression in the overlying epidermis of patients with dermatofibromas: Biochemical alterations in the epidermis as a consequence of dermal tumors. J Invest Dermatol. 1989;93:728–738. doi: 10.1111/1523-1747.ep12284397. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa K, Katagata Y, Kondo S. Relative amounts of keratin 17 are higher than those of keratin 16 in hair-follicle-derived tumors in comparison with nonfollicular epithelial skin tumors. J Invest Dermatol. 1995;104:396–400. doi: 10.1111/1523-1747.ep12665888. [DOI] [PubMed] [Google Scholar]
- 35.Swensson O, Langbein L, McMillan JR, Stevens HP, Leigh IM, McLean WH, Lane EB, Eady RA. Specialized keratin expression pattern in human ridged skin as an adaptation to high physical stress. Br J Dermatol. 1998;139:767–775. doi: 10.1046/j.1365-2133.1998.02499.x. [DOI] [PubMed] [Google Scholar]
- 36.McAleer MA, Jakasa I, Raj N, O'Donnell CPF, Lane ME, Rawlings AV, Voegeli R, McLean WHI, Kezic S, Irvine AD. Early-life regional and temporal variation in filaggrin-derived natural moisturizing factor, filaggrin-processing enzyme activity, corneocyte phenotypes and plasmin activity: Implications for atopic dermatitis. Br J Dermatol. 2018;179:431–441. doi: 10.1111/bjd.16691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thyssen JP, Godoy-Gijon E, Elias PM. Ichthyosis vulgaris: The filaggrin mutation disease. Br J Dermatol. 2013;168:1155–1166. doi: 10.1111/bjd.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamad J, Sarig O, Godsel LM, Peled A, Malchin N, Bochner R, Vodo D, Rabinowitz T, Pavlovsky M, Taiber S, et al. Filaggrin 2 deficiency results in abnormal cell-cell adhesion in the cornified cell layers and causes peeling skin syndrome type A. J Invest Dermatol. 2018;138:1736–1743. doi: 10.1016/j.jid.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BE, Howell MD, Guttman-Yassky E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, Krueger JG, Leung DY. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: Role for TNF-α antagonists to improve skin barrier. J Invest Dermatol. 2011;131:1272–1279. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/S0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y, Jang S, Min JK, Lee K, Sohn KC, Lim JS, Im M, Lee HE, Seo YJ, Kim CD, Lee JH. S100A8 and S100A9 are messengers in the crosstalk between epidermis and dermis modulating a psoriatic milieu in human skin. Biochem Biophys Res Commun. 2012;423:647–653. doi: 10.1016/j.bbrc.2012.05.162. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Meyer-Hoffert U, Reithmayer K, Paus R, Hansmann B, He Y, Bartels J, Gläser R, Harder J, Schröder JM. Highly complex peptide aggregates of the S100 fused-type protein hornerin are present in human skin. J Invest Dermatol. 2009;129:1446–1458. doi: 10.1038/jid.2008.370. [DOI] [PubMed] [Google Scholar]
- 43.Henry J, Hsu CY, Haftek M, Nachat R, de Koning HD, Gardinal-Galera I, Hitomi K, Balica S, Jean-Decoster C, Schmitt AM, et al. Hornerin is a component of the epidermal cornified cell envelopes. FASEB J. 2011;25:1567–1576. doi: 10.1096/fj.10-168658. [DOI] [PubMed] [Google Scholar]
- 44.Muñoz-Garcia A, Thomas CP, Keeney DS, Zheng Y, Brash AR. The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim Biophys Acta. 2014;1841:401–408. doi: 10.1016/j.bbalip.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pigors M, Kiritsi D, Krümpelmann S, Wagner N, He Y, Podda M, Kohlhase J, Hausser I, Bruckner-Tuderman L, Has C. Lack of plakoglobin leads to lethal congenital epidermolysis bullosa: A novel clinico-genetic entity. Hum Mol Genet. 2011;20:1811–1819. doi: 10.1093/hmg/ddr064. [DOI] [PubMed] [Google Scholar]
- 46.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111:2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- 47.Winter L, Wiche G. The many faces of plectin and plectinopathies: Pathology and mechanisms. Acta Neuropathol. 2013;125:77–93. doi: 10.1007/s00401-012-1026-0. [DOI] [PubMed] [Google Scholar]
- 48.Niehaus JZ, Good M, Jackson LE, Ozolek JA, Silverman GA, Luke CJ. Human SERPINB12 is an abundant intracellular serpin expressed in most surface and glandular epithelia. J Histochem Cytochem. 2015;63:854–865. doi: 10.1369/0022155415600498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galliano MF, Toulza E, Gallinaro H, Jonca N, Ishida-Yamamoto A, Serre G, Guerrin M. A novel protease inhibitor of the alpha2-macroglobulin family expressed in the human epidermis. J Biol Chem. 2006;281:5780–5789. doi: 10.1074/jbc.M508017200. [DOI] [PubMed] [Google Scholar]
- 50.Schepens I, Jaunin F, Begre N, Läderach U, Marcus K, Hashimoto T, Favre B, Borradori L. The protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in human. PLoS One. 2010;5:e12250. doi: 10.1371/journal.pone.0012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SH, Jung SH, Chung H, Jo DI, Kim CK, Park SH, Won KJ, Jeon HS, Kim B. Annexin A2 participates in human skin keloid formation by inhibiting fibroblast proliferation. Arch Dermatol Res. 2014;306:347–357. doi: 10.1007/s00403-014-1438-x. [DOI] [PubMed] [Google Scholar]
- 52.Tristan C, Shahani N, Sedlak TW, Sawa A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal. 2011;23:317–323. doi: 10.1016/j.cellsig.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramos D, Pellín-Carcelén A, Agustí J, Murgui A, Jordá E, Pellín A, Monteagudo C. Deregulation of glyceraldehyde-3-phosphate dehydrogenase expression during tumor progression of human cutaneous melanoma. Anticancer Res. 2015;35:439–444. [PubMed] [Google Scholar]
- 54.Noh JY, U Shin J, Kim JH, Kim SH, Kim BM, Kim YH, Park S, Kim TG, Shin KO, Park K, Lee KH. ZAG regulates the skin barrier and immunity in atopic dermatitis. J Invest Dermatol. 2019;139:1648–1657.e7. doi: 10.1016/j.jid.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 55.Chan I. The role of extracellular matrix protein 1 in human skin. Clin Exp Dermatol. 2004;29:52–56. doi: 10.1111/j.1365-2230.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- 56.Feng S, Fox D, Man SM. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol. 2018;430:3068–3080. doi: 10.1016/j.jmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Suga H, Oka T, Sugaya M, Sato Y, Ishii T, Nishida H, Ishikawa S, Fukayama M, Sato S. Keratinocyte proline-rich protein deficiency in atopic dermatitis leads to barrier disruption. J Invest Dermatol. 2019;139:1867–1875.e7. doi: 10.1016/j.jid.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 58.Schallreuter KU, Pittelkow MR, Wood JM. Free radical reduction by thioredoxin reductase at the surface of normal and vitiliginous human keratinocytes. J Invest Dermatol. 1986;87:728–732. doi: 10.1111/1523-1747.ep12456848. [DOI] [PubMed] [Google Scholar]
- 59.Schallreuter KU, Wood JM. Thioredoxin reductase-its role in epidermal redox status. J Photochem Photobiol B. 2001;64:179–184. doi: 10.1016/S1011-1344(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 60.Shin MH, Lee SR, Kim MK, Shin CY, Lee DH, Chung JH. Activation of peroxisome proliferator-activated receptor alpha improves aged and UV-irradiated skin by catalase induction. PLoS One. 2016;11:e0162628. doi: 10.1371/journal.pone.0162628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheppard HM, Feisst V, Chen J, Print C, Dunbar PR. AHNAK is downregulated in melanoma, predicts poor outcome, and may be required for the expression of functional cadherin-1. Melanoma Res. 2016;26:108–116. doi: 10.1097/CMR.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.