Abstract
Background
To analyze control measures used to eradicate a large vancomycin-resistant Enterococci (VRE) outbreak in a nonendemic 1600-bed tertiary care institution.
Methods
In mid-March 2005, VRE Van B was isolated from 2 clinical samples from different wards. Despite such measures as screening patients sharing rooms with index cases and isolating VRE patients, 43 isolates from different wards were detected by the end of March 2005. To eradicate a hospital-wide outbreak, a coordinated strategy between March and June 2005 comprised (1) formation of a VRE task force, (2) hospital-wide screening, (3) isolation of carriers, (4) physical segregation of contacts, (5) surveillance of high-risk groups, (6) increased cleaning, (7) electronic tagging of VRE status, and (8) education and audits. This is a retrospective study of this multipronged approach to containing VRE. The adequacy of rectal swab sampling for VRE was assessed in a substudy of 111 patients. The prevalence of methicillin-resistant Staphylococcus aureus (MRSA)/VRE co-colonization or co-infection also was determined.
Results
A total of 19,574 contacts were identified. Between April and June 2005, 5095 patients were screened, yielding 104 VRE carriers, 54 of whom (52%) were detected in the first 2 weeks of hospital-wide screening. The initial positive yield of 11.4% of persons actively screened declined to 4.2% by the end of June 2005. Pulsed-field typing revealed 1 major clone and several minor clones among the 151 total VRE cases, including 4 clinical cases. Hospital-wide physical segregation of contacts from other patients was difficult to achieve in communal wards. Co-colonization or co-infection with MRSA, which was present in 52 of 151 cases (34%) and the indefinite electronic tagging of positive VRE status strained limited isolation beds. Analysis of 2 fecal or rectal specimens collected 1 day apart may detect at least 83% of VRE carriers.
Conclusion
A multipronged strategy orchestrated by a central task force curbed but could not eradicate VRE. Control measures were confounded by hospital infrastructure and high MRSA endemicity.
Although vancomycin-resistant Enterococci (VRE) is endemic in many settings worldwide, with the Centers for Disease Control and Prevention (CDC) reporting an increasing prevalence in United States hospitals from 0.3% in 1989 to > 25% of all isolates in 1999, its prevalence in a tertiary care institution in Singapore in 2001 was < 0.01%.1 In 2004, a localized VRE outbreak in a hematology ward of Singapore General Hospital (SGH) involving 6 inpatients was blamed on overseas importation of the index case with breaches in infection control measures, resulting in subsequent dissemination in that ward.2 Enhanced infection control measures eradicated that outbreak. No more VRE cases were seen until a large hospital-wide outbreak in March 2005, for which there was no previous local precedence. Presuming background nonendemicity for VRE, we embarked on an eradication strategy modeled on a previously successful effort by Christiansen et al at Royal Perth Hospital.3 Here we review our infection control approach and its shortcomings in dealing with this large outbreak.
Methods
Facility characteristics
SGH has 1600 beds and offers comprehensive medical and surgical services, including acute trauma care, burn care, and solid organ and hematopoietic stem cell transplantation. Single rooms (class A) comprise 11% of the beds; 4-bed rooms (class B1), 21%. The majority of the beds (68%) are in either 6-bed rooms (classes B2 and B2+; 52%) or large open, communal wards with 8- or 9-bed class C cubicles (16%). The latter serve low-income populations, who receive an 80% government subsidy for medical care. For class B1 and B2 beds, patients pay 80% and 35% of the cost, respectively. Patients in class A pay 100% of the costs, with no government subsidy. Figure 1 shows a plan of a typical C class ward.
Fig 1.
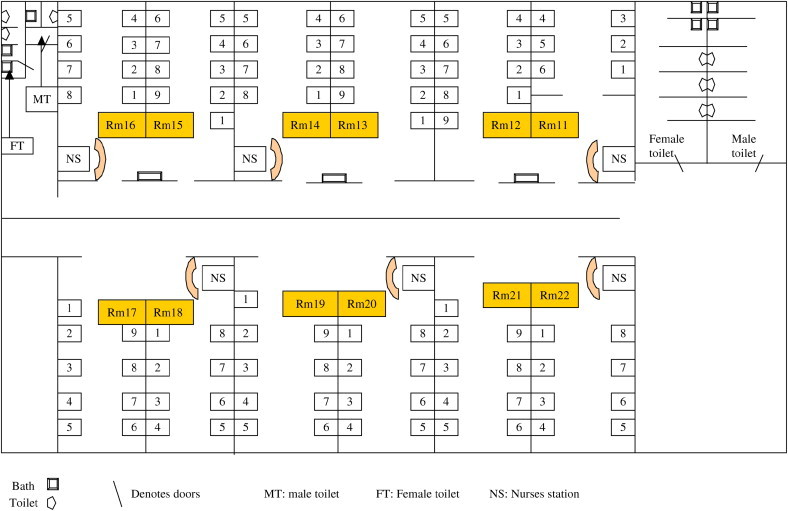
Geographic map of a typical C class ward (Ward 63C).
The outbreak
In mid-March 2005, Van B phenotype vancomycin-resistant Enterococcus faecium was isolated from wound samples of 2 patients from different wards. These isolates were resistant to ampicillin and had minimum inhibitory concentrations of 32 μg/mL for vancomycin and 1 to 2 μg/mL for teicoplanin. VRE-positive patients were isolated, and all neighboring contacts were screened. Despite this, by the end of March 2005, another 43 isolates from several different wards had been detected. Subsequently, measures were applied to eradicate a hospital-wide outbreak of VRE.
Outbreak control measures
A VRE Task Force reporting to senior management was formed on April 3, 2005, comprising representatives from various departments, including infectious diseases, microbiology, infection control, bed management, nursing, epidemiology, information technology, operations, and corporate communications. The Task Force met twice daily to review various details of the outbreak and was responsible for developing policies on control measures and communicating these to all staff.
Definitions
The first index case was traced to an inpatient in December 2004. Contacts were broadly defined as inpatients (n = 19,574) between December 1, 2004 and April 3, 2005. Patients with high-risk acquisition for VRE-like end-stage renal failure (ESRF) on dialysis, those with hematologic or oncologic malignancies, those transferred from other local or overseas hospitals, or those with hospital admissions after January 1, 2005 were defined as “unknowns.” Records of “contacts” and “unknowns” were electronically tagged to facilitate VRE screening by stool or rectal swab on 2 separate occasions at least 24 hours apart. All other patients were considered “clean” and not screened. Tagging of VRE-positive status prompted admission to an isolation or cohort room on admission.
Identification of VRE
Rectal swabs or stool samples from patients were either directly plated onto Enterococcosel agar (BD, Sparks, MD) containing 6 μg/mL of vancomycin (Sigma, St Louis, MO) or first inoculated into Enterococosel broth (BD). If the inoculated broth changed color after overnight incubation, then DNA was extracted and subjected to real-time polymerase chain reaction (PCR) for vanA/B genes (Roche Diagnostics, Mannheim, Germany). If the PCR was positive, then the broth was subcultured onto agar containing 6 μg/mL of vancomycin. VRE was identified using conventional methods4 and confirmed by Vitek 2 GP identification cards (bioMérieux, Marcy-l'Etoile, France). Susceptibility testing was done using Vitek 2 AST P535 cards and confirmed by Etest (AB Biodisk, Solna, Sweden) if necessary. All VRE isolates underwent molecular typing by pulsed-field gel electrophoresis (PFGE) following the protocol specified by Oon et al.5 In instances where PCR tests were discrepant with culture results, the culture results were used to guide infection control decisions.
Hospital-wide screening and physical segregation of patients
Active VRE screening of all inpatients was instituted on April 3, 2005, with physical segregation of inpatients into “clean,” “contact,” and “unknown” categories. Elective surgical admissions were cancelled on April 3 to 10 to facilitate this process. All existing inpatients on April 3 were classified as contacts and moved to predesignated cubicles, usually to 1 side of each ward, to facilitate new admissions (unknown or clean) to the other side. Screening was done in phases over 2 weeks to avoid overwhelming the microbiology laboratory with specimens. At least 2 negative stool or rectal swab cultures or PCR tests collected at least 1 day apart were required before a patient could be transferred to a clean section of the ward. All VRE-positive patients were isolated under strict contact precautions and screened for methicillin-resistant Staphylococcus aureus (MRSA) through a single nasal swab culture where feasible. Co-colonized individuals were not allowed into cohorts with those infected or colonized only with VRE.
Surveillance of risk groups
The “unknown” category was dropped in mid-April 2005. Thereafter, active surveillance for VRE involving a single rectal or stool swab for VRE PCR was instituted for all interhospital and overseas hospital transfers, ESRF patients on dialysis, and patients undergoing renal transplantation on admission.
Outpatient screening
VRE-positive patients were scheduled as the last cases of clinic sessions, followed by terminal cleaning of the consultation rooms. Only contacts already discharged to community dialysis centers and long-term care facilities were proactively screened as outpatients.
Electronic tagging
Electronic tagging of contacts and positive cases was implemented using the hospital's electronic medical record system. VRE-positive tagging continued indefinitely.
Cleaning
Cleaning of isolation, cohort, and “contact” areas involved a 2-step decontamination process with a quarternary ammonium detergent followed by a phenolic-based disinfectant twice daily on all surfaces in the room (ie, walls, furniture, mattress, sinks, doorknob). In all other areas, only quarternary ammonium detergent cleaning was used. Environmental screening was not attempted in this outbreak due to lack of resources.
Education and audit
Heightened infection control measures, especially hand hygiene, and enhanced auditing of these measures were enforced. Hospital-wide education regarding VRE, appropriate infection control measures, and vancomycin use involved both didactic lectures and information disseminated through the hospital's intranet. Strict criteria for vancomycin use were endorsed and circulated to all clinicians. The pharmacy department audited compliance. Education materials were also given to VRE carriers and their caregivers.
Results
On April 1, 19,574 contacts were identified for active screening. Between April and June 2005, 4934 patients were screened at least once, including 84% (912 of 1086) of inpatients on April 3. By the end of June, a total of 147 carriers and 4 clinical cases had been detected. The latter comprised 3 patients with bacteremia and 1 with wound infection, all of whom died from underlying comorbidities, which included metastatic carcinoma. PFGE analysis of these isolates demonstrated the presence of a major “outbreak” clone pattern, A, along with smaller clones, none of which bore any relation to the 2004 hematology outbreak. All of the clinical cases bore the “outbreak” clone pattern A. Of note, clone pattern B appeared to involve mainly ESRF patients ( Fig. 2).
Fig 2.
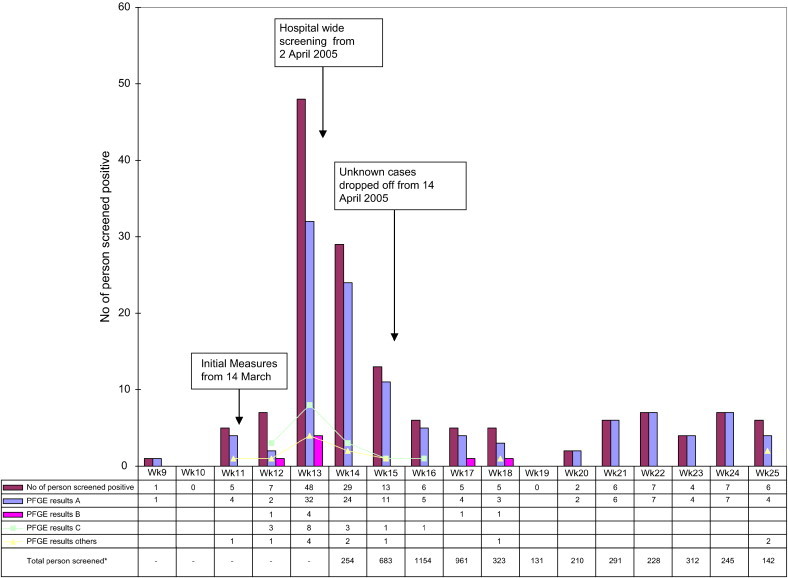
Time series showing distribution of positive cases with PFGE clone types interposed. ∗Data for total persons screened available only after hospital-wide screening was started at week 14.
Epidemiologic information
The epidemic curve is shown in Figure 2. A total of 43 carriers were detected before the creation of the VRE Task Force, followed by another 104 until the end of June 2005, 54 of which (52%) were detected during the first 2 weeks of the hospital-wide screening. Seven carriers were detected through outpatient screening. Multivariate analysis has revealed that VRE carriers are likely to be found among the elderly, diabetic, and female patients with recent and prolonged hospitalization stay particularly in communal wards.6 There was no association of VRE with any specific ward.
Adequacy of sampling
Between March 23, 2005 and May 31, 2005, 111 of 4149 the patients screened were positive for VRE. The first specimen detected 72 positive patients (65%); 2 specimens on different days detected 92 patients (83%). Nineteen patients (17%) underwent screening with 2 specimens on more than 1 occasion before VRE was detected. Subsequent specimens were collected as part of contact screening (4 cases) or active surveillance of high-risk groups (9 cases). In 6 cases, reasons for rescreening could not be determined. The specimens were collected an average of 17.5 days (range, 1 to 43 days) after initial VRE screening. The patients spent an average of 9 days (range, 1 to 33 days) in the hospital after their second negative stool test; 17 (90%) received various antibiotics for an average of 8.4 days (range, 2 to 27 days).
VRE/MRSA co-colonization or co-infection
Co-colonization and/or co-infection with MRSA was present in 52 of 151 (34%) VRE cases. This affected the creation of pure VRE cohorts and stretched the capacity of the existing 16-bed isolation ward. Consequently, the latter was extended to a 46-bed ward by temporarily displacing an adjacent colorectal ward.
End of the outbreak
At the peak of the outbreak, active screening for VRE revealed a positive yield of 11.4% (29 of 254) of persons actively screened. This fell to 4.2% (6 of 142) by late June 2005 (Fig 2). Note, however, that the denominator (ie, the number of patients screened each week during the outbreak) was higher before week 17 due to hospital-wide screening and declined thereafter due to cessation of screening of the “unknown” patient category. A prevalence study involving hematology and oncology wards and medical and surgical ICUs yielded no VRE cases. Physical segregation was discontinued in early July 2005, followed by discontinuation of contact screening. The restriction of vancomycin use and active surveillance of high-risk groups continued.
Discussion
VRE endemicity may have serious consequences in tertiary care institutions with immunocompromised patients. VRE has been independently associated with increased mortality among patients with enterococcal bloodstream infections.7 Infections caused by VRE also can result in increased morbidity, length of hospitalisation, and costs. The threat of vancomycin-resistant Staphylococcus aureus (VRSA) will be raised if VRE becomes as endemic as MRSA.
An aggressive approach to controlling VRE, akin to “nipping the problem in the bud,” requires identifying the reservoir while isolating positive patients. The numerous carriers from several different wards within 2 weeks of the outbreak suggested the existence of a large hospital-wide reservoir of VRE.8, 9 This triggered hospital-wide screening as part of a multipronged strategy to weed out carriers for isolation while maintaining surveillance of high-risk groups. In retrospect, it could be argued that we did not actually have a true nonendemic state, and that there was a transition from an initial monoclonal VRE outbreak in the hematology ward in 2004 to a multiclonal, hospital-wide spread in 2005. However, no VRE case was identified at any time between these 2 periods.
Controlling VRE in a nonendemic setting has little precedence. We tried to reproduce a successful containment effort at Royal Perth Hospital,2 albeit with numerous conundrums. To avoid overwhelming the laboratory, hospital-wide screening of the more than 2000 stool specimens was done in phases over 2 weeks. In contrast to the 4 separate screening stool samples used by Christiansen et al,2 only 2 samples per patient were usually analyzed, which detected 83% of carriers. Nineteen carriers were detected several days after they were “cleared” by 2 negative stool cultures; however, acquisition of VRE after initial screening could not be excluded.
The outbreak appeared to spare hematology, oncology, and ICU patients through limited surveillance. Although risks included coexisting diabetes and prolonged hospitalizations, the stay in crowded communal wards was the biggest infection control challenge. Interbed spacing of < 3 feet, limited toilet facilities (Fig 1), and a low nurse-to-patient ratio were among the problems. Physical segregation was artificial, and dedicating nursing teams to separate cohorts of patients was impossible. A transient attempt to improve interbed spacing by reducing the number of beds per cubicle was abruptly reversed by pressure from bed management and the high hospital occupancy rates. The cancellation of surgical electives to facilitate patient segregation movements affected costs and inconvenienced patients. To facilitate appropriate patient placement, long delays in admissions from the emergency department ensued. Similar bed “crunches” limited ring-fencing of vulnerable patients to parent wards and forced discontinuation of the “unknown” category 2 weeks into the outbreak. Bed management systems were stressed by the need to physically segregate patients according to the different outbreak categories while maintaining their class status. Lack of resources prevented us from doing environmental screening, although this would have been useful,10 because cleaning practices could not be supervised adequately. Proactive outpatient screening also was limited.
Some lessons were learned. Centralized planning, coordination, and dissemination of information from a multidisciplinary task force with a strong mandate from senior management were important. The experience dealing with the severe acute respiratory syndrome (SARS) outbreak in 2003 allowed us to respond quickly to this new threat. Coordinated outbreak control measures were introduced within hours of the first VRE Task Force meeting. Typing using PFGE provided insight into the outbreak and nonoutbreak strains but was limited in its turnaround time. Identifying the outbreak clone by a faster method could have prompted earlier focused strategies. The existence of a VRE clone specific to ESRF patients suggests the need for a coordinated national effort to study VRE prevalence in community dialysis centers. Given our high background MRSA endemicity, determining MRSA status before the creation of VRE cohorts was imperative, as evidenced by the fact that more than 1/3 of cases were co-colonized or co-infected with MRSA Although this and the indefinite tagging of VRE-positive status stretched our limited isolation facilities, it certainly highlighted the need to also control endemic MRSA. Educating staff unfamiliar with VRE was challenging, but at least half of the staff attended more than 20 lectures held throughout the outbreak. Although an audit showed a noncompliance rate of 23.4% of 260 vancomycin orders, it offered opportunities to further educate prescribers. The scale of the outbreak necessitated dissemination of information to the public through the media and other resources. Although this prompted several claims for compensation from affected patients, it made it easier for the public to understand the constrained processes within the hospital during the outbreak.
A multipronged strategy orchestrated by a central task force helped curb the outbreak at the expense of bed management systems, loss of revenue, and inconvenience to patients. The total cost of this strategy is still being computed. Eradicating VRE was hampered by our large hospital size, heterogenous patient mix with numerous inpatient movements, and communal wards. The 2006 VRE incidence in SGH was 0.04 per 1000 patient-days. Along with surveillance of risk groups including communal ward patients, the following measures are aimed at averting another outbreak:
-
•
Screening for VRE for all interhospital and overseas hospital transfers
-
•
Screening for VRE in all patients sharing the same cubicle as a newly diagnosed carrier
-
•
Isolation with contact precautions for all carriers
-
•
Vancomycin audit, as well as audits of other antibiotics
-
•
Hand hygiene vigilance
Acknowledgments
We thank the Epidemiolgy Unit of SGH for their kind assistance in providing the epidemiology data.
References
- 1.Chlebicki MP, Ling ML, Koh TH, Hsu LY, Tan BH, How KB, et al. National Nosocomial Infections Surveillance (NNIS) system report: data summary from January 1992-April 2000, issued June 2000. Am J Infect Control. 2000;28:429–448. doi: 10.1067/mic.2000.110544. [DOI] [PubMed] [Google Scholar]
- 2.Chlebicki M.P., Ling M.L., Koh T.H. First outbreak of vancomycin-resistant Enterococcus faecium in a tertiary-care hospital in Singapore. Infect Cont Hosp Epidemiol. 2006;27:991–993. doi: 10.1086/507289. [DOI] [PubMed] [Google Scholar]
- 3.Christiansen K.J., Tibbett P.A., Beresford W. Eradication of a large outbreak of a single strain of vanB vancomycin-resistant Enterococcus faecium at a major Australian teaching hospital. Infect Control Hosp Epidemiol. 2004;25:384–390. doi: 10.1086/502410. [DOI] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards . National Committee for Clinical Laboratory Standards; Wayne (PA): 2002. Performance standards for antimicrobial susceptibility testing: twelfth informational supplement. [Google Scholar]
- 5.Oon L.L., Ling M.L., Chiew Y.F. Gastrointestinal colonisation of vancomycin-resistant Enterococcus in a Singapore teaching hospital. Pathology. 2001;33:216–221. [PubMed] [Google Scholar]
- 6.Yang K.S., Fung YT., Lee H.Y., Kurup A. Predictors of vancomycin-resistant Enterococcus (VRE) carriage in the first major VRE outbreak in Singapore. Ann Acad Med Singapore. 2007;36:379–385. [PubMed] [Google Scholar]
- 7.DiazGranados C.A., Zimmer S.M., Klein M., Jernigan J.A. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41:327–332. doi: 10.1086/430909. [DOI] [PubMed] [Google Scholar]
- 8.Muto C.A., Jernigan J.A., Ostrowsky B.E., Richet H.M., Jarvis W.R., Boyce J.M. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 9.Farr B.M. What to think if the results of the National Institutes of Health randomized trial of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus control measures are negative (and other advice to young epidemiologists): a review and an au revoir. Infect Control Hosp Epidemiol. 2006;27:1096–1106. doi: 10.1086/508759. [DOI] [PubMed] [Google Scholar]
- 10.Hayden M.K., Bonten M.J., Blom D.W., Lyle E.A., van de Vijver D.A., Weinstein R.A. Reduction in acquisition of vancomycin-resistant Enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis. 2006;42:1552–1560. doi: 10.1086/503845. [DOI] [PubMed] [Google Scholar]


