Abstract
Treatment guidelines for acute rhinosinusitis (RS) recommend the use of intranasal corticosteroids (INSs) as monotherapy or adjunctive therapy. However, the adverse event (AE) profiles of oral glucocorticoids, which result largely from the systemic absorption of those agents, have engendered concerns about the safety of INSs. These concerns persist for INSs despite significant or marked clinical differences between them and systemic corticosteroids in systemic absorption and among the INSs in bioavailability, mechanism of action, and lipophilicity, which may contribute to differences in AEs. For example, the systemic bioavailability of the INSs as a percentage of the administered drug is less than 0.1% for mometasone furoate, less than 1% for fluticasone propionate, 46% for triamcinolone acetonide, and 44% for beclomethasone dipropionate. A review of the safety profiles of INSs, as reported in clinical trials in acute and chronic RS and allergic rhinitis, shows primarily local AEs (eg, epistaxis and headache) that are generally classified as mild to moderate, with occurrence rates that are similar to those with placebo. Studies of the safety of mometasone furoate, fluticasone propionate, budesonide, and triamcinolone acetonide did not identify any evidence of systemic AEs, such as growth retardation in children due to suppression of the hypothalamic-pituitary-adrenal axis, bone mineral density loss, or cataracts, which suggests that INSs can be safely administered in patients with acute RS without concern for systemic AEs.
1. Introduction
Rhinosinusitis (RS) is an inflammatory disorder of the upper respiratory tract affecting the nasal mucosa and paranasal sinuses. One of the most commonly reported diseases in the United States, RS is estimated to affect approximately 32 million people annually, or 16% of the adult population [1], [2], [3], and accounts for an estimated 15 million office visits annually [4]. Rhinosinusitis is usually classified, based on duration, as acute, subacute, chronic, and recurrent. Acute RS is characterized by symptoms lasting for less than 4 weeks, in contrast to subacute (symptoms lasting 4–8 weeks), chronic (symptoms for 8 weeks or longer), and recurrent (3 or more acute episodes per year) RS (Table 1 ) [5], [6]. In most cases, clinical interventions are directed toward the diagnosis and management of acute and chronic RS [5].
Table 1.
Classification of types of rhinosinusitis [5]
| Temporal designation | Description and duration of symptoms |
|---|---|
| Acute | Symptoms <4 wks' duration, including persistent upper respiratory tract infection, purulent rhinorrhea, postnasal drainage, anosmia, nasal congestion, facial pain, headache, fever, cough, and purulent discharge |
| Subacute | Unresolved acute symptoms of sinus inflammation lasting 4–8 wk |
| Chronic | Same symptoms as with acute rhinosinusitis but ≥8 wks' duration; degree of symptom severity varies |
| Recurrent | ≥3 episodes of acute rhinosinusitis annually; patients may be infected by different organisms at varying times |
Acute RS arises most frequently as a consequence of viral rhinitis (common cold), although bacterial infection can subsequently occur [7]. The incidence of acute RS is particularly high in children (who experience an estimated 7–10 colds per year), although it is also common in adults (who have 2–5 colds per year) [8]. The microbiology of acute RS is varied, with rhinovirus (found in 50% of cases) [9], [10], coronavirus (approximately 15%; also responsible for up to 18% of colds) [9], [11], and respiratory syncytial, parainfluenza, and influenza viruses being the most commonly isolated [12], [13], [14]. Bacterial infections are found in approximately 38% of adults presenting with RS symptoms in general medical practices and in 6% to 18% of children presenting with upper respiratory infections in the primary care setting [15]. However, studies suggest a positive bacterial culture is found in only about 0.5% to 2% of viral RS cases [16]. The bacterial species most frequently involved are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, the latter being more prevalent in children [5], [16], [17], [18].
1.1. Use of intranasal corticosteroids in acute RS
The treatment goals for acute RS are to eliminate infection when present, improve ostiomeatal patency as a means of restoring ventilation, promote drainage, reduce inflammation, and relieve symptoms, including pain and nasal congestion [17], [19]. Intranasal corticosteroids (INSs), with their recognized anti-inflammatory properties, have been shown to be effective in reducing mucosal swelling and improving sinus drainage, thereby hastening the elimination of pathogens [5], [19]. Consequently, the Joint Task Force on Practice Parameters for Allergy and Immunology recommends combining an INS with an antibiotic (mainly amoxicillin/potassium clavulanate) for the symptomatic treatment of recurrent acute or chronic RS. The Joint Task Force also notes that INS monotherapy may be helpful in patients with acute and chronic RS [5]. Similarly, the European Academy of Allergology and Clinical Immunology recommends INS therapy, either alone or as an adjunct to an antibiotic, for the treatment of moderate and severe RS [8].
The anti-inflammatory effects of INSs include decreased vascular permeability as well as inhibition of the release and/or formation of mucous secretogogues (eg, histamine, leukotrienes, prostanoids, platelet-activating factor) [5], [20]. Those effects are thought to result from inhibition of the release of proinflammatory mediators, such as adhesion molecules, cytokines, mast cells, basophils, and eosinophils [21]. By binding to the glucocorticoid receptor in the cytoplasm, the glucocorticoid molecule of an INS produces a complex that acts on a variety of transcriptional activities, leading to reductions in levels of proinflammatory molecules and cells (Table 2 ) [21], [22], [23], [24]. In acute RS, INSs have been shown to reduce the inflammation associated with symptoms such as congestion, headache, and facial pain [21], [25].
Table 2.
Proinflammatory mediators suppressed by INSs [21]
| Mediator | Components/role |
|---|---|
| Cytokines | Includes IL-6, IL-8; synthesis of IgE antibodies |
| Langerhans cells | IgE synthesis and stimulation of T cells |
| Lymphocytes | Activated T cells such as CD3+, CD4+, CD8+, and CD25+ cells |
| Mast cells | IgE-dependent histamine release |
| Basophils | Production of IL-4 and IL-13 and release of IgE-dependent histamine |
| Eosinophils | Cytokines such as IL-4 and IL-5 |
Concerns about the safety of corticosteroids in acute RS are related to the systemic absorption of oral corticosteroids, which may affect hypothalamic-pituitary-adrenal (HPA) axis function, bone metabolism, and ocular pressure [17], [26], [27], [28]. These effects may result in adverse events (AEs), such as growth inhibition in children [29], [30], bone mineral density loss [31], [32], [33], [34], hip fracture [35], cataracts [36], ocular hypertension or glaucoma [37], hypertension, hyperglycemia [38], and easily bruised skin [31], [39].
Given current recommendations for the use of INSs in acute RS and concerns about the safety of corticosteroids in general, this article will review data from clinical trials to help clarify the safety issues pertaining to the use of the INS drugs for acute RS, as well as the differences in systemic absorption between the older and newer INSs.
1.2. Assessment of systemic effects of INSs
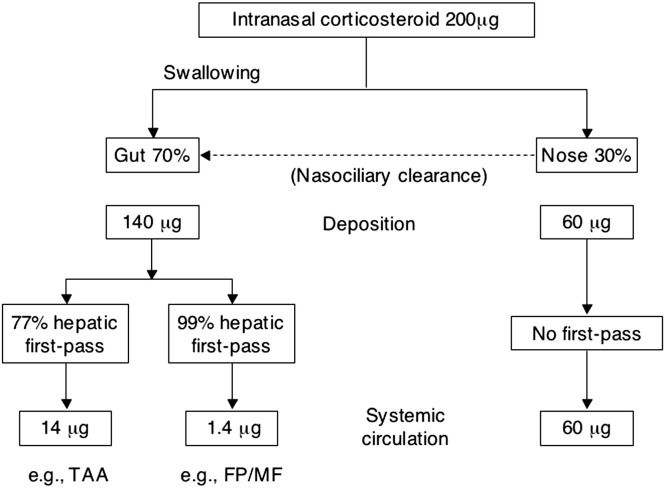
The motivation for the development of all the intranasal formulations of the corticosteroids—including the older INSs beclomethasone dipropionate (BDP), flunisolide (FLU), budesonide (BUD), and triamcinolone acetonide (TAA) and the more recently developed fluticasone propionate (FP), mometasone furoate (MF), fluticasone furoate, and ciclesonide—was to minimize the risk of systemic absorption and the resulting AEs (Fig. 1 ). The intranasal route of administration delivers drug directly to the target organ, allowing local therapeutic concentrations because of the high affinity of the agents for the glucocorticoid receptor. Approximately 30% of the administered dose is deposited in the nose, where it binds with the glucocorticoid receptor, while the remaining 70% is swallowed. The swallowed drug is subject to hepatic first-pass metabolism, which is about 90% with BUD and TAA, 2 agents with relatively lower lipophilicity (or lipid-partitioning potential), and 99% with MF and FP, which have higher lipophilicity [40]. The rank order of some of the currently available INSs according to lipophilicity (highest to lowest) is MF, FP, BDP, BUD, TAA, and FLU [41], [42].
Fig. 1.
This diagram of metabolism of a 200-μg dose of MF, FP, BUD, and TAA shows that total systemic absorption is 14 μg for BUD and TAA and 1.4 μg for MF and FP [40].
Concern about the risk of systemic side effects with INSs arises from the possibility that a portion of the drug may reach the systemic circulation through the airway and the gastrointestinal (GI) tract [43]. The main determinant of systemic bioavailability of these drugs is the amount directly absorbed from the lung or nose, which does not undergo first-pass hepatic inactivation as does most of the swallowed portion of the dose [40]. As shown in Table 3 , the estimated absolute bioavailability of an intranasal dose is highest with compounds with greater water solubility (eg, BUD and FLU, with 34% and 49% absolute bioavailability, respectively) [43], [45], [46] and lowest with the less water-soluble, more lipophilic agents (eg, FP with <1% and MF with <0.1% absolute bioavailability) [43], [45], [47], [48]. In a randomized, single-blind, placebo-controlled, 3-way, cross-over study in 15 healthy subjects, MF and FP (both administered at the higher than indicated doses of 400 μg/d for 4 days) produced mean peak plasma concentrations that were slightly above the assay's lower limit of detection [49].
Table 3.
| Corticosteroid | Systemic bioavailability |
|---|---|
| Flunisolide | 49% |
| Triamcinolone acetonide | 46% |
| Beclomethasone dipropionate | 44% |
| Budesonide | 34% |
| Fluticasone propionate | <1% |
| Mometasone furoate | <0.1% |
Investigators used a variety of markers or surrogates to determine the systemic presence of glucocorticoids in the circulation. An excessive level of systemic glucocorticoids would reduce the endogenous production of cortisol, which can be detected by evaluating basal HPA activity. Measurements of HPA function, such as area-under-the-curve cortisol concentrations and urinary free cortisol excretion, are considered the most sensitive indicators of INS systemic bioavailability. Stimulation tests of HPA-axis function, such as tests that measure serum cortisol levels after the administration of adrenocorticotropic hormone (ACTH) or cosyntropin, are not as sensitive in identifying the systemic bioavailability of the INSs, but they predict the likelihood of AEs more accurately [43], [50], [51]. Corticosteroids also may inhibit linear bone growth [52], an effect that has been assessed in short-term studies using knemometry (a precise measurement of lower-leg growth) [50], [53], [54] and surrogate markers of bone formation (eg, osteocalcin) [43], as well as in long-term studies using whole-body stadiometry [43], [50], [51].
2. Safety of INSs in clinical trials
Most of the knowledge about the safety of INSs is derived from studies in patients with allergic rhinitis (AR), which is a common indication for INSs, rather than in RS, for which a relatively small number of clinical studies have been conducted [17]. A correlation exists between RS and AR; AR may contribute from a quarter to more than a half of RS cases, and perennial AR (PAR) may be a predisposing factor for chronic RS [3], [55], [56], [57], [58], [59], [60]. Consequently, more information about the safety profiles of INSs is available from clinical studies for AR than for acute RS, especially with regard to systemic effects, such as HPA-axis suppression and inhibition of growth in children. This review will therefore summarize the clinical evidence from studies involving patients with AR and acute and chronic RS. Systemic AEs will be discussed first, followed by local effects.
2.1. Systemic effects
A relatively small amount of published data for only a few agents is available on the systemic safety of INSs in patients with RS. Giger et al [61], in a randomized, double-blind, parallel-group trial involving 112 patients with nonallergic chronic RS, did not detect any signs of adrenal suppression or significant changes in morning serum cortisol values with once- or twice-daily intranasal BDP (400 μg/d) administered for 12 weeks. A 3-week, randomized, double-blind, placebo-controlled, multicenter trial with MF 200 μg or 400 μg BID in 967 patients (aged 8–78 years) with acute RS did not find any clinically relevant decreases in plasma cortisol levels, based on 30-minute cosyntropin stimulation tests [62].
Studies in patients with AR also have demonstrated little evidence of systemic effects with most INSs (Table 4 ). In one of the earliest such studies, an open, longitudinal, multicenter trial involving 25 patients with PAR followed for up to 5.5 years, treatment with intranasal BUD (400 μg/d) did not affect HPA-axis activity, based on response to ACTH challenge. The investigators noted that plasma cortisol values were well within normal ranges, and increases in plasma cortisol levels after ACTH stimulation were high and remained unchanged regardless of duration of treatment [64].
Table 4.
Summary of systemic AEs in clinical trials of INS
| Author/year | N | INS Treatment regimen | Treatment duration | Patient population | Growth retardation/HPA axis (test) |
|---|---|---|---|---|---|
| Acute rhinosinusitis | |||||
| Nayak et al [62] | 967 | MF 200 or 400 μg BID | 21 d | Children and adults (8–78 y) | No decreases in cortisol (cosyntropin stimulation) |
| Chronic rhinosinusitis | |||||
| Giger et al [61] | 112 | BDP 400 μg QD or BID (no PBO arm) | 12 wk | Adults (19–66 y) | Minimal decrease in morning serum cortisol levels |
| Allergic rhinitis | |||||
| Pipkorn et al [64] | 24 | BUD 200–400 μg BID | Up to 5.5 y | Adolescents and adults (17–67 y) | No decreases in cortisol (ACTH challenge) |
| Grossman et al [67] | 250 | FP 100 or 200 μg QD | 14 d | Children (4–11 y) | No effect on morning cortisol levels |
| Brannan et al [69] | 96 | MF 50, 100, or 200 μg QD | 7 or 14 d | Children (3–12 y) | No effect on cortisol (cosyntropin stimulation in children aged 3–5 y only) |
| Nayak et al [68] | 80 | TAA 220 or 400 μg QD | 42 d | Children (6–12 y) | No effect on cortisol (cosyntropin stimulation) |
| Healthy subjects | |||||
| Wihl et al [63] | 14 | BUD or BDP 200, 400, and 800 μg QD | 3 wk | Men (18–47 y) | No significant influence on plasma cortisol, significant decrease in urinary cortisol with BUD 400 and 800 μg |
| 32 | BUD or BDP 100, 200, and 400 μg BID | 4 d | Men (19–41 y) | Significant reductions in urinary cortisol with all BUD doses and BDP 400 μg | |
| Allergic rhinitis | |||||
| Vargas et al [66] | 105 | FP 200 mcg QD or 400 μg BID; | 28 d | Adults (18–65 y) | [FP] No effect on cortisol (cosyntropin stimulation) |
| OR | [P] Significant reduction (P < .05) in cortisol (cosyntropin stimulation and morning urinary levels) | ||||
| Oral prednisone 7.5 or 15 mg QD | |||||
| Agertoft and Pederson [71]⁎ | 22 | MF 100 or 200 μg QD OR | 2 wk before crossover | Children (7–12 y) | No short-term effect on growth rate (knemometry) |
| BUD 400 μg QD | |||||
| Wolthers and Pedersen [53] | 44 | BUD 200 μg BID (n=14) vs IM methylprednisolone acetate (n=14) vs. terfenadine (n=16) | 6 wk | Children (6–15 y) | Suppressed short-term lower-leg growth with BUD and depot steroid (knemometry) |
| Schenkel et al [50] | 98 | MF 100 μg QD | 1 y | Children (3–9 y) | No effect on cortisol (cosyntropin stimulation) or growth rate (knemometry) |
| Allergic rhinitis | |||||
| Skoner et al [51] | 100 | BDP 168 μg BID | 1 y | Children (6–9 y) | No effect on morning cortisol levels or response to cosyntropin stimulation/growth suppression (stadiometry) |
| Allen et al [70] | 150 | FP 200 μg QD | 1 y | Children (3.5–9 y) | No growth changes |
| Kim et al [26] | 78 | BUD 64 μg QD | 42 d | Children (2–5 y) | No decreases in cortisol (cosyntropin stimulation) |
P indicates prednisone; PBO, placebo.
Four-way crossover study (results show no sequence or carryover effects).
Similarly, no significant differences were seen between 2 dosages of BDP nasal spray (336 μg QD and 168 μg BID) and placebo in plasma cortisol response to cosyntropin stimulation in a randomized, placebo- and positive-controlled, third party–blind, parallel-group, multiple-dose study of 64 adult men with AR who were treated for 36 days. In contrast, a significant (P < .01) difference between patients receiving prednisone or placebo was seen in the plasma cortisol response to cosyntropin stimulation [65]. Vargas et al [66] reported similar results from a 4-week, randomized, double-blind, double-dummy, placebo-controlled study (N = 105), in which the HPA-axis response to a 6-hour cosyntropin test was not altered with intranasal FP 200 μg QD or FP 400 μg BID, compared with placebo or oral prednisone. Prednisone (7.5 or 15 mg/d) was associated with a significant decline in HPA-axis function compared with placebo, as indicated by lower plasma cortisol levels (area under the curve and peak concentrations) after cosyntropin stimulation and reduced mean 24-hour urinary cortisol excretion [66]. The investigators concluded that FP, whether administered at the recommended dose of 200 μg QD or at 4 times that dose, does not alter HPA-axis response to the 6-hour cosyntropin test.
Studies in children with AR have generally been consistent with adult studies in terms of demonstrating a lack of HPA-axis suppression with INSs. A 2-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter study in children (N = 250; aged 4–11 years) with seasonal AR did not identify any significant differences between FP (100 and 200 μg QD) and placebo in morning plasma cortisol concentrations in all subject groups before and after treatment [67]. Similarly, no significant effects on adrenocortical function at 30 or 60 minutes after cosyntropin stimulation with either of 2 doses of intranasal TAA (220 and 440 μg QD) were seen in a 6-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter study of children (N=80; aged 6–12 years) with AR [68]. Another 6-week study with a similar design in 78 children (aged 2–5 years) demonstrated no HPA-axis suppression with BUD (64 μg QD), based on plasma cortisol levels at 0, 30, and 60 minutes after cosyntropin stimulation [26].
Brannan et al [69] have reported no clinically relevant systemic exposure to MF in children as young as 3 years of age. In the first phase of a randomized, placebo-controlled, parallel-group, multiple-dose study, 48 children (aged 6–12 years) received MFNS (50, 100, or 200 μg QD) or placebo for 7 days [69]. At the end of treatment, mean plasma cortisol concentrations were not significantly different from baseline values, nor were mean plasma cortisol and 24-hour urinary free cortisol values with MF significantly different from placebo. A second phase of the study was conducted in 48 children (aged 3–5 years) who received the same doses of MF or placebo for 14 days; HPA-axis function was assessed by response to a 30-minute cosyntropin stimulation test administered 2 to 3 hours after the last dose on the final day of treatment. All of the children experienced a normal plasma cortisol response to the cosyntropin challenge, and mean increases in plasma cortisol after cosyntropin stimulation were not significantly different between MF and placebo [69].
The INSs also have been evaluated for risk of growth suppression using stadiometry and knemometry, occasionally with results different from that seen in tests of HPA-axis suppression. For example, no HPA-axis suppression with BDP (168 μg BID), as measured by a 60-minute cosyntropin stimulation test, was reported in a randomized, double-blind, placebo-controlled, parallel-group, multicenter study in 100 children with AR [51]. However, stadiometry testing of these same children found a significantly slower rate of growth. The difference in growth rate was apparent as early as 1 month after the start of treatment and remained statistically significant over the last 6 months of the 1-year study.
Stadiometry studies of FP and MF did not uncover any evidence of growth suppression. Continuous treatment for 1 year with the maximum recommended dose of intranasal FP (200 μg QD) was found not to affect mean standing height, as measured by stadiometry, in 150 children (aged 3.5–9 years) with PAR [70]. In this randomized, double-blind, placebo-controlled study, FP was equivalent to placebo in effects on growth velocity.
A 1-year, randomized, double-blind, placebo-controlled, multicenter study found no growth retardation, as measured by stadiometry, in 98 children (aged 3–9 years) with PAR randomized to receive either MF 100 μg QD or placebo [50]. At all time points, the mean height of MF-treated patients was similar to that of the placebo group; although a significantly greater change in height from baseline was seen in the MF group at weeks 8 and 52, the rate of growth over 12 months was similar in both groups. In a subgroup of 38 subjects enrolled in the cosyntropin arm of the study, subjects receiving MF did not exhibit any evidence of HPA-axis suppression in a 30-minute cosyntropin stimulation test.
A 1993 parallel-group study found evidence of suppressed short-term lower-leg growth, as measured by knemometry, with BUD (200 μg BID) or intramuscular methylprednisolone acetate (60 mg QD) when compared to terfenadine tablets (60 mg QD) in 44 children (aged 6–15 years) with AR. Both of the corticosteroids, administered for 6 weeks, were associated with a significant reduction in lower-leg growth compared with terfenadine (P < .001) and with values observed during a 4-week run-in period (P < .01) [53]. Those findings contrast with a knemometry study conducted with MF in 22 children (aged 7–12 years) with AR. In this randomized, double-blind, placebo-controlled 4-way crossover study, no significant differences were observed in lower-leg growth rates in children treated for 2 weeks with once-daily MF 100 μg or 200 μg, BUD 400 μg, or placebo [71]. Pairwise comparisons showed that patients receiving a 100-μg QD dose of MF experienced greater growth than those receiving BUD (P = .033) or placebo (P = .024). Investigators did not detect any statistically significant sequence, carryover, overall treatment, or period effects on lower-leg growth rates.
2.2. Local adverse events
Table 5 summarizes local AEs observed in clinical trials of patients with acute and chronic RS. In general, the incidence of treatment-related local AEs with INSs was comparable to that found with placebo, and most events were mild or moderate in severity. The most commonly reported local AEs were headache, epistaxis, and GI complaints.
Table 5.
Summary of commonly reported local AEs in clinical trials of INS
| Author/year | N | INS Treatment regimen | Treatment duration | Patient population | Adverse events | Active treatment group | Placebo group | ||
|---|---|---|---|---|---|---|---|---|---|
| Acute rhinosinusitis | Vaginitis | 8% | 5% | ||||||
| Meltzer et al[77] | 407 | MF 400 μg BID as adjunct to ACP | 21 d | Adolescents and adults (12–73 y) | MF (n = 200) | PBO (n = 207) | |||
| Headache | 2% | 3% | |||||||
| Epistaxis | 3% | 1% | |||||||
| Nasal burning | 2% | 1% | |||||||
| Nasal irritation | 2% | 2% | |||||||
| Pharyngitis | 2% | 3% | |||||||
| Dolor et al [25] | 95 | FP 400 μg QD as adjunct to cefuroxime axetil and xylometazoline hydrochloride | 21 d | Adults (30–55 y) | FP (n=46) | PBO (n=46) | |||
| Headache | 6.5% | 6.5% | |||||||
| Epistaxis | 6.5% | 2.1% | |||||||
| Vaginal itching/yeast infection | 4.3% | 2.1% | |||||||
| Diarrhea | 2.1% | 4.3% | |||||||
| Nausea/stomach irritation | 4.3% | 0% | |||||||
| Nayak et al [62] | 967 | MF 200, 400 μg BID as adjunct to ACP | 21 d | Children and adults (8–78 y) | 200 μg (n = 318) | 400 μg (n = 324) | PBO (n = 325) | ||
| Epistaxis | 5% | 6% | 6% | ||||||
| Nasal burning | 1% | 1% | 2% | ||||||
| Nasal irritation | <1% | 2% | 0% | ||||||
| Headache | 2% | 1% | 2% | ||||||
| Meltzer et al [7] | 981 | MF 200, 400 μg QD | 15 d | Adolescents and adults (12–76 y) | Headache | Data not published | |||
| Epistaxis | |||||||||
| Chronic rhinosinusitis | |||||||||
| Lund et al [78] | 244 | BUD 128 μg BID | 20 wk | Adults (19–65 y) | BUD (n = 81) | PBO (n = 86) | |||
| Respiratory infection | 13.6% | 8.1% | |||||||
| Headache | 6.2% | 8.1% | |||||||
| Blood-tinged secretions | 9.9% | 3.5% | |||||||
| Viral infections | 6.2% | 4.7% | |||||||
| Pharyngitis | 3.37% | 4.7% | |||||||
| Sinusitis | 1.2% | 5.8% | |||||||
| Flu-like disorder | 4.9% | 2.3% | |||||||
| Pain | 4.9% | 2.3% | |||||||
| Rhinitis | 4.9% | 2.3% | |||||||
| External ear infection | 2.5% | 3.5% | |||||||
| Giger et al [61] | 112 | BDP 400 μg QD or BID (no placebo arm) | 12 wk | Adults (19–66 y) | QD arm (n=55) | BID arm (n=57) | |||
| Epistaxis | 46.2% | 43.8% | |||||||
| Dryness of nasal mucosa | 15.4% | 34.4% | |||||||
| Nasal burning | 3.85% | 9.38% | |||||||
| Nasal itching | 3.85% | 3.13% | |||||||
| Sinusitis | 7.69% | 0% | |||||||
| Pharyngitis | 3.85% | 0% | |||||||
| Otitis | 3.85% | 0% | |||||||
| Change of taste | 3.85% | 0% | |||||||
| Eczema | 3.85% | 0% | |||||||
| Nausea and diarrhea | 7.69% | 3.13% | |||||||
| Sinusitis | |||||||||
| Meltzer et al [74] | 180 | Phase I: FLU | 21 d | Adults (mean 36.8 y) | FLU (n = 89) | PBO (n = 86) | |||
| 300 μg TID as adjunct to ACP | Headache | 67% | 58% | ||||||
| Digestive system | 21% | 22% | |||||||
| Diarrhea | 16% | 16% | |||||||
| Nausea | 8% | 8% | |||||||
| Abdominal pain | 4% | 1% | |||||||
| Taste perversion | 10% | 8% | |||||||
| Vaginitis | 8% | 5% | |||||||
| Phase II: FLU | 28 d | FLU (n = 65) | PBO (n = 69) | ||||||
| 300 μg TID | Headache | 15% | 19% | ||||||
| Digestive system | 3% | 12% | |||||||
| Diarrhea | 2% | 3% | |||||||
| Nausea | 3% | 3% | |||||||
| Abdominal pain | 2% | 0% | |||||||
| Barlan et al [75] | 89 | BUD 100 μg BID as adjunct to ACP | 21 d | Children (1–15 y) | No adverse drug reactions observed with BUD (n = 43) | ||||
| Yilmaz et al [76] | 52 | BUD 200 μg BID or pseudoephedrine | 10 d | Children (6–16 y) | No adverse drug reactions observed with BUD (n = 26) | ||||
| 60 μg BID as adjunct to cefaclor | |||||||||
| Allergic rhinitis | |||||||||
| Grossman et al [67] | 250 | FP 100 or 200 μg | 14 d | Children (4–11 y) | 100 μg (n = 84) | 200 μg (n = 81) | PBO (n = 85) | ||
| QD | Nasal burning | 4% | 1% | 0% | |||||
| Epistaxis | 4% | 2% | 4% | ||||||
| Headache | 0% | 1% | 2% | ||||||
| Brannan et al [65] | 64 | BDP 336 μg QD (n=16) | 36 d | Men (19–44 y) | ⁎Headache 44% | ||||
| or BID (n = 16) | ⁎Pharyngitis 9% | ||||||||
| ⁎Nasal irritation 2% | |||||||||
| Munk et al [72] | 140 | TAA 220 μg QD | 14 d | Adults (20–65 y) | TAA (n = 69) | PBO (n = 70) | |||
| Headache | 1.4% | 29.% | |||||||
| Graft et al [73] | 349 | MF 200 μg QD or | MF: | Adolescents | |||||
| BDP 168 μg BID | 7 d | (12–69 y) | MF (n = 117) | BDP (n = 116) | PBO (n = 116) | ||||
| BDP: | Headache | 36% | 22% | 23% | |||||
| 14 d | Pharyngitis | 6% | 10% | 5% | |||||
| and adults | URTI | 6% | 3% | <1% | |||||
| Dysmenorrhea | 6% | 0% | 8% | ||||||
| Brannan et al [69] | 96 | MF 50, 100, or | 7 or 14 d | Children (3–12 y) | 50 μg0 (n = 24) | 100 μg (n = 24) | 200 μg (n = 24) | PBO (n = 24) | |
| 200 μg QD | Headache | 4% | 8% | 13% | 13% | ||||
| Schenkel et al [50] | 98 | MF 100 μg QD | 1 y | Children (3–9 y) | MF (n = 49) | PBO (n = 49) | |||
| Epistaxis | 12% | 8% | |||||||
| Nasal irritation | 8% | 6% | |||||||
| Headache | 0% | 2% | |||||||
| Pharyngitis | 0% | 2% | |||||||
| Rhinitis | 0% | 2% | |||||||
| Sneezing | 0% | 2% | |||||||
| Urticaria | 0% | 2% | |||||||
| Conjunctivitis | 2% | 0% | |||||||
| Skoner et al [51] | 100 | BDP 168 μg BID | 1 y | Children (6–9 y) | BDP (n = 49) | PBO (n = 51) | |||
| Epistaxis | 20% | 27% | |||||||
| Nasal burning | 8% | 14% | |||||||
| Nasal irritation | 6% | 8% | |||||||
| Rhinitis | 2% | 4% | |||||||
| Sneezing | 0% | 10% | |||||||
| Lacrimation | 0% | 4% | |||||||
| Increased appetite | 0% | 4% | |||||||
| Coughing | 0% | 4% | |||||||
| Allen et al [70] | 150 | FP 200 μg QD | 1 y | Children (3.5–9 y) | FP (n = 74) | PBO (n = 76) | |||
| Epistaxis | 9% | 8% | |||||||
| Nasal irritation | 3% | 0% | |||||||
| Headache | 1% | 1% | |||||||
| Gastric upset | 0% | 1% | |||||||
| Nasal burning | 0% | 1% | |||||||
| Nasal soreness | 1% | 0% | |||||||
| Vestibulitis of nose | 0% | 1% | |||||||
| Kim et al [26] | 78 | BUD 64 μg QD | 6 wk | Children (2–5 y) | BUD (n = 39) | PBO (n = 39) | |||
| Respiratory infection | 5.1% | 10.3% | |||||||
| Otitis media | 7.7% | 5.1% | |||||||
| Accident and/or injury | 10.3% | 0% | |||||||
| Fever | 7.7% | 2.6% | |||||||
| Gastroenteritis | 5.1% | 5.1% | |||||||
| Headache | 2.6% | 7.7% | |||||||
| Insect bite/scratch | 2.6% | 5.1% | |||||||
| Parasitosis | 5.1% | 2.6% | |||||||
| Rash | 2.6% | 5.1% | |||||||
| Coughing | 5.1% | 0% | |||||||
URTI indicates upper respiratory tract infection.
The incidence of these AEs was similar in active treatment and placebo groups.
The first double-blind, randomized trial of an INS as adjunctive therapy for acute or chronic RS was a parallel-group, multicenter study (N = 180) with FLU (300 μg TID) or placebo as an adjunct to amoxicillin/clavulanate potassium (ACP) for 3 weeks (phase 1), followed by monotherapy with either FLU or placebo for an additional 4 weeks (phase 2) [74]. Approximately two thirds of patients in phase 1 and half of those in phase 2 complained of at least one AE. Most complaints were attributed to the RS itself, to ineffective therapy, or to GI side effects of the antibiotic. During phase 2, headache was the most frequently reported side effect with FLU. The incidence of AEs was similar in the active treatment and placebo groups.
Since that initial study, clinical trials have been conducted with 4 other INSs—MF (200 or 400 μg BID), FP (200 μg QD), BDP (400 μg QD), and BUD (50 μg QD and 200 μg QD in separate studies)—as adjunctive therapy with an antibiotic for acute RS [25], [62], [75], [76].
Similar AEs were seen in 2 studies with MF as adjunctive therapy to oral antibiotics. In two 3-week, double-blind, placebo-controlled, multicenter studies, in which MF (200 or 400 μg twice daily) was given with ACP in patients (N = 407 and N = 967) with acute or acute recurrent RS, the most commonly reported AEs were headache, epistaxis, nasal burning/irritation, and pharyngitis. Most AEs were mild or moderate in severity, and their incidence was similar in the MF and placebo groups [62], [77].
In 2 separate studies of BUD as an adjunct to oral antibiotics in children with acute RS, no AEs associated with the INS were reported [75], [76].
Only one study reported a greater incidence of AEs with INS-antibiotic adjunctive therapy than with placebo, although not all of the AEs may have been due to INS therapy. In a double-blind, randomized, placebo-controlled trial (N = 95), a greater number of local AEs (eg, headache, epistaxis, vaginal itching/yeast infection, and nausea or stomach irritation) were observed in patients receiving a 21-day course of FP (200 μg/d) as an adjunct to the cephalosporin antibiotic cefuroxime axetil and the topical decongestant xylometazoline hydrochloride than in those receiving placebo. However, investigators noted that the AEs observed with FP may have been a result of the combination of medications or one of the other medications [25].
To date, MF is the only INS to have been investigated in a large-scale clinical trial as monotherapy for acute RS. The randomized, double-blind, double-dummy, dose-ranging study (N = 981) compared MF (200 μg QD and BID) for 15 days both with placebo and with amoxicillin (500 mg TID) [7]. Investigators observed a similar incidence of mild or moderate local AEs in all treatment groups and with placebo; the most common treatment-related events were headache and epistaxis.
Studies in patients with chronic RS have yielded similar information on the local effects of INSs. Only minor differences in AE profiles were observed between patients treated with BUD (128 μg/d) and placebo in a randomized, double-blind, multicenter trial (N = 244; aged 19–65 years). Most AEs (eg, respiratory infection, headache, blood-tinged secretions) were reported as mild or moderate. Although respiratory infection was the most commonly reported AE, there was no statistically significant difference between groups in the incidence of this AE [78]. In a randomized, double-blind, parallel-group comparison of once- or twice-daily BDP (400 μg/d) in 112 patients (aged 19–66 years) with nonallergic chronic RS, Giger et al observed a similar number of local AEs (eg, epistaxis, dryness of nasal mucosa, nasal burning/itching) in the once- and twice-daily groups. Slight differences were seen between groups in terms of the severity of AEs (once-daily: mild, 61.6%; moderate, 34.6%; severe, 3.8%; twice-daily: mild, 53.1%; moderate, 43.8%; severe, 3.1%) [61].
3 Summary and conclusions
The safety profiles of the INSs in the treatment of acute RS have been well established. Reported AEs have been primarily local (eg, epistaxis and headache), generally classified as mild to moderate, and similar in incidence to that of placebo. Based on the results of clinical studies, concerns about possible systemic effects with intranasal use have not been justified. Studies that tested for INSs in the systemic circulation and possible effects arising from such exposure showed no evidence of HPA-axis suppression with administration of MF, FP, and BUD at doses as high as 400 μg twice daily for 4 weeks. Studies after administration of similar doses of MF, BUD, and FP to children for as long as 1 year showed no evidence of growth retardation. No clinically relevant systemic exposure resulting from intranasal administration of these agents was observed. For patients receiving an INS at the recommended dose, there appears to be little risk of HPA-axis suppression or disturbed bone metabolism. The mild side effect profile for newer agents such as MF, which is the only INS that has been studied as adjunctive therapy to antibiotics and as monotherapy for acute RS, appears to be related to their relatively low systemic bioavailability. Thus, physicians should feel confident in prescribing newer agents for long-term treatment of acute RS.
References
- 1.Anand V.K. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol. 2004;113(Suppl 193):3–5. doi: 10.1177/00034894041130s502. [DOI] [PubMed] [Google Scholar]
- 2.Pleis J.R., Coles R. Summary health statistics for U.S. adults: National Health Interview Survey, 1998. National Center for Health Statistics. Vital Health Stat. 2002;10:1–113. [PubMed] [Google Scholar]
- 3.Kaliner M.A., Osguthorpe J.D., Fireman P. Sinusitis: bench to bedside. Current findings, future directions. Otolaryngol Head Neck Surg. 1997;116(6 Pt 2):S1–S20. [PubMed] [Google Scholar]
- 4.Schappert S.M., Burt C.W. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001-02. National Health Center for Health Statistics. Vital Health Stat 13. 2006;159:1–66. [PubMed] [Google Scholar]
- 5.Slavin R.G., Spector S.L., Bernstein I.L. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116:S13–S47. doi: 10.1016/j.jaci.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer E.O., Hamilos D.L., Hadley J.A. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:S155–S212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer E.O., Bachert C., Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J Allergy Clin Immunol. 2005;116:1289–1295. doi: 10.1016/j.jaci.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Fokkens W., Lund V., Mullol J., on behalf of the European Position Paper on Rhinosinusitis and Nasal Polyps group European position paper on rhinosinusitis and nasal polyps 2007. Rhinology. 2007;1-136(Suppl 20) [PubMed] [Google Scholar]
- 9.Pitkaranta A., Arruda E., Malmberg H. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–1793. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitkaranta A., Starck M., Savolainen S. Rhinovirus RNA in the maxillary sinus epithelium of adult patients with acute sinusitis. Clin Infect Dis. 2001;33:909–911. doi: 10.1086/322678. [DOI] [PubMed] [Google Scholar]
- 11.MacNaughton M.R. Occurrence and frequency of coronavirus infections in humans as determined by enzyme-linked immunosorbent assay. Infect Immun. 1982;38:419–423. doi: 10.1128/iai.38.2.419-423.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwaltney J.M., Jr, Sydnor A., Jr, Sande M.A. Etiology and antimicrobial treatment of acute sinusitis. Ann Otol Rhinol Laryngol. 1981;90:68–71. doi: 10.1177/00034894810903s216. [DOI] [PubMed] [Google Scholar]
- 13.Wald E.R., Milmoe G.J., Bowen A. Acute maxillary sinusitis in children. N Engl J Med. 1981;304:749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- 14.Gwaltney J.M., Jr . The common cold. In: Mandell G.L., Douglas R.G. Jr, Bennett J.E., editors. Principles and practice of infectious diseases. 3rd ed. Churchill Livingstone; New York (NY): 1990. pp. 489–493. [Google Scholar]
- 15.Lau J., Zucker D., Engels E.A. Agency for Health Care Policy and Research; Rockville (Md): 1999. Diagnosis and treatment of acute bacterial rhinosinusitis. Evidence Report/Technology Assessment No. 9 (Contract 290-97-0019 to the New England Medical Center) Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1.chapter.13219 Accessed April 17, 2007. [Google Scholar]
- 16.Gwaltney J.M. Acute community-acquired sinusitis. Clin Infect Dis. 1996;23:1209–1223. doi: 10.1093/clinids/23.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Cauwenberge P., Van Hoecke H., Vandenbulcke L. Glucocorticosteroids in allergic inflammation: clinical benefits in allergic rhinitis, rhinosinusitis, and otitis media. Immunol Allergy Clin North Am. 2005;25:489–509. doi: 10.1016/j.iac.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Berg O., Carenfelt C., Kronvall G. Bacteriology of maxillary sinusitis in relation to character of inflammation and prior treatment. Scand J Infect Dis. 1988;20:511–516. doi: 10.3109/00365548809032499. [DOI] [PubMed] [Google Scholar]
- 19.Slavin R.G. Sinusitis in adults and its relation to allergic rhinitis, asthma, and nasal polyps. J Allergy Clin Immunol. 1988;82:950–956. doi: 10.1016/0091-6749(88)90038-3. [DOI] [PubMed] [Google Scholar]
- 20.Spector S.L., Bernstein I.L., Li J.T. Parameters for the diagnosis and management of sinusitis. J Allergy Clin Immunol. 1998;102(6 Pt 2):S107–S144. doi: 10.1016/s0091-6749(98)70045-4. [DOI] [PubMed] [Google Scholar]
- 21.Mygind N., Nielsen L.P., Hoffmann H.J. Mode of action of intranasal corticosteroids. J Allergy Clin Immunol. 2001;108:S16–S25. doi: 10.1067/mai.2001.115561. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison K.A., Scherrer L.C., Czar M.J. Regulation of glucocorticoid receptor function through assembly of a receptor-heat shock protein complex. Ann NY Acad Sci. 1993;684:35–48. doi: 10.1111/j.1749-6632.1993.tb32269.x. [DOI] [PubMed] [Google Scholar]
- 23.Wissink S., van de Stolpe A., Caldenhoven E. NF-kappa B/Rel family members regulating the ICAM-1 promoter in monocytic THP-1 cells. Immunobiology. 1997;198:50–64. doi: 10.1016/s0171-2985(97)80026-5. [DOI] [PubMed] [Google Scholar]
- 24.LeVan T.D., Behr F.D., Adkins K.K. Glucocorticoid receptor signaling in a bronchial epithelial cell line. Am J Physiol. 1997;272:L838–L843. doi: 10.1152/ajplung.1997.272.5.L838. [DOI] [PubMed] [Google Scholar]
- 25.Dolor R.J., Witsell D.L., Hellkamp A.S., for the Ceftin and Flonase for Sinusitis (CAFFS) Investigators Comparison of cefuroxime with or without intranasal fluticasone for the treatment of rhinosinusitis: The CAFFS trial: a randomized controlled trial. JAMA. 2001;286:3097–3105. doi: 10.1001/jama.286.24.3097. [DOI] [PubMed] [Google Scholar]
- 26.Kim K.T., Rabinovitch N., Uryniak T. Effect of budesonide aqueous nasal spray on hypothalamic-pituitary-adrenal axis function in children with allergic rhinitis. Ann Allergy Asthma Immunol. 2004;93:61–67. doi: 10.1016/S1081-1206(10)61448-2. [DOI] [PubMed] [Google Scholar]
- 27.Bross-Soriano D., Hanenberg-Milver C., Schimelmitz-Idi J. Effects of three nasal topical steroids in the intraocular pressure compartment. Otolaryngol Head Neck Surg. 2004;130:187–191. doi: 10.1016/j.otohns.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Opatowsky I., Feldman R.M., Gross R. Intraocular pressure elevation associated with inhalation and nasal corticosteroids. Ophthalmology. 1995;102:177–179. doi: 10.1016/s0161-6420(95)31039-1. [DOI] [PubMed] [Google Scholar]
- 29.The Childhood Asthma Management Program Research Group Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 30.Pauwels R.A., Pedersen S., Busse W.W., on behalf of the START Investigators' Group Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 31.The Lung Health Study Research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 32.Tattersfield A.E., Town G.I., Johnell O. Bone mineral density in subjects with mild asthma randomised to treatment with inhaled corticosteroids or non-corticosteroid treatment for two years. Thorax. 2001;56:272–278. doi: 10.1136/thorax.56.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Israel E., Banerjee T.R., Fitzmaurice G.M. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med. 2001;345:941–947. doi: 10.1056/NEJMoa002304. [DOI] [PubMed] [Google Scholar]
- 34.Wong C.A., Walsh L.J., Smith C.J.P. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet. 2000;355:1399–1403. doi: 10.1016/S0140-6736(00)02138-3. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard R.B., Smith C.J.P., Smeeth L. Inhaled corticosteroids and hip fracture: a population-based case-control study. Am J Respir Crit Care Med. 2002;166:1563–1566. doi: 10.1164/rccm.200206-606OC. [DOI] [PubMed] [Google Scholar]
- 36.Cumming R.G., Mitchell P., Leeder S.R. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337:8–14. doi: 10.1056/NEJM199707033370102. [DOI] [PubMed] [Google Scholar]
- 37.Garbe E., LeLorier J., Biovin J.F. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. JAMA. 1997;277:722–727. [PubMed] [Google Scholar]
- 38.Haynes R.C. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Rall T.W., Nies A.S., Taylor P., editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. Pergamon Press; New York (NY): 1990. pp. 1431–1462. [Google Scholar]
- 39.Pauwels R.A., Lofdahl C.G., Laitinen L.A., for the European Society Respiratory Study on Chronic Obstructive Pulmonary Disease Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 40.Lipworth B.J., Jackson C.M. Safety of inhaled and intranasal corticosteroids: lessons for the new millennium. Drug Saf. 2000;23:11–33. doi: 10.2165/00002018-200023010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Corren J. Intranasal corticosteroids for allergic rhinitis: how do different agents compare? J Allergy Clin Immunol. 1999;104:S144–S149. doi: 10.1016/s0091-6749(99)70310-6. [DOI] [PubMed] [Google Scholar]
- 42.Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. J Allergy Clin Immunol. 1996;97:169–176. doi: 10.1016/s0091-6749(96)80217-x. [DOI] [PubMed] [Google Scholar]
- 43.Allen D.B. Systemic effects of intranasal steroids: an endocrinologist's perspective. J Allergy Clin Immunol. 2000;106:S179–S190. doi: 10.1067/mai.2000.110038. [DOI] [PubMed] [Google Scholar]
- 44.Daley-Yates P.T., Richards H.R. Pharmacokinetic and pharmacodynamic relationships for intranasal corticosteroids (INCS) J Allergy Clin Immunol. 2001;107:S313. [Google Scholar]
- 45.Richards D.H., Daley-Yates P.T. Choice of inhaled and intranasal steroids when used in combination for asthma and rhinitis in children. Allergy. 2000;55:61. (abstr 185) (suppl) [Google Scholar]
- 46.Pakes G.E., Brogden R.N., Heel R.C. Flunisolide: a review of its pharmacological properties and therapeutic efficacy in rhinitis. Drugs. 1980;19:397–411. doi: 10.2165/00003495-198019060-00001. [DOI] [PubMed] [Google Scholar]
- 47.Lumry W.R. A review of the preclinical and clinical data of newer intranasal steroids used in the treatment of allergic rhinitis. J Allergy Clin Immunol. 1999;104(Suppl):S150–S158. doi: 10.1016/s0091-6749(99)70311-8. [DOI] [PubMed] [Google Scholar]
- 48.Daley-Yates P.T., McAllister T. Systemic bioavailability of fluticasone propionate administered as nasal drops (FP-Drops) and aqueous nasal spray formulations (FPANS) Allergy. 1998;53:412. doi: 10.1046/j.0306-5251.2001.01325.x. (abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daley-Yates P.T., Kunka R.L., Yin Y. Bioavailability of fluticasone propionate and mometasone furoate aqueous nasal sprays. Eur J Clin Pharmacol. 2004;60:265–268. doi: 10.1007/s00228-004-0763-y. [DOI] [PubMed] [Google Scholar]
- 50.Schenkel E.J., Skoner D.P., Bronsky E.A. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics. 2000;105:E22. doi: 10.1542/peds.105.2.e22. [DOI] [PubMed] [Google Scholar]
- 51.Skoner D.P., Rachelefsky G.S., Meltzer E.O. Detection of growth suppression in children during treatment with intranasal beclomethasone dipropionate. Pediatrics. 2000;105:e23. doi: 10.1542/peds.105.2.e23. [DOI] [PubMed] [Google Scholar]
- 52.Allen D.B. Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am. 1996;25:699–717. doi: 10.1016/s0889-8529(05)70348-0. [DOI] [PubMed] [Google Scholar]
- 53.Wolthers O.D., Pedersen S. Short-term growth in children with allergic rhinitis treated with oral antihistamines, depot and intranasal glucocorticosteroids. Acta Paediatr. 1993;82:635–640. doi: 10.1111/j.1651-2227.1993.tb18030.x. [DOI] [PubMed] [Google Scholar]
- 54.Wolthers O.D. Long-, intermediate- and short-term growth studies in asthmatic children treated with inhaled glucocorticosteroids. Eur Respir J. 1996;9:821–827. doi: 10.1183/09031936.96.09040821. [DOI] [PubMed] [Google Scholar]
- 55.Kaliner M. Rhinosinusitis—the role of the allergist in diagnosis and treatment. Allergy Clin Immunol Int. 1998;10:141–148. [Google Scholar]
- 56.McNally P.A., White M.V., Kaliner M.A. Sinusitis in an allergists' office: analysis of 200 consecutive cases. Allergy Asthma Proc. 1997;18:169–175. doi: 10.2500/108854197778984374. [DOI] [PubMed] [Google Scholar]
- 57.Shapiro G.G., Virant F.S., Furukawa C.T. Immunologic defects in patients with refractory sinusitis. Pediatrics. 1991;87:311–316. [PubMed] [Google Scholar]
- 58.Savolainen S. Allergy in patients with acute maxillary sinusitis. Allergy. 1989;44:116–122. doi: 10.1111/j.1398-9995.1989.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 59.Kogutt M.S., Swischuk L.E. Diagnosis of sinusitis in infants and children. Pediatrics. 1973;52:121–124. [PubMed] [Google Scholar]
- 60.Slavin R.G. Complications of allergic rhinitis: implications for sinusitis and asthma. J Allergy Clin Immunol. 1998;101(2 pt 2):S357–S360. doi: 10.1016/s0091-6749(98)70219-2. [DOI] [PubMed] [Google Scholar]
- 61.Giger R., Pasche P., Cheseaux C. Comparison of once- versus twice-daily use of beclomethasone dipropionate aqueous nasal spray in the treatment of allergic and non-allergic chronic rhinosinusitis. Eur Arch Otorhinolaryngol. 2003;260:135–140. doi: 10.1007/s00405-002-0543-1. [DOI] [PubMed] [Google Scholar]
- 62.Nayak A.S., Settipane G.A., Pedinoff A., and the Nasonex Sinusitis Group Effective dose range of mometasone furoate nasal spray in the treatment of acute rhinosinusitis. Ann Allergy Asthma Immunol. 2002;89:271–278. doi: 10.1016/S1081-1206(10)61954-0. [DOI] [PubMed] [Google Scholar]
- 63.Wihl J.A., Andersson K.E., Johansson S.A. Systemic effects of two nasally administered glucocorticosteroids. Allergy. 1997;52:620–626. doi: 10.1111/j.1398-9995.1997.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 64.Pipkorn U., Pukander J., Suonpaa J. Long-term safety of budesonide nasal aerosol: a 5.5-year follow-up study. Clin Allergy. 1988;18:253–259. doi: 10.1111/j.1365-2222.1988.tb02867.x. [DOI] [PubMed] [Google Scholar]
- 65.Brannan M.D., Herron J.M., Reidenberg P. Lack of hypothalamic-pituitary-adrenal axis suppression with once-daily or twice-daily beclomethasone dipropionate aqueous nasal spray administered to patients with allergic rhinitis. Clin Ther. 1995;17:637–647. doi: 10.1016/0149-2918(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 66.Vargas R., Dockhorn R.J., Findlay S.R. Effect of fluticasone propionate aqueous nasal spray versus oral prednisone on the hypothalamic-pituitary-adrenal axis. J Allergy Clin Immunol. 1998;102:191–197. doi: 10.1016/s0091-6749(98)70085-5. [DOI] [PubMed] [Google Scholar]
- 67.Grossman J., Banov C., Bronsky E.A. Fluticasone propionate aqueous nasal spray is safe and effective for children with seasonal allergic rhinitis. Pediatrics. 1993;92:594–599. [PubMed] [Google Scholar]
- 68.Nayak A.S., Ellis M.H., Gross G.N. The effects of triamcinolone acetonide aqueous nasal spray on adrenocortical function in children with allergic rhinitis. J Allergy Clin Immunol. 1998;101(2 Pt 1):157–162. doi: 10.1016/S0091-6749(98)70379-3. [DOI] [PubMed] [Google Scholar]
- 69.Brannan M.D., Herron J.M., Affrime M.B. Safety and tolerability of once-daily mometasone furoate aqueous nasal spray in children. Clin Ther. 1997:1330–1339. doi: 10.1016/s0149-2918(97)80008-2. [DOI] [PubMed] [Google Scholar]
- 70.Allen D.B., Meltzer E.O., Lemanske R.F., Jr No growth suppression in children treated with the maximum recommended dose of fluticasone propionate aqueous nasal spray for one year. Allergy Asthma Proc. 2002;23:407–413. [PubMed] [Google Scholar]
- 71.Agertoft L., Pedersen S. Short-term lower leg growth rate in children with rhinitis treated with intranasal mometasone furoate and budesonide. J Allergy Clin Immunol. 1999;104:948–952. doi: 10.1016/s0091-6749(99)70073-4. [DOI] [PubMed] [Google Scholar]
- 72.Munk Z.M., LaForce C., Furst J.A. Efficacy and safety of triamcinolone acetonide aqueous nasal spray in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1996;77:277–281. doi: 10.1016/S1081-1206(10)63320-0. [DOI] [PubMed] [Google Scholar]
- 73.Graft D., Aaronson D., Chervinsky P. A placebo- and active-controlled randomized trial of prophylactic treatment of seasonal allergic rhinitis with mometasone furoate aqueous nasal spray. J Allergy Clin Immunol. 1996;98:724–731. doi: 10.1016/s0091-6749(96)70119-7. [DOI] [PubMed] [Google Scholar]
- 74.Meltzer E.O., Orgel H.A., Backhaus J.W. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. J Allergy Clin Immunol. 1993;92:812–823. doi: 10.1016/0091-6749(93)90058-n. [DOI] [PubMed] [Google Scholar]
- 75.Barlan I.B., Erkan E., Bakir M. Intranasal budesonide spray as an adjunct to oral antibiotic therapy for acute sinusitis in children. Ann Allergy Asthma Immunol. 1997;78:598–601. doi: 10.1016/S1081-1206(10)63223-1. [DOI] [PubMed] [Google Scholar]
- 76.Yilmaz G., Varan B., Yilmaz T. Intranasal budesonide spray as an adjunct to oral antibiotic therapy for acute sinusitis in children. Eur Arch Otorhinolaryngol. 2000;257:256–259. doi: 10.1007/s004050050234. [DOI] [PubMed] [Google Scholar]
- 77.Meltzer E.O., Charous B.L., Busse W.W., and the Nasonex Sinusitis Group Added relief in the treatment of acute recurrent sinusitis with adjunctive mometasone furoate nasal spray. J Allergy Clin Immunol. 2000;106:630–637. doi: 10.1067/mai.2000.109056. [DOI] [PubMed] [Google Scholar]
- 78.Lund V.J., Black J.H., Szabo L.Z. Efficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patients. Rhinology. 2004;42:57–62. [PubMed] [Google Scholar]