Abstract
Background
Community determinants of antibiotics nonadherence, an important contributor of antibiotics resistance, remained unclear.
Objectives
Our objective was to investigate whether deficient antibiotics knowledge could contribute to nonadherence in a community with high prevalence of antibiotics resistance.
Methods
We recruited 465 people by random sampling from 5 urban areas in Hong Kong. A structured questionnaire was used to assess antibiotics knowledge and adherence. Adherence was defined as completing the most recent course of antibiotics entirely according to physicians’ instructions. An antibiotics knowledge score ranging from 0 to 3 (highest) was composed based on the number of correctly answered questions.
Results
Of the 465 participants interviewed, 96.3% had heard of the term “antibiotics,” and 80.6% recalled having previously received antibiotics prescription. Among the eligible 369 subjects, 32.9% showed nonadherence. Percentages of participants with antibiotics knowledge scores of 0, 1, 2, and 3 were 11%, 27%, 33%, and 29%, respectively. There was a higher prevalence of nonadherence among people with lower antibiotics knowledge score (P < .001). Furthermore, people with nonadherence had a significantly lower mean antibiotics knowledge score (1.3 ± 1.0 versus 2.0 ± 0.9, P < .001), with no interaction with education (P < .05). Adjusted for potential confounders, antibiotics knowledge scores of 2, 1, and 0 independently predicted increased risk of nonadherence by 1-fold (odds ratio [OR], 2.00; 95% confidence interval [CI]: 1.01-3.94; P = .047), 4-fold (OR, 4.77; 95% CI: 2.30-9.92; P < .001), and 17-fold (OR, 18.41; 95% CI: 6.92-48.97; P < .001) respectively, compared with the maximum score of 3.
Conclusion
Lack of antibiotics knowledge is a critical determinant of nonadherence independent of education in the community.
Key Words: Nonadherence, Antibiotics resistance, Knowledge, Community, Education
In today’s postantimicrobial era,1 antibiotic resistance has emerged into a threatening trend of reversing our decades’ progress of therapeutic success.2 Antibiotics resistance causes substantially increased infectious disease morbidity and mortality, as well as huge burden of cumulative socioeconomic costs.3, 4 With the alarming emergence of bacteria multiresistant to potent antibiotics,5, 6 the search for new antibiotics has become an issue of urgency.7 At the same time, there is a pressing need to identify the causes of antibiotics resistance and to devise effective interventions accordingly.8, 9
The causes of antibiotic resistance are multifactorial, representing a complex interplay among physician practices, patient behavior, and environmental factors.5 Along with the frequent emergence of resistant microbial strains in the tertiary care setting, such resistant strains are also increasingly found in the community.10, 11, 12 Larson has emphasized the importance of addressing the community’s role in the development of antibiotics resistance including behavioral, environmental, and policy factors.13 Although previous studies have attempted to understand the dynamics of physicians stewardship9, 14 and infection control in the hospital setting,15 research studies that addressed patients’ behavioral factors in the community have been scarce. In particular, it remained unclear as to what determines nonadherence behavior among patients who are prescribed antibiotics in the community setting. Unnecessarily long course of antibiotics (overuse) and nonindicated use of antibiotics (eg, for uncomplicated viral infections) are known to be important contributors to the development of bacterial resistance.13, 16, 17 Although without substantive evidence and requires further proof, nonadherence to prescribed antibiotics may potentially also play an important role. Thus, understanding the determinants of patient adherence could have profound implications for formulating effective public health interventions and policy making. We hypothesize that general knowledge of the appropriate use of antibiotics is important for patients in the community to ensure adherence to antibiotic prescriptions and that lack of this knowledge could negatively impact on adherence.
Hong Kong has prevalence of resistant microbial strains consistently ranked amongst the highest worldwide18, 19, 20, 21, 22, 23, 24, 25, 26, 27 and increasing numbers of potent antibiotics reported to be ineffective.6, 24, 27 We performed a cross-sectional study in this setting of highly prevalent antibiotics resistance to investigate the prevalence of antibiotics nonadherence and to assess the association between general knowledge of antibiotics and adherence.
Materials and methods
Study design and subjects
Participants were recruited from 5 randomly selected geographical areas in Hong Kong. Twenty eligible urban areas were determined a priori based on a maximum deviation of <10% from the census data in Hong Kong (2001) in terms of age groups, gender, and education levels. From these 20 areas, 5 were selected randomly with the constraint that at least 1 area from each of the 4 administrative districts of Hong Kong Island had to be included.
Pedestrians in these study areas were invited to complete a structured questionnaire. Interviews at the 5 study areas were simultaneously conducted over 4 consecutive weeks. A random sampling method was used for recruitment: every fifth pedestrian encountered by the interviewers exiting a zebra crosswalk was recruited. If the person refused to participate or did not meet the inclusion criteria, the immediate next person so encountered was recruited. The interviewers were trained to ask the questions in a standard format with a fixed order. Inclusion criteria were as follows: (1) aged 18 years or above; (2) able to speak Cantonese; (3) aware of the term “antibiotics”; and (4) previously prescribed antibiotics by a medical professional. All study participants gave an oral informed consent. The study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Hong Kong.
Assessment of demographics and antibiotics knowledge and adherence
A structured short questionnaire consisting of 11 items was used to assess demographic characteristics, antibiotic usage behavior, and knowledge. “Adherence” is defined as “the extent to which a person’s behavior corresponds to medical authoritative demand.”28 We defined adherence as (1) completing the most recent course of antibiotics prescribed in its entirety; (2) taking the antibiotics according to the physicians’ instructions. We assessed subjects’ general knowledge of antibiotics by asking 3 related questions (Table 1 ): 1. “Do you think it is essential to finish the whole course of antibiotics prescription?” 2. “What is/are the potential consequence(s) of not finishing the whole course of antibiotics?” 3. “Are antibiotics equivalent to anti-inflammatory agents?” Each correctly answered question was scored as 1, and wrong answers were scored as 0. A 4-point scale (0 to 3) was generated by summing the scores.
Table 1.
Antibiotics usage behavior and knowledge
| No. (%) | |
|---|---|
| Antibiotics usage behavior | |
| Having been prescribed antibiotics in the past 2 years | 210 (57) |
| Completed entire course of antibiotics in most recent prescription | 276 (76) |
| Took antibiotics on time according to physicians’ instructions | 305 (84) |
| Antibiotics nonadherence | 126 (33) |
| Assessment of antibiotics knowledge (Correct answer in bold) | Answered correctly |
| 1. Do you think it is essential to finish the whole course of antibiotics prescription? (Answers: Yes/No/Don’t know) | 320 (87) |
| 2. What is/are the potential consequence(s) of not finishing the whole course of antibiotics? (Answers: Nil/Emergence of resistance strains/Don’t know) | 195 (53) |
| 3. Are antibiotics equivalent to anti-inflammatory agent? (Answers: Yes/No/Don’t know) | 52 (14) |
| Antibiotics knowledge score | |
| 3 | 107 (29) |
| 2 | 121 (33) |
| 1 | 99 (27) |
| 0 | 42 (11) |
Statistical analysis
Student t test and analysis of variance were used for comparison of antibiotics knowledge score in adherent versus nonadherent participants. The χ2 was used for comparisons of categorical variables. We used multivariable logistic regression to examine the adjusted association of antibiotics knowledge score with adherence. The maximum antibiotics knowledge score of 3 with abundant cases was used as the reference group for comparison. Based on multivariable regression model of 4 predictors (age, gender, education, and antibiotics knowledge score) and assuming type I error <.05, a sample size of 312 is required to detect a small effect size of 0.05 with power of 90%. Statistical significance was defined at the level of P value < .05.
Results
A total of 465 people was interviewed. Of them, 96.3% had heard of the term “antibiotics,” whereas 80.6% recalled having been prescribed antibiotics at least once before the interview. The 96 individuals who had never heard of the term “antibiotics” and/or had never been prescribed antibiotics were excluded from the analysis.
The demographic characteristics of the 369 included participants are shown in Table 2 . Most 57.1% (210) of the participants had been prescribed antibiotics in the 2 years before the interview. Of these, 75.8% (276) had completed the entire course of their most recent antibiotic prescription, and 83.6% (305) had taken the antibiotics on time according to physicians’ instructions. Of the 83 participants who did not complete the course of antibiotics, 66.3% deemed it unnecessary to complete the course, 15.7% stopped the course because of adverse effects, and 7.2% reported no clear instructions from their physicians on the need for completion. Thus, 67.1% (243) met the criteria for adherence (Table 1).
Table 2.
Demographic characteristics of study participants: N = 369
| Characteristics | No. (%) |
|---|---|
| Age, yr | |
| 18–28 | 50 (14) |
| 29–39 | 70 (19) |
| 40–50 | 126 (34) |
| 51–60 | 65 (18) |
| 60 or above | 55 (15) |
| Sex | |
| Female | 200 (54) |
| Education level | |
| Up to primary school | 47 (13) |
| Forms 1-5 | 165 (45) |
| Forms 6-7 | 46 (13) |
| Tertiary education | 104 (28) |
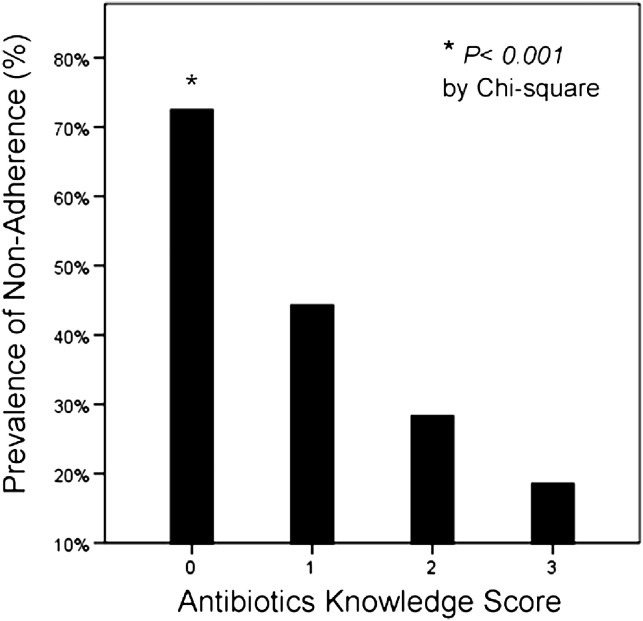
The antibiotics knowledge score had a normal distribution, with a score of 2 most frequent (33%). Less than 30% answered all 3 questions correctly. Figure 1 shows that nonadherence was associated with lower antibiotics knowledge scores with a clear dose response (P for trend < .001). Nonadherent participants had a lower mean antibiotics knowledge score (mean, 1.3; standard deviation [SD], 1.0; compared with mean, 2.0; SD, 0.9, P < .001). People with a score ≤1 accounted for most (58%) of the nonadherence, and this relationship persisted across all education levels (Fig 2 , primary school: P = .037; forms 1 to 5: P < .001; advanced levels and tertiary: P = .001). Test of interaction was negative between education and antibiotics knowledge score (P > .05). After adjusting for age, gender, and education, lower antibiotics knowledge scores of 2, 1, and 0 remained independently predictive of increased risk of nonadherence by 1-fold (odds ratio [OR], 2.00; 95% confidence interval [CI]: 1.01-3.94; P = .047), 4-fold (OR, 4.77; 95% CI: 2.30-9.92; P < .001), and 17-fold (OR, 18.41; 95% CI: 6.92-48.97; P < .001) respectively, as compared with the maximum score of 3 (Table 3 ).
Fig 1.
Percentage of subjects with antibiotics nonadherence in relation to antibiotics knowledge score. Score = 0: 69% (27/39); score = 1: 42% (42/99); score = 2: 26% (32/121); score = 3: 17% (18/107). P value for χ test < .001.
Fig 2.
Mean antibiotics knowledge score in patients demonstrating adherence versus nonadherence across different education levels. Primary school: 1.3 ± 0.9 versus 0.7 ± 0.7, respectively, P = .037; form 5-6: 1.9 ± 0.8 versus 1.1 ± 0.9, respectively, P < .001; advanced levels and tertiary: 2.4 ± 0.7 versus 1.8 ± 1.0, respectively, P = .001. Overall mean antibiotics knowledge score across different education levels: 1.1 ± 0.9 (primary school) versus 1.6 ± 0.9 (form 5-6) versus 2.2 ± 0.8 (advanced levels and tertiary). All comparisons, P ≤ .001; overall trend, P < .001. All comparisons by t test.
Table 3.
Multivariable logistic regression: Predictor for antibiotics nonadherence
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Age, yr | ||
| 18–28 | Reference | – |
| 29–39 | 0.73 (0.31-1.69) | .46 |
| 40–50 | 0.41 (0.19-0.92) | .03 |
| 51–60 | 0.49 (0.20-1.19) | .11 |
| 60 or above | 0.57 (0.21-1.54) | .57 |
| Sex | ||
| Male | Reference | – |
| Female | 0.93 (0.56-1.52) | .76 |
| Education level | ||
| Up to primary school | Reference | – |
| Forms 1-5 | 1.77 (0.76-4.11) | .18 |
| Advanced levels or tertiary | 2.18 (0.85-5.55) | .10 |
| Antibiotics knowledge score | ||
| 3 | Reference | – |
| 2 | 2.00 (1.01-3.94) | .047 |
| 1 | 4.77 (2.30-9.92) | <.001 |
| 0 | 18.41 (6.92-48.97) | <.001 |
Although a significant positive relationship was observed between subjects’ education level and antibiotics knowledge score (P < .001), there was no significant difference between the prevalence of nonadherence across different background education levels (P > .05).
Discussion
To our knowledge, this is the first study to investigate the determinants of community antibiotics nonadherence in a location with endemic of antibiotics resistance. More than 30% of participants reported nonadherence to a complete course of prescribed antibiotics in the community setting. Lack of knowledge about antibiotics was associated with nonadherence.
In a metropolitan setting such as Hong Kong where agriculture and animal husbandry are uncommon, behavioral patterns of physicians and the community are the major factors governing the dynamics of antibiotics resistance. First, in this densely populated area, high rates of antibiotic usage and selection pressure11 contribute to rapid development of resistant microbial strains. Furthermore, the public sector’s dominance29, 30 in primary health care reduces continuity of physician care in general. Doctor shopping31 is common, and the patient’s bargaining power for self-perceived treatment needs is high. Physicians may feel pressured by the patient’s expectation of an antibiotic prescription even in the absence of clinical indications,32 although that could be an overestimation of the patients’ expectations.33 However, in a competitive, mixed medical ecology, physicians may be driven to respond to patient’s wishes and to be overly cautious to avoid potential medico-legal consequences in an atmosphere of protective medicine.34 The challenge and the need for appropriate physician stewardship has been recognized.35 On the other hand, several participants reported lack of clear instructions from their physicians as the reason why they did not complete the course of antibiotics. Limited consultation time is often a constraint for physicians in practice,36 hindering clear explanations for each prescription. Measures to foster communications between physicians and patients and to reconcile their expectations could be useful.
Given the lack of previous data on the behavioral dynamics of antibiotics use in the community, this study adds by providing evidence that lack of antibiotics knowledge is associated with nonadherence. There was a linear dose-response relation between antibiotics knowledge and nonadherence, which did not vary with educational level. The differential impact of antibiotics knowledge and background education level on nonadherence suggests that traditional school education have failed to adequately equip the community to demonstrate proper behavior in antibiotics usage. Antibiotic overuse and misuse (and possibly that of nonadherence, which should be confirmed in further studies) are important contributors to the development of bacterial resistance.13, 16, 17 Targeted and multilevel community education in the appropriate use of antibiotics could be a valuable public health intervention in our combat against the ever-increasing threat of antibiotics resistance. To generate a real impact, it is equally critical that physicians should take the initiative to actively educate their individual patients about the importance of adherence to prescribed antibiotic therapy. Clearly defined, culturally relevant educational interventions targeted at both clinicians and the community can result in significantly improved knowledge regarding antibiotics resistance and indications,37 and a holistic, multilevel ecologic approach targeting the whole community should be emphasized.13
Of note, Hong Kong has been threatened by infectious diseases in recent years, including severe acute respiratory syndrome,38 bird flu,39 and H1N1 pandemic.40 The heightened awareness for personal hygiene and infection prevention strategies41 are likely to be beneficial for preventing further antibiotic resistance and could serve as a platform to promote structural as well as personal solutions. However, the related lower threshold of antibiotics prescription in anticipation of potentially severe infections and infection control practices may produce an undesirable counteracting effect.42 The recent experience from European countries such as Norway43 has shown that antibiotic resistance can be halted, and potentially reversed, with comprehensive, appropriate legislative measures restricting antibiotics use, which are appropriately implemented and enforced. The need for active surveillance systems of antibiotics use and enforced legislative measures in metropolitans such as Hong Kong needs to be critically reviewed.
There are several limitations in this study. First, we did not include people, albeit a minority, who did not recognize the term “antibiotics.” Some of these could have been prescribed antibiotics, but their behavior was not assessed. This should lead to a more conservative estimate of effect size observed. Second, we cannot exclude misclassification error at the initial recruitment based on their prior knowledge of the term “antibiotics.” To reduce this potential misclassification, we also included prior experience of physician-prescribed antibiotics as another inclusion criterion. There may be recall error regarding previous antibiotic prescriptions, but any such effect is likely to equally distribute in the sample. Furthermore, if there is a bias because of prompting, it is likely to affect predominantly subjects with poorer knowledge in antibiotics, which would be expected to drive the results towards null, as opposed to the observed results in this study. Third, because the study was conducted in a rather challenging setting (nearby the zebra cross), we had to strike a balance between the depth of inquiry and the need to minimize the interview duration for each subject, so as to ease administration and enhance the response rate. For this reason, we could not inquire further into other important areas including knowledge of treatment failure as an important possible outcome of nonadherence and the (in)appropriateness of using antibiotics for viral infections/ influenza. Finally, this study was conducted in a location with high antibiotic resistance and may not apply to developing countries or suburban areas. Finally, this is a cross-sectional study, and, thus, causality cannot be confirmed. Nevertheless, given the nature of parameters studied—nonadherence and antibiotics knowledge—reverse causality is unlikely. Future large prospective interventional studies would be of great interest.
Conclusion
In an urban location with a high prevalence of antibiotic resistance, more than 30% of participants in the community were nonadherent to course of antibiotics. Lack of knowledge about antibiotics was associated with nonadherence behavior, independent of education. This suggests that multilevel, culturally relevant public education on the proper use of antibiotics to promote adherence should be a major target of public health interventions against the development of antibiotics resistance.
Footnotes
Conflicts of interest: None to report.
References
- 1.Cohen M.L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 2.Knobler S., Institute of Medicine (US). Forum on Emerging Infections . National Academies Press; Washington, DC: 2003. The resistance phenomenon in microbes and infectious disease vectors: implications for human health and strategies for containment. Workshop summary. [PubMed] [Google Scholar]
- 3.Gums J.G. Assessing the impact of antimicrobial resistance. Am J Health Syst Pharm. 2002;59(8 Suppl. 3):S4–S6. doi: 10.1093/ajhp/59.suppl_3.S4. [DOI] [PubMed] [Google Scholar]
- 4.Holmberg S.D., Solomon S.L., Blake P.A. Health and economic impacts of antimicrobial resistance. Rev Infect Dis. 1987;9:1065–1078. doi: 10.1093/clinids/9.6.1065. [DOI] [PubMed] [Google Scholar]
- 5.Gould I.M. The epidemiology of antibiotic resistance. Int J Antimicrob Agents. 2008;32(Suppl 1):S2–S9. doi: 10.1016/j.ijantimicag.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Seaworth B.J. Multidrug-resistant tuberculosis. Infect Dis Clin North Am. 2002;16:73–105. doi: 10.1016/s0891-5520(03)00047-3. [DOI] [PubMed] [Google Scholar]
- 7.The Lancet. Urgently needed: new antibiotics. Lancet. 2009;374:1868. doi: 10.1016/S0140-6736(09)62076-6. [DOI] [PubMed] [Google Scholar]
- 8.Levy S.B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(Suppl 12):S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 9.Harbarth S., Samore M.H. Antimicrobial resistance determinants and future control. Emerg Infect Dis. 2005;11:794–801. doi: 10.3201/eid1106.050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho P.L., Wong R.C., Yip K.S., Loke S.L., Leung M.S., Mak G.C. Antimicrobial resistance in Escherichia coli outpatient urinary isolates from women: emerging multidrug resistance phenotypes. Diagn Microbiol Infect Dis. 2007;59:439–445. doi: 10.1016/j.diagmicrobio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Levy S.B. Antibiotic resistance: an ecological imbalance. Ciba Found Symp. 1997;207:1–14. doi: 10.1002/9780470515358.ch1. [DOI] [PubMed] [Google Scholar]
- 12.Ho P.L., Cheng V.C., Chu C.M. Antibiotic resistance in community-acquired pneumonia caused by Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii. Chest. 2009;136:1119–1127. doi: 10.1378/chest.09-0285. [DOI] [PubMed] [Google Scholar]
- 13.Larson E. Community factors in the development of antibiotic resistance. Annu Rev Public Health. 2007;28:435–447. doi: 10.1146/annurev.publhealth.28.021406.144020. [DOI] [PubMed] [Google Scholar]
- 14.Pulcini C., Williams F., Molinari N., Davey P., Nathwani D. Junior doctors’ knowledge and perceptions of antibiotic resistance and prescribing: a survey in France and Scotland. Clin Microbiol Infect. 2011;17:80–87. doi: 10.1111/j.1469-0691.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 15.Junior M.S., Correa L., Marra A.R., Camargo L.F., Pereira C.A. Analysis of vancomycin use and associated risk factors in a university teaching hospital: a prospective cohort study. BMC Infect Dis. 2007;7:88. doi: 10.1186/1471-2334-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin D.J., Kristinsson K.G., Anderson R.M. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A. 1999;96:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunin C.M., Liu Y.C. Excessive use of antibiotics in the community associated with delayed admission and masked diagnosis of infectious diseases. J Microbiol Immunol Infect. 2002;35:141–146. [PubMed] [Google Scholar]
- 18.Canton R., Morosini M., Enright M.C., Morrissey I. Worldwide incidence, molecular epidemiology and mutations implicated in fluoroquinolone-resistant Streptococcus pneumoniae: data from the global PROTEKT surveillance programme. J Antimicrob Chemother. 2003;52:944–952. doi: 10.1093/jac/dkg465. [DOI] [PubMed] [Google Scholar]
- 19.Song J.H., Jung S.I., Ko K.S., Kim N.Y., Son J.S., Chang H.H. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study) Antimicrob Agents Chemother. 2004;48:2101–2107. doi: 10.1128/AAC.48.6.2101-2107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright A., Zignol M., Van Deun A., Falzon D., Gerdes S.R., Feldman K. Epidemiology of antituberculosis drug resistance 2002-2007: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–1873. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- 21.Ho P.L., Yung R.W., Tsang D.N., Que T.L., Ho M., Seto W.H. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J Antimicrob Chemother. 2001;48:659–665. doi: 10.1093/jac/48.5.659. [DOI] [PubMed] [Google Scholar]
- 22.Hirakata Y., Matsuda J., Miyazaki Y., Kamihira S., Kawakami S., Miyazawa Y. Regional variation in the prevalence of extended-spectrum β-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998-2002) Diagn Microbiol Infect Dis. 2005;52:323–329. doi: 10.1016/j.diagmicrobio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Aziz M.A., Wright A., Laszlo A., De Muynck A., Portaels F., Van Deun A. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet. 2006;368:2142–2154. doi: 10.1016/S0140-6736(06)69863-2. [DOI] [PubMed] [Google Scholar]
- 24.Chu Y.W., Cheung T.K., Chu M.Y., Lo J.Y., Dijkshoorn L. OXA-23-type imipenem resistance in Acinetobacter baumannii in Hong Kong. Int J Antimicrob Agents. 2009;34:285–286. doi: 10.1016/j.ijantimicag.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Boost M.V., O’Donoghue M.M., Dooley J.S. Prevalence of carriage of antimicrobial resistant strains of Streptococcus pneumoniae in primary school children in Hong Kong. Epidemiol Infect. 2001;127:49–55. doi: 10.1017/s0950268801005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canton R., Loza E., Morosini M.I., Baquero F. Antimicrobial resistance amongst isolates of Streptococcus pyogenes and Staphylococcus aureus in the PROTEKT antimicrobial surveillance programme during 1999-2000. J Antimicrob Chemother. 2002;50(Suppl 1):9–24. doi: 10.1093/jac/dkf811. [DOI] [PubMed] [Google Scholar]
- 27.Ho P.L., Que T.L., Chiu S.S., Yung R.W., Ng T.K., Tsang D.N. Fluoroquinolone and other antimicrobial resistance in invasive pneumococci, Hong Kong, 1995-2001. Emerg Infect Dis. 2004;10:1250–1257. doi: 10.3201/eid1007.030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes C.M. Medication non-adherence in the elderly: how big is the problem? Drugs Aging. 2004;21:793–811. doi: 10.2165/00002512-200421120-00004. [DOI] [PubMed] [Google Scholar]
- 29.Yam H.K., Mercer S.W., Wong L.Y., Chan W.K., Yeoh E.K. Public and private healthcare services utilization by non-institutional elderly in Hong Kong: is the inverse care law operating? Health policy (Amsterdam, Netherlands) 2009;91:229–238. doi: 10.1016/j.healthpol.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Leung G.M., Yeung R.Y., Wong I.O., Castan-Cameo S., Johnston J.M. Time costs of waiting, doctor-shopping and private-public sector imbalance: microdata evidence from Hong Kong. Health policy (Amsterdam, Netherlands) 2006;76:1–12. doi: 10.1016/j.healthpol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Yeung R.Y., Leung G.M., McGhee S.M., Johnston J.M. Waiting time and doctor shopping in a mixed medical economy. Health Econ. 2004;13:1137–1144. doi: 10.1002/hec.871. [DOI] [PubMed] [Google Scholar]
- 32.Coenen S., Michiels B., Renard D., Denekens J., Van Royen P. Antibiotic prescribing for acute cough: the effect of perceived patient demand. Br J Gen Pract. 2006;56:183–190. [PMC free article] [PubMed] [Google Scholar]
- 33.Britten N. Patients’ demands for prescriptions in primary care. BMJ. 1995;310:1084–1085. doi: 10.1136/bmj.310.6987.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam T.P., Lam K.F. What are the non-biomedical reasons which make family doctors over-prescribe antibiotics for upper respiratory tract infection in a mixed private/public Asian setting? J Clin Pharm Ther. 2003;28:197–201. doi: 10.1046/j.1365-2710.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- 35.Cheng V.C., To K.K., Li I.W., Tang B.S., Chan J.F., Kwan S. Antimicrobial stewardship program directed at broad-spectrum intravenous antibiotics prescription in a tertiary hospital. Eur J Clin Microbiol Infect Dis. 2009;28:1447–1456. doi: 10.1007/s10096-009-0803-8. [DOI] [PubMed] [Google Scholar]
- 36.Deveugele M., Derese A., van den Brink-Muinen A., Bensing J., De Maeseneer J. Consultation length in general practice: cross-sectional study in six European countries. BMJ. 2002;325:472. doi: 10.1136/bmj.325.7362.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trepka M.J., Belongia E.A., Chyou P.H., Davis J.P., Schwartz B. The effect of a community intervention trial on parental knowledge and awareness of antibiotic resistance and appropriate antibiotic use in children. Pediatrics. 2001;107:E6. doi: 10.1542/peds.107.1.e6. [DOI] [PubMed] [Google Scholar]
- 38.Leung G.M., Ho L.M., Lam T.H., Hedley A.J. Epidemiology of SARS in the 2003 Hong Kong epidemic. Hong Kong Med J. 2009;15(Suppl 9):12–16. [PubMed] [Google Scholar]
- 39.Guan Y., Chen H., Li K., Riley S., Leung G., Webster R. A model to control the epidemic of H5N1 influenza at the source. BMC Infect Dis. 2007;7:132. doi: 10.1186/1471-2334-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipsitch M., Hayden F.G., Cowling B.J., Leung G.M. How to maintain surveillance for novel influenza A H1N1 when there are too many cases to count. Lancet. 2009;374:1209–1211. doi: 10.1016/S0140-6736(09)61377-5. [DOI] [PubMed] [Google Scholar]
- 41.Cowling B.J., Leung G.M., Seto W.H. Hand hygiene and virus transmission. CMAJ. 2009;181:716. doi: 10.1503/cmaj.109-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yap F.H., Gomersall C.D., Fung K.S., Ho P.L., Ho O.M., Lam P.K. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004;39:511–516. doi: 10.1086/422641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnsen P.J., Townsend J.P., Bohn T., Simonsen G.S., Sundsfjord A., Nielsen K.M. Factors affecting the reversal of antimicrobial-drug resistance. Lancet Infect Dis. 2009;9:357–364. doi: 10.1016/S1473-3099(09)70105-7. [DOI] [PubMed] [Google Scholar]