Highlights
-
•
Bacterial concentrations in a bathroom with an ultraviolet C device and a control bathroom are compared.
-
•
The ultraviolet C–treated bathroom had significantly lower airborne and surface bacterial levels.
-
•
Ultraviolet C has the potential to be used in a wide range of decontamination applications.
Key Words: Ultraviolet C, bioaerosol, environmental cleaning, contact surface, toilet plume, bathroom
Abstract
Toilet flushing can contribute to disease transmission by generating aerosolized bacteria and viruses that can land on nearby surfaces or follow air currents. Aerobic and anaerobic bacterial bioaerosol loads, and bacterial counts on 2 surfaces in a bathroom with a permanently installed, automated ultraviolet C (UVC) irradiation device, were significantly lower than in a comparable bathroom without the UVC device. Permanently installed UVC lights may be a useful supplementary decontamination tool in shared patient bathrooms.
Toilet flushing generates airborne particles that contain bacteria and viruses.1 Larger droplets can travel short distances and settle on surfaces and may also rapidly form smaller droplet nuclei that follow air currents potentially for long distances.2, 3 The fate of the toilet plume will depend on the distribution of droplets within it, the environmental conditions (temperature and relative humidity), and room air currents dictated by local ventilation. Despite this variable distribution of microorganisms, both aerosol and surface contamination may increase disease transmission risk. One cost-effective decontamination tool used in hospitals as an adjunct to traditional cleaning and disinfection is ultraviolet C (UVC) irradiation.4, 5, 6 The goal of this study was to evaluate the incremental antimicrobial effect of an automated, permanently installed UVC device with a short run time (5 minutes) on surface and airborne bacterial levels in a shared patient bathroom (BR).
Materials and methods
This study was conducted in November 2015 in a 268-bed community hospital medical ward with common hallway bathrooms shared by up to 8 patients. All samples were collected by a trained graduate student in a shared BR without a UVC device and in a shared BR with a permanently installed, wall-mounted automated UVC (254-nm) device (ASEPT.1X; Sanuvox, Saint-Laurent, QC, Canada). The decontamination cycle initiates only after there has been 30 seconds of no motion detected by infrared sensors, after which a UVC cycle will run for 5 minutes. The device is located directly above the door and has an automatic shutoff safety feature that terminates the cycle if the BR door is opened midrun. Both the UVC-treated BR and control BR contain a 6.0 L per flush, wash down toilet (TOTO, Morrow, GA) and have similar room volumes (12.7 and 11.7 m3, respectively). Air and surface samples were collected in each BR 5 minutes and 30 seconds after each patient use—a time that accounts for the decontamination cycle and permits comparison of the samples between each BR. A dual-headed SAS 360 bioaerosol sampler (Bioscience International, Rockville, MD) set to collect 150 L of air over 50 seconds was used to permit simultaneous collection of aerobic and anaerobic bacterial cultures. The anaerobic Brucella plates (Oxoid, Nepean, ON, Canada) were immediately placed in a candle jar postsampling and subsequently incubated anaerobically at 37°C for 48 hours prior to counting bacterial colonies. The aerobic 5% sheep blood agar plates (Oxoid) were incubated at 37°C for 24-48 hours prior to counting.
Surface bacteria on the toilet seat and handwashing sink counter were sampled using 65-mm Replicate Organism Detection and Counting (RODAC) Contact Plates (American Precision Plastics, NorthGlenn, CO) containing trypticase soy agar (BD, Mississauga, ON, Canada), with a contact time of 10-12 seconds, and were incubated aerobically at 37°C overnight. Surface sample locations were matched between the 2 bathrooms, with samples taken at random locations on the counter, approximately 1.5 m away from the toilet, and on the top of the toilet seat. We collected a total of 66 air samples (32 aerobic and 34 anaerobic) and 64 surface samples (32 counter and 32 toilet seat). Mean bacterial concentrations were compared between the 2 bathrooms for each of the different sample types using Welch t tests.
Results
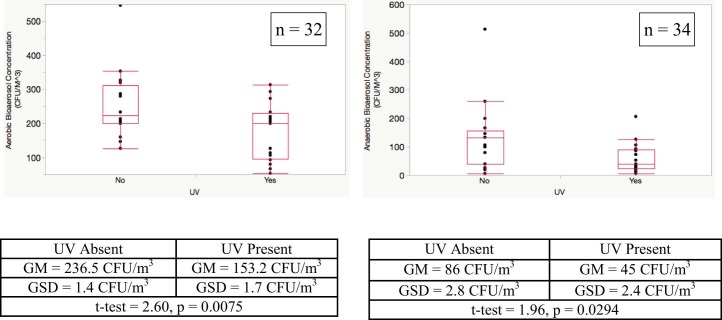
The UVC-treated BR (geometric mean [GM] = 153.2 colony forming units [CFU]/m3, geometric SD [GSD] = 1.7) had a 35.2% reduction in aerobic bacterial bioaerosol concentration compared with the control BR (GM = 236.5 CFU/m3, GSD = 1.44) (Fig 1 ). This difference was even more pronounced for anaerobic bacterial bioaerosols, where the UVC-treated BR (GM = 45 CFU/m3, GSD = 2.4) had a 47.7% reduction compared with the control BR (GM = 86 CFU/m3, GSD = 2.8).
Fig 1.
Effect of ultraviolet C light on aerobic and anaerobic bacterial bioaerosol levels. Lower, middle, and upper lines of the box indicate the 25th, 50th, and 75th percentiles, respectively. The upper and lower whiskers represent plus and minus 1.5× the interquartile range. CFU, colony forming units; GM, geometric mean; GSD, geometric SD; UV, ultraviolet.
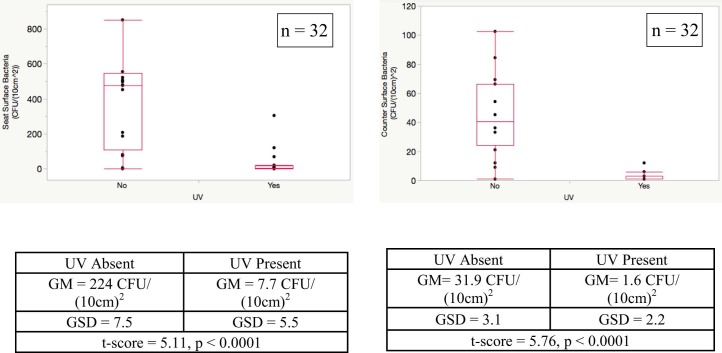
The mean bacterial concentration on the UVC-treated BR counter (GM = 1.6 CFU/[10 cm]2, GSD = 2.2) was reduced by approximately 95% compared with the control BR counter (GM = 31.0 CFU/10 cm2, GSD = 3.1) (Fig 2 ). The greatest effect was seen for surface seat bacteria, with a 97% reduction in the UVC-treated BR (GM = 7.7 CFU/[10 cm]2, GSD = 5.5) compared with the control BR (GM = 224 CFU/[10 cm]2, GSD = 7.5). Two outliers collected from the toilet seat of the control BR had concentrations >2,000 CFU/(10 cm)2 (not shown in Fig 2). These outlier samples may represent highly contaminated droplets deposited onto the seat after flushing.
Fig 2.
Effect of ultraviolet C light on surface toilet seat and counter bacterial levels. Lower, middle, and upper lines of the box indicate the 25th, 50th, and 75th percentiles, respectively. The upper and lower whiskers represent plus and minus 1.5× interquartile range. Two outliers (2,019 CFU/[10 cm]2 and (2,305 CFU/[10 cm]2) are not shown for the seat. CFU, colony forming units; GM, geometric mean; GSD, geometric SD; UV, ultraviolet.
Discussion
The toilet plume can be a vector for disease transmission. The severe acute respiratory syndrome outbreak at the Amoy Gardens apartment complex in Hong Kong was caused by contamination of the sewage drainage system by the index patient.3 The dry U-tube drains allowed virus containing–droplet nuclei to be pulled by air currents from the bathroom fan to neighboring apartments, with subsequent secondary severe acute respiratory syndrome cases.1 On an international flight, a probable norovirus outbreak involved 8 crew members experiencing vomiting or diarrhea symptoms in the bathroom.7 Although there was no obvious soiling of the bathroom, 5 passengers who visited it significantly more than the others developed norovirus-like symptoms. Shared BRs, specifically among patients in health care facilities, represent a significant challenge for controlling disease transmission. Frequent use by patients not only increases the microbial burden, but sequential flushing can actually cause release of organisms from a previous contamination; viral bioaerosols can be generated up to 7 flushes after initial contamination.8 Patients entering the BR may be exposed to pathogens in the toilet plume originating from multiple previous patients, and because it is not practical to clean the BR after every patient use, these organisms may remain in the BR for some time. One limitation of this study was that consideration was not given to the nature of the patient's use of the washroom.
Portable UVC devices have been used as effective decontamination tools and have been shown to be more effective than manual cleaning alone.5 Recently, UVC significantly outperformed manual cleaning using accelerated hydrogen peroxide in the removal of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and Clostridium difficile.5 Similar results have been seen with the use of short exposure time UVC devices (5-10 minutes), significantly reducing C difficile and methicillin-resistant S aureus levels.6 However, there is a paucity of studies looking at the antimicrobial efficacy of permanently installed UVC devices. To our knowledge, this study is the first to evaluate the use of such a device in a shared BR. These results demonstrate the potential utility of permanently installed UVC lights to supplement decontamination efforts. Specifically, the short run time and automatic shutoff safety feature, in addition to its antimicrobial efficacy, make this device an ideal decontamination adjunct in shared BRs, where high microbial burden and frequent occupant use pose significant challenges to current manual cleaning and disinfection efforts. The potential to use this technology both in health care (bathrooms and procedure rooms) and in commercial applications (aircraft, train, and cruise ship bathrooms) is intriguing.
Acknowledgments
We thank the staff on the medical floor at Lion's Gate Hospital for accommodating the sampling for this project and Rosma Facundo from Infection Control and the members of the Vancouver General Hospital Medical Microbiology Laboratory team for assisting with the project.
Footnotes
Funding/Support: Supported by the Canadian Institutes of Health Research and the University of British Columbia.
Conflicts of Interest: None to report.
Disclaimer: The ASEPT.1X UV device was donated from Class 1 Inc, with full awareness of the manufacturer Sanuvox (Montreal). Neither had input into the study design, analysis, or writing.
References
- 1.Johnson D.L., Mead K.R., Lynch R.A., Hirst D.V.L. Lifting the lid on toilet plume aerosol: a literature review with suggestions for future research. Am J Infect Control. 2013;41:254–258. doi: 10.1016/j.ajic.2012.04.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells W. On air-bourne infection. Study II: droplets and droplet nuclei. Am J Hyg. 1934;20:1–8. [Google Scholar]
- 3.Hung L.S. The SARS epidemic in Hong Kong: what lessons have we learned? J R Soc Med. 2003;96:374–378. doi: 10.1258/jrsm.96.8.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nerandzic M.M., Cadnum J.L., Pultz M.J., Donskey C.J. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis. 2010;10:197. doi: 10.1186/1471-2334-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong T., Woznow T., Petrie B., Murzello E., Muniak A., Kadora A. Postdischarge decontamination of MRSA, VRE, and Clostridium difficile isolation rooms using 2 commercially available automated ultraviolet-C–emitting devices. Am J Infect Control. 2016;44:416–420. doi: 10.1016/j.ajic.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Rutala W.A., Gergen M.F., Tande B.M., Weber D.J. Room decontamination using an ultraviolet-C device with short ultraviolet exposure time. Infect Control Hosp Epidemiol. 2014;35:1070–1072. doi: 10.1086/677149. [DOI] [PubMed] [Google Scholar]
- 7.Widdowson M., Glass R., Monroe S., Beard S., Bateman J., Lurie P. Probable transmission of norovirus on an airplane. JAMA. 2005;293:1859–1860. doi: 10.1001/jama.293.15.1859. [DOI] [PubMed] [Google Scholar]
- 8.Yahya M., Cassells J., Straub T., Gerba C. Reduction of microbial aerosols by automatic toilet bowl cleaners. J Environ Health. 1991;55:32–34. [Google Scholar]