Abstract
Background
The removal of personal protective equipment (PPE) after patient care may result in transfer of virus to hands and clothing of health care workers (HCWs). The risk of transfer can be modeled using harmless viruses to obtain quantitative data. To determine whether double-gloving reduces virus transfer to HCWs’ hands and clothing during removal of contaminated PPE, we conducted a human challenge study using bacteriophages to compare the frequency and quantity of virus transfer to hands and clothes during PPE removal with single-gloving and double-gloving technique.
Methods
Each experiment had a double-gloving phase and a single-gloving phase. Participants donned PPE (ie, contact isolation gown, N95 respirator, eye protection, latex gloves). The gown, respirator, eye protection, and dominant glove were contaminated with bacteriophage. Participants then removed the PPE, and their hands, face, and scrubs were sampled for virus.
Results
Transfer of virus to hands during PPE removal was significantly more frequent with single-gloving than with double-gloving. Transfer to scrubs was similar during single-gloving and double-gloving. The amount of virus transfer to hands ranged from 0.15 to 2.5 log10 most probable number. Significantly more virus was transferred to participants’ hands after single-gloving than after double-gloving.
Conclusions
Our comparison of double-gloving and single-gloving using a simulation system with MS2 and a most-probable number method suggests that double gloving can reduce the risk of viral contamination of HCWs’ hands during PPE removal. If incorporated into practice when full PPE is worn, this practice may reduce the risk of viral contamination of HCWs’ hands during PPE removal. The use of double gloves should be explored in larger controlled studies.
Key Words: Gloving, Occupational health, Infection control
Caring for patients with communicable diseases places health care workers (HCWs) at risk for exposure to respiratory viruses, such as severe acute respiratory syndrome coronavirus (SARS Co-V) and influenza, that spread via contact, droplets, and aerosols. Exposure during patient care activities can result in infection, illness or death, and HCWs can spread infectious agents to other HCWs, their families, or other patients. Protecting HCWs from occupationally acquired respiratory infections uses a barrier approach, with personal protective equipment (PPE) to protect HCWs from exposure to pathogens during patient care.1 PPE may include gowns, gloves, eye protection, masks, and respirators to protect HCWs’ mucous membranes, airways, skin, and clothing from contact with infectious agents.
The risks of occupationally acquired respiratory infections and the importance of PPE for HCWs was graphically illustrated by the worldwide outbreak of SARS. HCWs represented approximately 20% of cases,2 and failure to properly and consistently use PPE was a risk factor for infection of HCWs.2, 3, 4, 5, 6 As new risks from potential pandemic human and avian-derived influenza emerge, protecting HCWs from respiratory infection will be increasingly important.
The SARS outbreak reinforced the vital role of PPE in protecting HCWs from occupationally acquired infection, but also led to the realization that the step of equipment removal was potentially a neglected source of contamination and an infection risk. PPE must be removed after each patient encounter, and transfer of organisms from contaminated PPE to hands or clothing could be a source of infection for HCWs as well as others. The Centers for Disease Control and Prevention (CDC) responded by asking experts to design a protocol to minimize contamination of the wearer’s hands and clothing during PPE removal.1
This protocol was based on expert opinion and knowledge, but was not empirically validated when it was designed. Whether these PPE removal practices effectively protect HCWs is an empirical question that is not easily answered in real-world health care settings, especially in the context of an ongoing outbreak. Data may be affected by problems with recall and variations in PPE use among health care facilities and among HCWs within the same facility. Outbreak settings do not allow for rigorous comparisons of PPE use practices, because obviously staff cannot be assigned or randomized to practices that might expose them to infection.
Empirical data on the effectiveness of PPE removal protocols and other aspects of PPE use can be objectively improved by using model systems with human volunteers and surrogate microorganisms. Modeling viral contamination and transfer events using harmless viruses in controlled settings allow investigators to obtain quantitative data on virus transfer events and risks to HCWs without exposing participants to the risk of infection. Bacteriophages are candidate surrogates for human viral pathogens. They are nonpathogenic, posing no risk to study participants. They are structurally similar to nonenveloped human viruses, including norovirus and hepatitis A,7, 8 and because of these similarities have been previously used as surrogates to examine aspects of health care hand hygiene9 and virus transfer10 with human volunteers. A model system with human volunteers and the MS2 bacteriophage has been used to evaluate the CDC’s PPE removal protocol. That study found that removing contaminated PPE according to the protocol still resulted in virus transfer to the wearer’s hands and clothing.11
The results of the previous volunteer study indicate the need for alternative PPE removal protocols to reduce the risk of wearer contamination during removal. Using the same model system as used in that study, we can empirically test such alternatives. One such alternative is double-gloving, in which 2 pairs of gloves are worn one on top of the other. When removing PPE, the outer pair of gloves is removed first, followed by the rest of the PPE items, and the inner pair of gloves is removed last. The HCW never actually touches any contaminated PPE item with bare hands. To examine whether double-gloving reduces the probability of virus transfer to HCWs’ hands and clothing during the removal of contaminated PPE, we conducted a human challenge study using the bacteriophage MS2. This study compared the frequency and quantity of virus transfer to hands and clothes during PPE removal using single-gloving and double-gloving techniques.
Methods
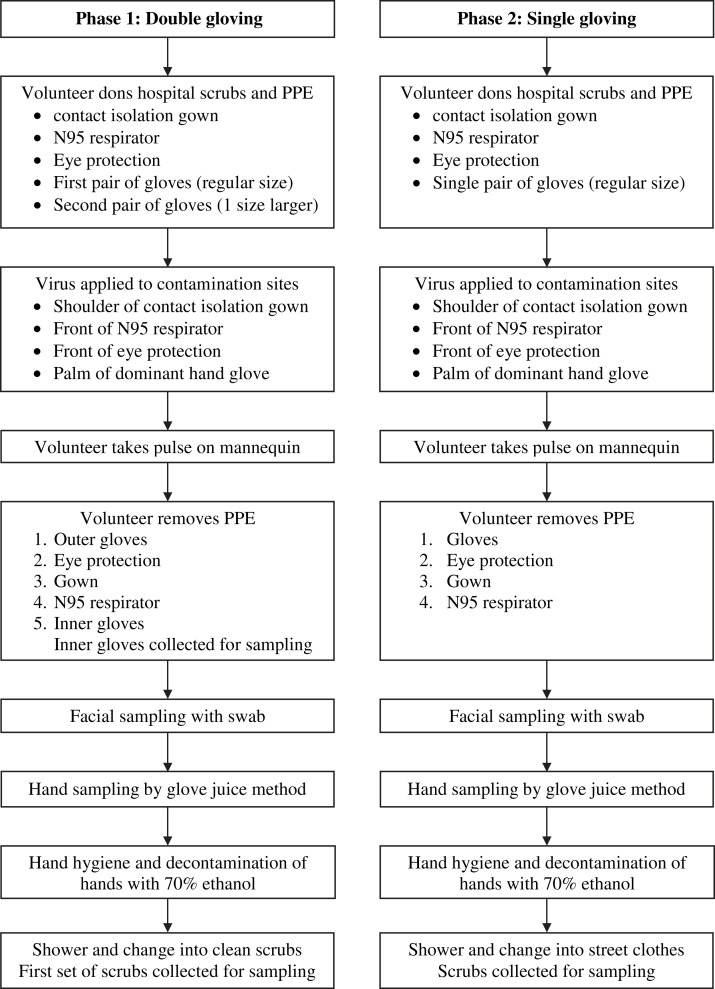
The study protocol was approved by the University of North Carolina’s Biomedical Institutional Review Board (Study 05-2856), and written informed consent was obtained from each participant. The study population was individuals working as health care providers. The inclusion criteria for enrollment were age >18 years, not pregnant, no latex allergy, no active skin disorders, and previous fit testing for an N95 respirator. Experiments were performed in a patient care room in the University of North Carolina Hospital Clinical and Translational Research Center. The experimental protocol is shown in Figure 1 .
Fig 1.
Experimental protocol.
Each experiment comprised 2 phases: a double-gloving phase and a single-gloving phase. Before beginning, participants were shown a poster presenting of the CDC’s PPE removal protocol and given an opportunity to read it and ask questions. The double-gloving phase was performed first. Participants changed into a scrub shirt and pants and donned a full set of PPE, consisting of a contact isolation gown, an N95 respirator, eye protection, and 2 pairs of latex gloves. The first (inner) pair of gloves was put on so that the wrist of the glove was under the elastic cuff at the wrist of the gown sleeve. The second (outer) pair, one size larger, was worn over the first pair so that the wrist of the glove was over the elastic cuff at the end of the gown sleeve. Although the CDC protocol calls for donning and doffing PPE at the door of the patient’s room, participants carried out these activities in the center of the room, to minimize the possibility of accidental touching of room surfaces or objects.
After donning, PPE was contaminated with bacteriophage MS2 suspended in 0.01 M phosphate-buffered saline. Sites of contamination were the front shoulder of the gown, right side of the N95 respirator, upper right front of the eye protection, and palm of the dominant hand. Each site was contaminated with a total of 5 log10 plaque-forming units (PFU) of MS2 in 5 drops of 5 μL each to simulate droplet contamination. To simulate typical physical movement that would occur while wearing PPE, the participant then performed a routine health care task (assessing neck and wrist pulses on a mannequin).
The participant then removed the PPE according to the CDC protocol, with modifications. The participant was verbally instructed to remove the outer pair of gloves first and discard them, then remove the remaining items of PPE according to the protocol. Once the gown, eye protection, and respirator were removed according to the protocol, the inner pair of gloves was removed last. The protocol was available to the participant for reference at all times during PPE removal. During the removal process, the investigator observed the participant and noted any deviations from the CDC removal protocol on a data sheet.
After removal, the inner gloves were immediately placed in containers of eluent liquid. The participant was instructed to stand in the center of the room without touching the hair or face. Hands were sampled using the glove juice method.12 Each hand was placed inside a bag containing 75 mL of stripping solution (0.4 g of KH2PO4, 10.1 g of Na2HPO4, and 1.0 mL of Triton-X/L of reagent water) and massaged for 60 seconds to cover all hand surfaces with solution. The nondominant hand was sampled first, followed by the dominant hand. The face was sampled by dipping a polyester-tipped swab in stripping solution and swabbing a 1-cm2 area of each cheek where the edge of the N95 respirator had rested. The swab was immediately placed in a tube of eluent liquid. The hands were decontaminated by washing with soap and water and rubbing with 70% ethanol. The removed scrub shirt and pants were collected for sampling immediately and placed in containers of eluent liquid. The participant took a shower with full body washing and then donned a clean pair of scrubs.
The single-gloving phase was performed next. The participant donned a single glove on each hand and followed the CDC removal protocol as written. Sampling was identical to that in the double-gloving phase. The hands were decontaminated by washing with soap and water and rubbing with 70% ethanol, and the participant showered before changing back into street clothes. The removed scrubs were collected for sampling in the same manner as in the double-gloving phase. Samples were transported to the laboratory and assayed within 4 hours of collection. No more than 20 minutes elapsed between application of virus and placement of hand, face, glove, and scrub samples in eluent liquid.
Gloves and scrubs were assayed as described previously.13 Items were immersed in 0.5-2 L of 1.5% beef extract (pH 7.5) and agitated on a shaker for 20 minutes. Eluent from hands, face, and PPE was assayed by a most probable number (MPN) enrichment infectivity assay.14 To prevent cross-contamination, only one participant was assayed and processed each day, and individual eluent samples were processed separately in a biological safety cabinet, with UV light decontamination between samples. Using an a priori value of 20% for the acceptable 95% upper confidence limit for the probability of virus transfer by a given participant when P (transfer) = 0, the minimum sample size was determined to be n = 15.
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC) and GraphPad Prism 5 (GraphPad, LaJolla, CA). All statistical tests were 2-tailed and used a significance level of α = 0.05.
Results
A total of 18 volunteers participated, all HCWs with previous experience in using PPE. Seventeen were right-handed, and 1 was left-handed. The frequency of virus transfer to hands, face, clothing, and inner gloves (during double-gloving) is shown in Table 1 . Transfer of virus to participants’ hands during PPE removal was significantly more frequent in the single-gloving phase than in the double-gloving phase (14/18 vs 5/18; P = .006, Fisher’s exact test). Transfer of virus to scrub shirt and pants worn underneath PPE was observed with similar frequency during both study phases. Transfer to the inner gloves worn during double-gloving was detected in 17/18 participants. Only 1 participant had virus transfer to the face during the double-gloving phase.
Table 1.
Virus transfer during PPE removal (α = 0.05)
| Site | Participants transferring virus (n = 18), n (%) |
P value | |
|---|---|---|---|
| Double-gloving | Single-gloving | ||
| Inner gloves | 17 (94) | — | — |
| Hands | 5 (23) | 14 (78) | .007 |
| Face | 1 (6) | 0 (0) | — |
| Shirt | 17 (94) | 16 (89) | 1.00 |
| Pants | 10 (56) | 11 (61) | 1.00 |
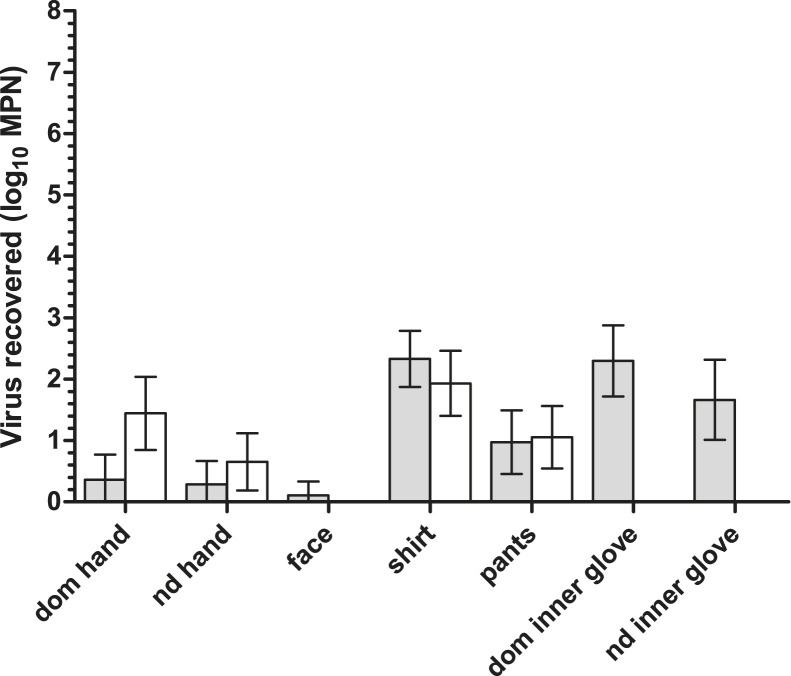
The quantities of infectious virus recovered from previously uncontaminated sampling sites were measured (Fig 2 ). The mean amount of virus applied per site across all PPE contamination sites was 5.13 log10 PFU (95% CI, 5.14-5.46). The amount of virus transferred to hands after PPE removal ranged from 0.15 log10 MPN to 2.5 log10 MPN. In the single-gloving phase, larger quantities of virus were transferred to participants’ dominant hands compared to their nondominant hands. In the double-gloving phase, more virus was transferred to inner gloves than to hands. The paired t test for dependent samples was used to compare the quantity of infectious virus transferred to each site during the single-gloving phase versus the double-gloving phase (Table 2 ). Significantly more infectious viruses were transferred to participants’ hands during PPE removal after single-gloving than after double-gloving. Double-gloving also was associated with significantly less transfer of virus to participants’ scrub shirts; however, virus transfer to pants did not differ significantly between the study phases.
Fig 2.
Quantity of infectious virus transferred from PPE to different sites during PPE removal (gray, double-gloving; white, single-gloving). The quantity of virus originally applied to each site was 5.13 log10 PFU. Lower detection limits of virus assay: hands, 0.15 log10 MPN; shirts/pants, 0.60 log10 MPN; gloves, 0.30 log10 MPN.
Table 2.
Errors observed during PPE removal
| Error type | Participants making any error (n = 18), n (%) |
|
|---|---|---|
| Double-gloving | Single-gloving | |
| Any error | 13 (72) | 12 (67) |
| Gown removal | 3 (17) | 3 (17) |
| N95 respirator removal | 9 (50) | 5 (28) |
| Eye protection removal | 10 (56) | 6 (33) |
| Removal of items in wrong order | 2 (11) | 6 (33) |
Despite the fact that participants reviewed the protocol before beginning PPE removal and had it available during removal, deviations from the protocol were common during removal (Table 3 ). Errors included removal of PPE items in the wrong order and touching of an area of an item identified by the protocol as contaminated. The most common error was touching the N95 respirator and eye protection on the front (where it is most likely to be contaminated), rather than by the straps or edges. The paired t test was used to compare the quantity of infectious virus transferred to hands by participants who deviated from the protocol and by participants who did not. A comparison of the quantity of virus transferred to the dominant hand in participants who made errors and those who did not showed that only errors in gown removal (most frequently grabbing the gown and pulling it off by the sleeves instead of the neckline) were associated with a significant increase in the quantity of virus transferred (P < .0001).
Table 3.
Statistical comparisons of quantity of virus transferred to sampling sites during PPE removal using the single- and double-gloving protocols (α = 0.05)
| Site | Virus transferred (log10 MPN) |
P value | |
|---|---|---|---|
| Single | Double | ||
| Dominant hand | 1.44 | 0.36 | .0055 |
| Nondominant hand | 0.65 | 0.28 | .026 |
| Face | 0.11 | 0 | .33 |
| Shirt | 1.93 | 2.33 | .05 |
| Pants | 0.97 | 1.05 | .77 |
Discussion
This comparison of double- and single-gloving using a model simulation system has demonstrated that removal of contaminated PPE results in contamination of HCWs’ hands, and that double-gloving can reduce this risk. When double gloves were worn with PPE and the inner glove was removed last, significantly fewer participants transferred virus to their hands, and significantly fewer viruses were transferred to participants’ hands compared to when single gloves were worn and removed first. The difference in virus transfer to the inner glove and hands from double-gloving suggests that the inner gloves receive much of the virus transferred from contaminated PPE items, reducing the amount reaching the surface of the hands. The finding that viruses on PPE transfer to hands is consistent with previous studies using model viruses11 and fluorescent tracers.15 This transfer poses an increased risk of self-inoculation, environmental contamination, or transfer of virus to others if an HCW touches his or her face, surfaces, objects, or other patients without first performing hand hygiene.
Our results indicate that the time of PPE removal is a risk point for hand contamination and a potentially important point at which to target messages about hand hygiene and infection prevention. However, observations of participants’ actions in this simulation of PPE use and removal suggest that a multistep removal protocol might not be an optimal approach. Although the participants in this study were given an opportunity to read the CDC PPE removal protocol poster and ask questions, and the poster was in front of them at all times for reference, most participants still made some type of error during removal. These errors, including handing PPE by touching the front areas (eg, handling N95 respirator by the front instead of the straps), are targeted by the CDC protocol because they are likely to result in hand contamination. In practice, HCWs are unlikely to have the protocol in front of them each time they need to remove PPE. Achieving adherence to the protocol may require multiple educational messages and feedback techniques targeting each item of PPE, making it a complex undertaking and not necessarily an efficient use of resources if some elements of the protocol are not actually protective.
A better approach may be to target high-risk points and high-risk situations for specific actions that reduce risk. For example, the significant difference in contamination levels observed for gown removal suggests that this may be a high-risk point in the PPE removal process. The gown has a large surface area for contamination to spread across, as well as a large surface area for which the wearer to accidentally come in contact with during removal. Observations of participants making gown removal errors showed a tendency for the participant to remove the gown in a manner typical of the way in which one would remove a piece of clothing worn on the torso, rather than by grabbing the neckline and pulling it off inside out. These findings suggest that gown removal could be targeted as a high-risk point in PPE removal. This point could be addressed using messages targeting one specific action (eg, always grab the gown by the neck), rather than multiple steps, all of which might not have protective effects.
Our results demonstrate that double-gloving is a protective measure; however, the low rate of compliance with recommended double-gloving practices among nonsurgical specialties, such as emergency medicine,16 suggests that targeted use of double gloves is already challenging and may complicate promotion of routine use of double-gloving while wearing PPE. Double-gloving has some drawbacks, including greater cost due to the use of twice as many gloves, the extra time required to don extra gloves, and issues with HCWs’ perception of impaired sensitivity and dexterity during double-gloving.16, 17 An alternative targeted measure might be to encourage HCWs to double-glove during certain high-risk situations that are likely to result in heavily contaminated PPE, such as droplet- and aerosol-generating procedures like intubation that require gowns and respirators and have previously been observed to carry infection risks.4
Although targeted measures such as the aforementioned may protect HCWs from hand contamination, the need to emphasize hand hygiene after PPE removal remains. According to the CDC protocol, hand hygiene should immediately follow each incidence of PPE removal. Little data are available on hand hygiene compliance specifically after removal of PPE. No study has found that glove use reduces hand hygiene compliance compared with situations in which gloves are not worn,18 but research has shown that HCWs’ general hand hygiene compliance is suboptimal. One multicenter study found a compliance rate of 36% in non-ICU settings, which increased to only 50% after intervention19; another study found a rate of 32% before interventions designed to increase compliance, increasing to 63% after these interventions.20 The risk of hand contamination during PPE removal underscores the crucial need to reinforce messages about the importance of hand hygiene when PPE is worn. Analyses of factors that contribute to the success of hand hygiene interventions suggest that incorporating “vicarious experience” and “introduction of experiential elements” as part of educational efforts can improve compliance.21 Simulations such as the one used in the present study demonstrate how these types of virus transfers can occur during real-world use. Presenting information from and encouraging HCWs to participate in such simulations can help reinforce the importance of hand hygiene after PPE removal and thereby improve compliance.
Transfer of virus to the scrubs worn beneath PPE occurred at similarly high frequencies during both the single-gloving and double-gloving phases of the study. The amount of virus transferred to scrubs did not differ significantly in the 2 study phases, and transfer to scrubs occurred in the absence of detectable hand contamination (data not shown). Contamination of the clothing worn under PPE may occur when an item of PPE, such as the gown, contacts the scrubs during removal. This can occur even in the absence of transfer of virus to hands. Our findings suggest that double-gloving did not affect the transfer of virus to scrubs. In this study, the most likely cause of the greater contamination of scrub shirts was accidental contact between shirts and hands or gowns. Although according to protocol, the gown should be grasped by the neckline and pulled off inside out, this still provides an opportunity for the hands or gloves to come in contact with the gown’s fabric, especially near the neck and shoulders. This study was not designed to determine whether penetration of viruses through gown material, in contrast to direct contact with the outside of contaminated PPE items, might be a factor in the contamination of scrubs. The contamination of scrubs observed here suggests that the potential risks posed by handling scrubs that have been worn during patient care, as well as wearing them outside the health care facility after work, merit further examination. Further research is needed to explore the need for measures to prevent the transmission of viruses via contaminated clothing after PPE use.
The results of the present study must be interpreted with caution because of the small sample size. Nonetheless, they suggest that the PPE removal protocol itself has little or no effect on the transfer of virus to HCWs’ hands. If the currently recommended removal protocol still results in virus transfer, then double-gloving can provide an alternative technique to decrease the risk of hand contamination during PPE removal. Whether or not double-gloving is used, reinforcing the importance of hand hygiene after PPE removal is vital. Even if effective removal protocols can be formulated, the errors in adhering to the removal protocol observed in this study suggest that a single behavioral change message, such as the wearing of a second pair of gloves in high-risk situations, or a message targeting the importance of hand hygiene specifically after PPE removal, may be simpler than trying to achieve universal adherence to a multistep removal protocol that is then followed by hand hygiene.
This study has several limitations. The order in which the single-gloving and double-gloving techniques were tested was the same for all participants (double-gloving first, followed by single-gloving). Because this was a pilot study, we decided that all participants should perform exactly the same activities in exactly the same order. This introduced the possibility that participant fatigue could affect the single-gloving phase. However, we found more errors in PPE removal during the double-gloving phase, suggesting that fatigue may have no affect on errors during the removal process. Future studies should incorporate random assignment of the order of activities to address participant fatigue as a potential variable.
A second limitation of this study is that the virus was applied to PPE items in individual drops to simulate droplet transmission during patient care. This method of application allows for precise targeting of the parts of PPE that are contaminated; however, it might not accurately simulate the patterns of virus deposition that would result from aerosol transmission of virus from a patient to a HCW’s PPE. Work is ongoing on application systems that replicate aerosol deposition of viruses on PPE.
Double-gloving is not used routinely in health care; however, it may be used in surgery, where compliance is not universal and depends largely on the surgeon’s specialty area.22 When incorporated into patient care encounters for which full PPE (ie, gowns, N95 respirators, and eye protection) are worn, double-gloving may provide greater protection than multistep removal protocols. Although it may provide greater protection against contamination during PPE removal, double-gloving promoted as part of routine barrier precautions still must be accompanied by an emphasis on hand hygiene. This pilot study suggests that double-gloving can reduce the risks of viral contamination of HCWs’ hands during removal of PPE after patient care activities. The double-gloving technique is a promising alternative that should be explored in larger controlled studies of PPE removal and virus transfer risks.
Footnotes
This work was supported by the Centers for Disease Control and Prevention (Grant 1-U01 CI000299-01) and the General Clinical Research Centers Program, Division of Research Resources, National Institutes of Health (Grant R00046). The funding agencies had no role in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the manuscript for publication.
Conflict of interest: None to report.
References
- 1.Siegel J., Rhinehart E., Jackson M., Chiarello L., and the Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan-Yeung M. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10:421–427. doi: 10.1179/oeh.2004.10.4.421. [DOI] [PubMed] [Google Scholar]
- 3.Lau J., Fung K., Tze Wai W., Kim J., Wong E., Chung S. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis. 2004;10:280–286. doi: 10.3201/eid1002.030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb M., McGeer A., Henry B., Ofner M., Rose D., Hlywka T. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ofner-Agostini M., Gravel D., McDonald L.C., Lem M., Sarwal S., McGeer A. Cluster of cases of severe acute respiratory syndrome among Toronto healthcare workers after implementation of infection control precautions: a case series. Infect Control Hosp Epidemiol. 2006;27:473–478. doi: 10.1086/504363. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.C., Chen P.J., Chang S.C., Kao C.L., Wang S.H., Wang L.H. Infection control and SARS transmission among healthcare workers. Taiwan. Emerg Infect Dis. 2004;10:895–898. doi: 10.3201/eid1005.030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae J., Schwab K.J. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl Environ Microbiol. 2008;74:477–484. doi: 10.1128/AEM.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewitt J., Rivera Aban M., Greening G. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. J Appl Microbiol. 2009;107:65–71. doi: 10.1111/j.1365-2672.2009.04179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sickbert-Bennett E.E., Weber D.J., Gergen-Teague M.F., Sobsey M.D., Samsa G.P., Rutala W.A. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control. 2005;33:67–77. doi: 10.1016/j.ajic.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rusin P., Maxwell S., Gerba C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J Appl Microbiol. 2002;93:585–592. doi: 10.1046/j.1365-2672.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- 11.Casanova L., Alfano-Sobsey E., Rutala W., Weber D., Sobsey M. Virus transfer from personal protective equipment to healthcare employees’ skin and clothing. Emerg Infect Dis. 2008;14:1291–1293. doi: 10.3201/eid1408.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ASTM International . ASTM International; West Conshohocken [PA]: 2006. Standard test method for evaluation of the effectiveness of health care personnel handwash formulations. [Google Scholar]
- 13.Casanova L., Rutala W., Weber D., Sobsey M. Methods for the recovery of a model virus from healthcare personal protective equipment. J Appl Microbiol. 2009;106:1244–1251. doi: 10.1111/j.1365-2672.2008.04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Environmental Protection Agency . Environmental Protection Agency; Cinncinati [OH]: 2001. Method 1601: male-specific (F+) and somatic coliphage in water by a two-step enrichment procedure. [Google Scholar]
- 15.Zamora J., Murdoch J., Simchison B., Day A. Contamination: a comparison of 2 personal protective systems. CMAJ. 2006;175:249–254. doi: 10.1503/cmaj.060094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim L.E., Evanoff B.A., Parks R.L., Jeffe D.B., Mutha S., Haase C. Compliance with universal precautions among emergency department personnel: Implications for prevention programs. Am J Infect Control. 1999;27:453–455. doi: 10.1016/s0196-6553(99)70014-3. [DOI] [PubMed] [Google Scholar]
- 17.St Germaine R.L., Hanson J., de Gara C.J. Double-gloving and practice attitudes among surgeons. Am J Surg. 2003;185:141–145. doi: 10.1016/s0002-9610(02)01217-5. [DOI] [PubMed] [Google Scholar]
- 18.Zimakoff J., Stormark M., Larsen S. Use of gloves and handwashing behaviour among health care workers in intensive care units: a multicentre investigation in four hospitals in Denmark and Norway. J Hosp Infect. 1993;24:63–67. doi: 10.1016/0195-6701(93)90090-m. [DOI] [PubMed] [Google Scholar]
- 19.McGuckin M., Waterman R., Govednik J. Hand hygiene compliance rates in the United States—a one-year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual. 2009;24:205–213. doi: 10.1177/1062860609332369. [DOI] [PubMed] [Google Scholar]
- 20.Randle J., Clarke M., Storr J. Hand hygiene compliance in healthcare workers. J Hosp Infect. 2006;64:205–209. doi: 10.1016/j.jhin.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Nicol P., Watkins R., Donovan R., Wynaden D., Cadwallader H. The power of vivid experience in hand hygiene compliance. J Hosp Infect. 2009;72:36–42. doi: 10.1016/j.jhin.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Akduman D., Kim L., Parks R., L’Ecuyer P., Mutha S., Jeffe D. Use of personal protective equipment and operating room behaviors in four surgical subspecialties: personal protective equipment and behaviors in surgery. Infect Control Hosp Epidemiol. 1999;20:110–114. doi: 10.1086/501601. [DOI] [PubMed] [Google Scholar]