Highlights
-
•
A 30-bed negative-pressure isolation ward was established on a functioning hospital.
-
•
The pressure relative to the main hospital was −29 Pa by adjusting the ventilation.
-
•
No occurrences of pressure reversal occurred at ward entrance.
-
•
Pressures on the ward changed to slightly positive.
-
•
Health care personnel should wear personal protective equipment on the ward.
Key Words: Airborne infection isolation room, Respiratory infection control, Pandemic preparedness, Surge capacity, Bioterrorism, Biodefense
Abstract
Background
During a large-scale airborne infectious disease outbreak, the number of patients needing hospital-based health care services may exceed available negative-pressure isolation room capacity.
Methods
To test one method of increasing hospital surge capacity, a temporary negative-pressure isolation ward was established at a fully functioning hospital. Negative pressure was achieved in a 30-bed hospital ward by adjusting the ventilation system. Differential pressure was continuously measured at 22 locations, and ventilation airflow was characterized throughout the ward.
Results
The pressure on the test ward relative to the main hospital hallway was −29 Pa on average, approximately 10 times higher than the Centers for Disease Control and Prevention guidance for airborne infection control. No occurrences of pressure reversal occurred at the entrances to the ward, even when staff entered the ward. Pressures within the ward changed, with some rooms becoming neutrally or slightly positively pressurized.
Conclusions
This study showed that establishing a temporary negative-pressure isolation ward is an effective method to increase surge capacity in a hospital.
Background
Infectious disease epidemics, such as severe acute respiratory syndrome in 2003, H1N1 influenza in 2009, and the outbreak of Middle East respiratory syndrome starting in 2012, are public health threats that are best mitigated by deliberate planning at the health system level.1, 2, 3 A robust response to a large-scale infectious disease outbreak is predicated, in part, on coordination between public health and health care delivery systems.1, 4, 5 Hospital pandemic preparedness plans typically include protocols for handling a surge of infectious patients.6 Hospitals need to respond rapidly if they are among the first impacted by a highly contagious outbreak.7
Most U.S. hospitals use negative-pressure airborne infection isolation rooms (AIIRs) to house patients with suspected or confirmed airborne transmissible infections. The pressure difference between an AIIR and the hospital corridor is recommended to be −2.5 Pa in the United States.8, 9 It is also recommended to have an air exchange rate (AER) of 12 air changes per hour (ACH), of which 2 ACHs must be outside air in an AIIR.2, 8 In approximately one-half of urban hospitals, only 2%-4% of rooms are equipped with negative pressure.10 The number of patients needing health care services may rapidly exceed such a small AIIR capacity during an airborne transmissible pandemic or bioterror event.11
There are no regulations stipulating surge capacity requirements for U.S. hospitals. Guidance for intensive care unit capacity has been published, ranging from a 20%-300% increase in bed numbers, depending on the type of incident.5, 6, 11, 12, 13, 14 One option to meet capacity needs would be to implement a temporary negative-pressure isolation ward that could house a large number of patients. To date, there are few studies detailing the effectiveness of temporary isolation wards to be used during a surge. Rosenbaum et al demonstrated during a hospital disaster preparedness drill that multiple high-efficiency particulate air (HEPA)–filtered negative air machines placed in a physical therapy gymnasium produced the recommended pressure and AER for negative-pressure isolation.15 In another demonstration, a 3-unit temporary patient shelter was constructed out of plastic sheeting and ventilated using negative-air machines.16 Containment was estimated using fluorescent tracer particles, and very high levels of containment were achieved (>99%) with AERs of 15 ACHs.
Although it is recognized that increased surge capacity is an important component of hospital preparedness, more knowledge and field experience are needed to guide decisions about increasing airborne surge capacity.17 The purpose of this project was to demonstrate and test whether a functional hospital wing could be operated effectively as a negative-pressure isolation ward for an entire day. Data collected included the following: pressure differentials at the isolation ward's outer envelope, internal variability of pressure on the ward, performance of the temporary anteroom (ANT), pressure fluctuations when ingress or egress events occurred, flow rates and AERs in bedrooms, and ultraviolet (UV)-C fluxes in stairwells.
Materials and methods
Isolation ward layout
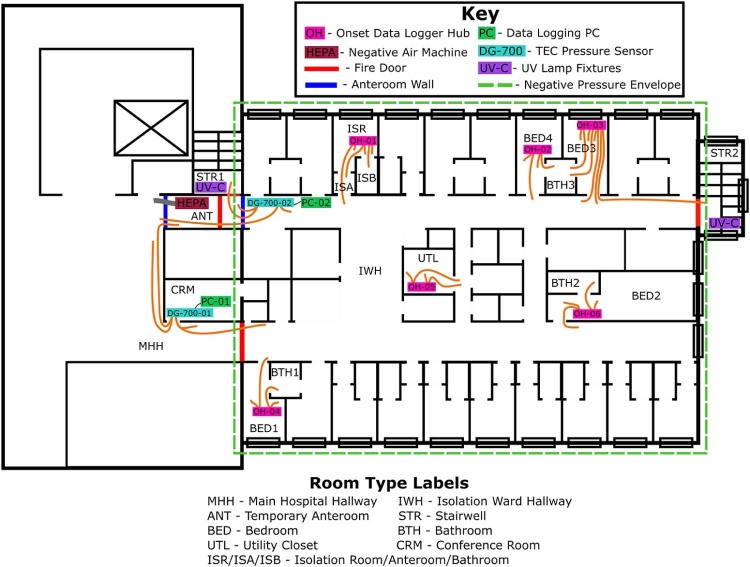
A functioning hospital in the San Francisco Bay Area, Northern California, was chosen as the study site. The project was completed in March 2015. A temporary negative-pressure isolation ward was located where it could be effectively isolated from the rest of the hospital. A ward on the top floor of the hospital was chosen because it had a dedicated air handling unit (AHU), a dedicated bathroom exhaust system, a separate dedicated exhaust system for return registers in existing isolation rooms (ISRs), and a firewall separating the ward from the rest of the hospital. Figure 1 depicts the ward layout.
Fig 1.
Isolation ward layout and instrument locations. PC, personal computer; TEC, the energy conservatory; UV, ultraviolet.
The ward was sealed from the rest of the hospital by closing the fire doors in one hallway (main hospital hallway [MHH]) (Fig 1) and by setting up an ANT in the other hallway (Fig 1). The ANT was constructed of a wood frame bolted to the ceiling. Plastic sheeting was taped to the ceiling frame, walls, and floors and fitted with 2 zippered openings for doors. All doorways with access to the ward, and internal bedroom and bathroom doors, were kept closed during the study except for brief times during staff ingress or egress.
Ventilation design and control
During the demonstration, the AHU was operated with supply airflow reduced to 60% of its normal operating speed and exhaust airflow operating at capacity. The AHU was an air-to-air, constant-air-volume system, set to 100% outside air and 100% exhaust manually for this study. All return and exhaust air was directly released through on-roof stacks with no mixing or recirculation. This ventilation scheme generated −29 Pa of pressure across closed fire doors in the MHH, while limiting nuisance noise on the ward produced by the AHU.
Two HEPA-filtered negative-air machines (MICROCON MAP800; Biological Controls, Eatontown, NJ) were operated at 1,104 m3/h to establish negative pressure in the ANT and were exhausted into the MHH. Negative-air machine flow rates were set such that the anteroom pressure was highly negative relative to the MMH, yet not as negatively pressurized as the isolation ward, to direct air flow toward the isolation ward.
During planning visits, pressure measurements collected from the stairwells indicated that they were positively pressurized relative to the ward, limiting the possibility of infectious particles escaping through these spaces except when stairwell doors were opened. One solution to ensure any escaping particles are disinfected was to install upper room germicidal UV lamps. These lamps (nonlouvered GL-188; Lumalier, Memphis, TN) were installed near the door in each stairwell internal to the ward at a height of 2.1 m. UV-C fluxes were measured in both stairwells using a radiometer (Model IL1400A; International Light, Peabody, MA) with an SEL240 UV-C sensor. UV-C measurements were collected in a grid at 2 distances away from each lamp with the radiometer probe facing the wall on which the lamps were hung. Prior to the demonstration, UV-C lamps were burnt-in for >100 hours.
Instrumentation and data collection
Two pressure sensors (DG-700; The Energy Conservatory, Minneapolis, MN) were used to monitor the ward's outer negative-pressure envelope. Fifteen pressure sensors (Model T-VER-PXU-X by Veris Industries; Onset Computer Corporation, Bourne, MA) were connected to 6 data loggers (Model UX120-006M; Onset Computer Corporation, Bourne, MA) and monitored internal pressure variability on the ward between bedrooms, bathrooms, and the isolation ward hallway (IWH). Reported accuracy for the DG-700 is 0.15 Pa for pressures <1.5 Pa and 1% of the reading at higher pressures. Three side-by-side comparisons for the 2 DG-700 sensors resulted in excellent agreement. Reported accuracy for the onset pressure (OP) sensors is 0.5-1 Pa. In preliminary side-by-side comparisons, good agreement was observed between the DG-700 and OP sensors.
A balometer (Model ABT701; TSI, Shoreview, MN) was used to measure supply, return, and exhaust register flow rates. One return register in the ISR could not be accessed, and the return register could also not be accessed in the utility closet (UTL). AERs were calculated by dividing the highest summed register flow (supply, return, or exhaust) by the room volume.
Data analysis
Data time series were split into 5 time periods for analysis: pretest (March 17, 2015, 5:05 PM-March 18, 2015, 1:10 PM; 20 hours), ramp-up (March 18, 2015, 1:10 PM-1:53 PM; 42 minutes), negative-pressure demonstration (March 18, 2015, 1:53 PM-March 19, 2015, 1:14 PM; 23 hours), ramp-down (March 19, 2015 1:14 PM-1:54 PM; 40 minutes), and posttest (March 19, 2015, 1:54 PM-March 20, 2015 9:32 AM; 20 hours). Ramp-up and ramp-down periods are not considered for data summaries because they include transition periods when the isolation ward, ANT, and UV-C lamps were being setup or taken down. The ANT and UV luminaries were operated throughout the 23-hour negative-pressure demonstration phase.
Door-opening events were separated from the static pressures on the ward using the average static pressure conditions. All data outside of boundaries along a smoothed line fit through the data were identified as door-opening events, and all data within the boundaries were considered static pressure conditions. Internal pressures were typically smaller, more uncertain, and less temporally variable than outer envelope pressures.
Results
AERs, pressures, and UV-C flux
Table 1 contains room size, sums of supply, return, and exhaust flow rates and the estimated AER for each room during each phase of the project. Bedrooms 1 and 3 had AERs near or above the suggested AER for hospital bedrooms of 4-6 ACHs.8 Bedrooms lacking supply flow (bedrooms 2 and 4) had reduced AERs.
Table 1.
Volumetric flow (cubic meters per hour) and air exchange rates (air changes per hour) measured during the demonstration
| Parameter | BED1 | BTH1 | BED2 | BTH2 | BED3 | BTH3 | BED4 | ISR* | ISA | ISB | UTL† | ANT | IWH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface area (m2) | 25.5 | 6.9 | 29.8 | 5.3 | 25.5 | 6.9 | 25.5 | 18.1 | 5.7 | 6.3 | 15.6 | 11.9 | — | |
| Volume (m3) | 69.9 | 16.9 | 81.8 | 13.0 | 69.9 | 16.9 | 69.9 | 49.6 | 15.6 | 15.3 | 47.5 | 32.6 | — | |
| Pretest | ∑Supply | 505 | — | 0 | — | 395 | — | 0 | 327 | 121 | — | 154 | — | 4745 |
| ∑Return | 319 | — | 443 | — | 356 | — | 270 | 529 | 337 | — | N/A | — | — | |
| ∑Exhaust | — | 189 | — | 230 | — | 172 | — | — | — | 398 | — | — | — | |
| AER | 7.2 | 11.1 | 5.4 | 17.7 | 5.6 | 10.1 | 3.9 | 10.7 | 21.6 | 26.0 | 3.2 | — | — | |
| Negative-pressure demonstration | ∑Supply | 432 | — | 0 | — | 396 | — | 0 | 346 | 135 | — | 164 | — | 3781 |
| ∑Return | 343 | — | 482 | — | 386 | — | 325 | 563 | 347 | — | N/A | — | — | |
| ∑Exhaust | — | 161 | — | 200 | — | 159 | — | — | — | 385 | — | 2209 | — | |
| AER | 6.2 | 9.5 | 5.9 | 15.4 | 5.7 | 9.4 | 4.6 | 11.4 | 22.2 | 25.2 | 3.5 | 67.7 | — | |
| Posttest | ∑Supply | 391 | — | 0 | — | 433 | — | 0 | N/A | 136 | — | N/A | — | N/A |
| ∑Return | 340 | — | 425 | — | 391 | — | 297 | N/A | 306 | — | N/A | — | — | |
| ∑Exhaust | — | 170 | — | 195 | — | 170 | — | — | — | 382 | — | — | — | |
| AER | 5.6 | 10.0 | 5.2 | 15.0 | 6.2 | 10.0 | 4.3 | N/A | 19.6 | 25.0 | N/A | — | — | |
AER, air exchange rate; ANT, temporary anteroom; BED1, bedroom 1; BED2, bedroom 2; BED3, bedroom 3; BED4, bedroom 4; BTH1, bathroom 1; BTH2, bathroom 2; BTH3, bathroom 3; ISA, isolation anteroom; ISB, isolation bathroom; ISR, isolation room; IWH, isolation ward hallway; N/A, not applicable; UTL, utility closet.
Only 2 of 3 return registers were measured; therefore, the total return and AERs listed here are underestimates of the actual rates. Estimating the AER for the ISR using the design flow rate for the unmeasured register resulted in pretest and demonstration phase AERs of 15.5 and 16.1 air changes per hour, respectively.
UTL return register could not be accessed for measurements.
Means and SDs of static pressures are presented in Table 2 . Mean isolation ward pressures during the negative-pressure demonstration were about −29 Pa, both across the closed fire doors and the ANT. The pressure gradient across the anteroom had higher-pressure differences on the ANT-MHH side than the IWH-ANT side, which was the intended design.
Table 2.
Static pressure data measured during the demonstration
| Location | Instrument name (hub/channel) |
Dataset name, − and + probe locations | Pretest phase, mean ± SD (Pa) |
Negative-pressure demonstration, mean ± SD (Pa) | Posttest phase, mean ± SD (Pa) |
Comments |
|---|---|---|---|---|---|---|
| Outer envelope | DG-700-01(Ch.A) | IWH-MHH1 | 0.0±0.1 | −28.9±0.9 | — | Across fire doors |
| DG-700-02(Ch.B) | IWH-MHH2 | — | −28.8±0.9 | — | Across anteroom | |
| DG-700-01(Ch.B) | ANT-MHH | 0.0±0.2 | −17.5±2.4 | — | ||
| — | IWH-ANT | — | −11.2±1.9 | — | Sub. estimate | |
| Stairwells | DG-700-02(Ch.A) | ANT-STR1 | — | −20.9±2.6 | — | |
| — | IWH-STR1 | — | −32.2±1.7 | — | Sub. estimate | |
| OP-08(OH-03) | IWH-STR2 | −4.4±1.3 | −22.2±0.9 | −3.4±0.9 | ||
| ISR | OP-01(OH-01) | ISR-IWH | −19.1±3.1 | −17.7±0.2 | −19.5±0.2 | |
| OP-02(OH-01) | ISR-ISA | −7.4±1.2 | −7.1±0.1 | −7.7±0.1 | ||
| — | ISA-IWH | −11.7±1.9 | −10.7±0.2 | −11.8±0.2 | Sub. estimate | |
| OP-03(OH-01) | ISB-ISR | −4.4±0.7 | −4.1±0.1 | −4.5±0.1 | ||
| Bedrooms and bathrooms | OP-11(OH-04) | BED1-IWH | 0.0±0.1 | 0.5±0.1 | −0.1±0.1 | |
| OP-10(OH-04) | BTH1-BED1 | −1.4±0.2 | −1.3±0.2 | −1.4±0.2 | ||
| OP-14(OH-06) | BED2-IWH | −0.5±1.2 | −0.6±1.0 | −0.1±0.1 | ||
| OP-15(OH-06) | BTH2-BED2 | −1.6±1.4 | −1.7±0.7 | −1.7±1.6 | ||
| OP-07(OH-03) | BED3-IWH | −0.6±0.1 | −0.1±0.2 | −0.6±0.1 | ||
| OP-06(OH-03) | BTH3-BED31 | −1.5±0.3 | −1.4±0.2 | −1.7±0.2 | ||
| OP-09(OH-03) | BTH3-BED32 | −1.3±0.3 | −1.2±0.2 | −1.4±0.2 | Duplicate | |
| OP-04(OH-02) | BED4-IWH | −1.6±0.6 | −1.2±0.1 | −1.7±0.5 | ||
| OP-05(OH-02) | BTH3-BED4 | −0.3±0.3 | 0.0±0.1 | −0.3±0.3 | ||
| UTL | OP-12(OH-05) | UTL-IWH1 | 0.0±0.1 | 0.2±0.1 | −0.1±0.1 | |
| OP-13(OH-05) | UTL-IWH2 | 0.1±0.1 | 0.3±0.1 | 0.1±0.1 | Duplicate |
NOTE. Duplicate datasets are noted with subscripts 1, 2.
ANT, temporary anteroom; BED1, bedroom 1; BED2, bedroom 2; BED3, bedroom 3; BED4, bedroom 4; BTH1, bathroom 1; BTH2, bathroom 2; BTH3, bathroom 3; ISA, isolation anteroom; ISB, isolation bathroom; ISR, isolation room; IWH, isolation ward hallway; MHH, main hospital hallway; STR1, stairwell 1; STR2, stairwell 2; UTL, utility closet.
Many internal pressures measured between bedrooms and the IWH became less negative during the negative-pressure demonstration. Pressure differences across the AIIR anteroom were higher on the isolation anteroom (ISA)–IWH side than on the ISR-ISA side. Bedroom-IWH pressures were much smaller than those measured on the ward's outer envelope.
In stairwell 1, the UV-C flux ranged from 10-20 µW/cm2 at a height of 2.4 m. An exponential decline in UV-C flux was observed with height in both stairwells, as expected. At a height of 1.8 m, the UV-C flux ranged from 0.2-0.4 µW/cm2. At lower heights, fluxes were less impacted by the distance away from the lamp, likely because much of the light at lower heights was the result of reflection from upper room surfaces, resulting in a homogenized spatial variability. UV-C fluxes of 20-40 µW/cm2 are recommended for disinfecting tuberculosis.18 Flux levels at lower heights were within recommended levels for human safety.19
Temporal variability of pressure differentials
To explore temporal variability, smoothed pressure time series are plotted in Figures 2A and 2B . Figure 2A shows that the IWH-MHH and IWH–stairwell 2 were relatively unchanged throughout the negative-pressure demonstration. There was also typically little temporal variability in internal pressures, with the exception of bedroom 2. Bedroom 2 was used as a family and visitor room, and it was not possible to keep the door of this room closed throughout the demonstration.
Fig 2.
Smoothed pressure time series of (A) outer envelope and isolation room pressure differentials and (B) internal pressure differentials. Vertical lines split pretest, ramp-up, demonstration, ramp-down, and posttest time periods. ANT, temporary anteroom; BED1, bedroom 1; BED2, bedroom 2; BED3, bedroom 3; BED4, bedroom 4; BTH1, bathroom 1; BTH2, bathroom 2; BTH3, bathroom 3; ISA, isolation anteroom; ISB, isolation bathroom; ISR, isolation room; IWH, isolation ward hallway; MHH, main hospital hallway; STR1, stairwell 1; STR2, stairwell 2; UTL, utility closet. Note. Duplicate datasets are noted with subscripts 1, 2.
Door opening events
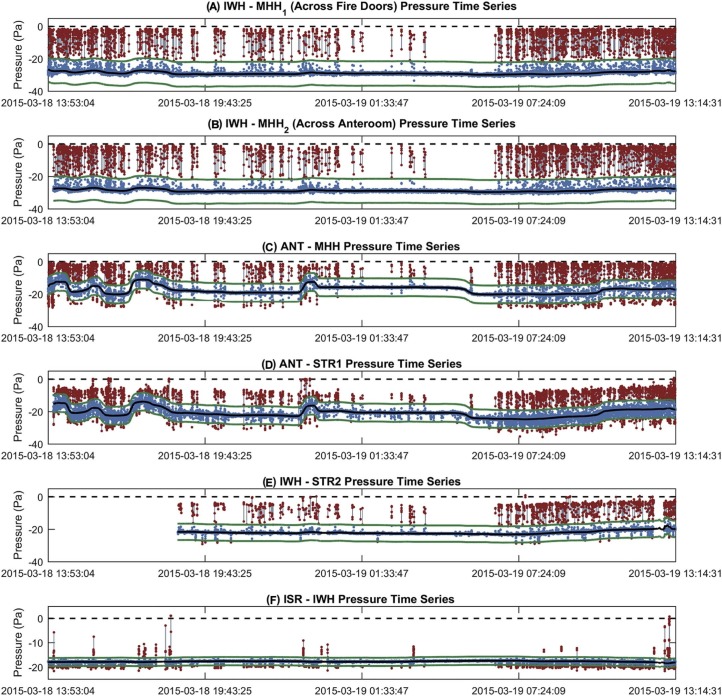
Figure 3 depicts the door opening events compared with the steady-state pressure conditions on the ward for the outer envelope and the ISR-IWH pressure differences. Door opening events made up 5.7% of the outer envelope pressure time series and 2.3% of the ISR-IWH time series. Besides the ISR-IWH pressure difference, other internal pressures did not vary with door opening events that occurred at the outer edge of the ward's pressure envelope. Internal pressures were impacted when bedrooms and bathrooms were entered, but these were rare compared with frequent traffic by hospital staff in and out of the ward. Ward door opening events resulted in pressures typically changing to around 0 to −5 Pa. Most ingress and egress events occurred on the fire door hallway side, the side without the anteroom, because this allowed easier access. The ANT–stairwell 1 and IWH–stairwell 2 differences tended to only reduce to near-zero values when stairwell doors were opened, otherwise negative-pressure was maintained even when the ward was opened at other locations. The ISR-IWH pressure difference typically became more negative when the ward was depressurized, and only decreased when the AIIR was entered.
Fig 3.
Static pressure time series (blue markers), door opening events (red markers, gray line), trimmed mean time series (black line), and door opening event identification boundaries (green lines) for the outer pressure envelope during the negative-pressure demonstration. (A) IWH-MHH1 (across fire doors) pressure time series. (B) IWH-MHH2 (across anteroom) pressure time series. (C) ANT-MHH pressure time series. (D) ANT-STR1 pressure time series. (E) IWH-STR2 pressure time series. (F) ISR-IWH pressure time series. ANT, temporary anteroom; ISR, isolation room; IWH, isolation ward hallway; MHH, main hospital hallway; STR1, stairwell 1; STR2, stairwell 2. Note. Duplicate datasets are noted with subscripts 1, 2.
To understand the dynamics of pressure changes during door opening events, we calculated the length of each event, the maximum pressure reached (Fig 4 ), the median pressure during the event, and whether the event resulted in a positive pressure. These parameters helped identify potential deficits in ability to contain airborne infectious particles on the ward during health care worker (HCW) ingress or egress. Door opening events lasted 7.5 second on average, and the longest event lasted 50 seconds. Events where fire doors were not closed tightly were >30 seconds. Brief pressure fluctuation events with negative median and maximum pressures are pictured as blue clusters in Figures 4C-F. For the IWH-MHH time series (Figs 4A and 4B), only one event was identified where pressures became slightly positive. No events were identified where ANT-MHH pressures became positive. Stairwells had more positive-pressure–generating door opening events. The ISR-ISA pressure difference exhibited the highest number of positive-pressure–generating events.
Fig 4.
Door opening event maximum pressures and event lengths, with markers colored by the median pressure measured during the event. (A) IWH-MHH1 (across anteroom). (B) IWH-MHH2 (across anteroom). (C) ANT-MHH. (D) ANT-STR1. (E) IWH-STR2. (F) ISR-IWH. (G) ISR-ISA. (H) ISB-ISR. ANT, temporary anteroom; ISA, isolation anteroom; ISB, isolation bathroom; ISR, isolation room; IWH, isolation ward hallway; Max, maximum; MHH, main hospital hallway; Press., pressure; STR1, stairwell 1; STR2, stairwell 2. Note. Duplicate datasets are noted with subscripts 1, 2.
Discussion
This project demonstrated that a temporary negative-pressure isolation ward capable of sustained negative pressure in excess of national infection control guidelines can be designed and operated for 24 hours. In a real-life scenario, there will most likely be a need for increasing surge capacity for much longer periods. The successful maintenance of a negatively pressurized ward over long durations is achievable from an engineering standpoint following the data presented here, but there may be other clinical factors that need to be addressed for this approach to be successful in reality. More studies may be needed to show the effectiveness of such an isolation ward in maintaining surge capacity over longer periods and in terms of clinical end points of infection control.
The pressure difference between an AIIR and hospital corridor is recommended to be −2.5 Pa in the United States, with an AER of 12 ACHs, of which 2 ACHs must be outside air.2, 8, 9 Through dilution of airborne particles and limiting air migration volume, ISRs significantly reduce the likelihood of airborne particles escaping into adjacent corridors.20 Although it is clear from previous studies that increased containment is observed with AIIR pressure differentials >−2.5 Pa,20 an optimal pressure has not been determined.21
It was decided for this project to achieve a sizeable pressure difference on the ward while keeping nuisance noise to the staff, patients, and visitors at a minimum. We were able to attain a pressure difference of −29 Pa before the noise on the ward became an issue. It was determined that this approach was warranted considering the ramifications of failing to contain an airborne disease. Using this approach, we demonstrated negative pressure could be maintained throughout the ward, even during door opening and dynamic HCW movements.
During the demonstration, all but one bathroom on the ward stayed negatively pressurized relative to the adjacent bedrooms (bathroom 3–bedroom 4 became neutrally pressured). Bathrooms must be kept pressurized to prevent odors and bathroom-related contamination from escaping.22 Bathroom AERs were particularly high to remove odors, whereas bedrooms were at the recommended level of ≤6 ACHs (Table 2).8
A main goal of a ventilation system is to provide thermal comfort for building occupants. An additional goal in a hospital is infection control; therefore, many systems are 100% outside air and have higher AERs than typical office buildings. When supply air is reduced, there may not be sufficient conditioned air serving the rooms, and the occupants may feel more uncomfortable. This situation would be less in milder climates. This project was conducted in a milder climate, the San Francisco Bay Area, where at the time of the study in March 2015 the mean temperature for the week of the study was 16°C, with a minimum of 8°C and a maximum of 24°C. During the study, we received one complaint from a nurse who commented that the air felt dry.
The speed of the ward's AHU supply fan was reduced for the demonstration to control ventilation rates. Another option would be to control individual room dampers, which for this hospital would have added an additional layer of complexity that was beyond the scope of the demonstration. As a result, some room airflow changes within the ward were not entirely predictable. As expected, an overall reduction in supply flows was observed during the negative-pressure demonstration, but there was significant room-to-room variability. This variability resulted in 2 rooms within the ward (bedroom 1 and UTL) becoming neutrally or positively pressurized during the demonstration. In bedroom 1, the difference between the supply and return flow decreased from 186 to 89 m3/h during the pretest and demonstration phases, respectively. Interestingly, room-to-room variability in ventilation flow changes was not limited to supply flow changes, but often return flows increased and exhaust flows decreased when negative pressure was implemented. Despite our findings that airflow reversals were rarely encountered, they are possible, even when pressure gradients far exceed Centers for Disease Control and Prevention guidelines (as seen on the ward in bedroom 1 and UTL). Therefore, it is prudent for HCWs and visitors to wear airborne precautions (eg, N95 respirator) while residing on these wards, whether in patient rooms or common areas.
During a surge of ill patients, a hierarchy of hospital infection control measures should be implemented,23 including engineering controls, administrative controls, and personal protective equipment (PPE). This approach was used to help curtail the resurgence of tuberculosis in the 1990s. Although engineering controls are important for the creation of an effective negative-pressure isolation ward, administrative controls (eg, patient triage, proper ingress and egress of patients and visitors) and proper donning and doffing of PPE are essential components of infection control and prevention that work in concert. Early in the course of a high-consequence infectious disease outbreak when large numbers of ill patients require health care services, it may be necessary for hospital engineers to rapidly convert a routinely functioning ward to a negative-pressure isolation ward. We have demonstrated that this type of conversion may be achieved in approximately 40 minutes, including installation and troubleshooting of the anteroom.
At our demonstration site, project personnel and hospital staff decided that in addition to demonstrating the temporary isolation ward, supplemental infection control strategies would be included. These strategies included a temporary hall anteroom and UV-C lamps in stairwells. The ANT showed appropriate pressure and ventilation conditions to contain airborne contamination, although at times during door-opening events, the anteroom-associated pressure differences were highly variable, probably because of its design and construction. In 6 minutes, 99.9% removal efficiency in the ANT could be achieved, assuming unobstructed air movement.2
Anteroom use is often recommended for airborne infection control.20, 24 The optimal anteroom pressure differentials and flow rates for aerosol containment with consideration of HCWs moving through doorways have not been determined. Studies have shown that opening the doors of ISRs can generate flow across the doorway.25, 26, 27 Inducing a pressure difference, however, across a door can decrease the air volume exchange across the door.25, 28 For this demonstration, it would have been optimal to construct an anteroom at each hallway entrance to the temporary isolation ward (we only constructed one to minimize project complexity). With 2 hallway anterooms, one would be used as a clean anteroom for ingress and PPE donning, and the other would be a potentially contaminated anteroom for egress and PPE doffing.
Upper room germicidal UV-C fluxes were appropriate for disinfecting any escaping contamination. Lamps were installed as close to doors as possible to irradiate any air volume exchange because of door opening. They were accepted by the staff on the ward, which contributed to the knowledge gained about how surge capacity interventions are viewed by staff.
According to the Institute of Medicine's report on medical surge capacity,5 cost of pandemic preparedness is important to consider when developing a plan, and tents, temporary housing materials, disaster response trailers, and HEPA-filtered negative-air machines are expensive purchases. Temporary patient housing options and gymnasiums also do not typically provide amenities found in hospital bedrooms, such as oxygen supply lines, various medical devices and equipment, and a bathroom with a toilet and shower. Because of these limitations, using existing hospital spaces and ventilation systems to establish a surge ward could be an improvement on previous negative-pressure isolation ward designs. Supplemental methods to increase surge capacity, such as reverse triage,29 reducing nonurgent hospital admissions,12 and delaying certain types of surgery,30 could provide the room availability needed to establish a surge ward in a functioning hospital.
In contradistinction, the key challenges we faced in this project were months of planning and coordination with hospital administrative processes that are typical for any U.S. health care facility. Close collaboration and cooperation involved numerous departments and disciplines, including infection control and prevention, nursing and hospice services, occupational health, environmental agents service, safety services, medical center leadership, and engineering services. The engineering and hospital infection control departments helped design the temporary ward plan, and input from nursing leadership on the ward was vital for determining what would be possible during the surge demonstration. Hospital leadership was briefed with the full plan in the weeks prior to the demonstration. When conducting such a project at a functioning hospital, it is essential to balance the needs of the patients, needs of the hospital staff, and requirements for a successful demonstration.
Conclusions
Our demonstration affirms that a temporary negative-pressure isolation ward may be an effective way to increase surge capacity during a large-scale outbreak of an airborne transmissible infectious disease. Even though air pressure differentials well exceeded Centers for Disease Control and Prevention guidelines, airflow reversals still occurred. These reversals only occurred within the ward and not between the hall anteroom and the rest of the hospital, therefore still containing a possible outbreak. Accordingly, it is prudent for health care personnel to wear PPE when working on temporary negative-pressure isolation wards.
Acknowledgments
We thank the hospital administrators, staff, and patients that supported this project and made it possible. We also thank the facility's engineering staff, who were excellent and helped with all the ventilation adjustments. We thank the staff who built the anteroom and helped to hang all the sampling lines within the ward. We also thank all the students who supported this project along the way.
Footnotes
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the position of the U.S. Department of Veterans Affairs or its affiliates.
Conflicts of interest: None to report.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ajic.2017.01.029.
Supplementary data
The following is the supplementary data to this article:
Figures S1 and S2.
References
- 1.Lurie N., Dausey D.J., Knighton T., Moore M., Zakowski S., Deyton L. Community planning for pandemic influenza: lessons from the VA health care system. Disaster Med Public Health Prep. 2008;2:251–257. doi: 10.1097/DMP.0b013e31817dd143. [DOI] [PubMed] [Google Scholar]
- 2.Mead K.R., Feng A., Hammond D., Shulman S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Atlanta (GA): 2012. Expedient methods for surge airborne isolation within healthcare settings during response to a natural or manmade epidemic. EPHB Report No. 301-05f. [Google Scholar]
- 3.Frieden T.R., Damon I., Bell B.P., Kenyon T., Nichol S. Ebola 2014 – new challenges, new global response and responsibility. N Engl J Med. 2014;371:1177–1180. doi: 10.1056/NEJMp1409903. [DOI] [PubMed] [Google Scholar]
- 4.IOM . Institute of Medicine, The National Academies Press; Washington (DC): 2010. Crisis standards of care: summary of a workshop series. [PubMed] [Google Scholar]
- 5.IOM . Institute of Medicine, The National Academies Press; Washington (DC): 2010. Medical surge capacity: workshop summary. [PubMed] [Google Scholar]
- 6.Hick J.L., Barbera J.A., Kelen G.D. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(Suppl):S59–67. doi: 10.1097/DMP.0b013e31819f1ae2. [DOI] [PubMed] [Google Scholar]
- 7.US Homeland Security Council . US Homeland Security Council; Washington (DC): 2006. National strategy for pandemic influenza: implementation plan. [Google Scholar]
- 8.The Facility Guidelines Institute . 2014 ed. American Society for Healthcare Engineering of the American Hospital Association; Chicago (IL): 2009. Guidelines for design and construction of hospital and outpatient facilities; pp. 422–427. [Google Scholar]
- 9.ASHRAE . American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.; Atlanta (GA): 2013. Standard 170-2013. Ventilation for health care facilities. [Google Scholar]
- 10.GAO Hospital preparedness. Most urban hospitals have emergency plans but lack certain capacities for bioterrorism response. 2003. www.gao.gov/cgi-bin/getrpt?GAO-03-924 GAO-03-924, Report to Congressional Committees; Available from. Accessed February 20, 2017.
- 11.Rubinson L., Nuzzo J.B., Talmor D.S., O'Toole T., Kramer B.R., Inglesby T.V. Augmentation of hospital critical care capacity after bioterrorist attacks or epidemics: recommendations of the Working Group on Emergency Mass Critical Care. Crit Care Med. 2005;33:E2393. doi: 10.1097/01.ccm.0000173411.06574.d5. [DOI] [PubMed] [Google Scholar]
- 12.Schull M.J., Stukel T.A., Vermeulen M.J., Guttmann A., Zwarenstein M. Surge capacity associated with restrictions on nonurgent hospital utilization and expected admissions during an influenza pandemic: lessons from the Toronto severe acute respiratory syndrome outbreak. Acad Emerg Med. 2006;13:1228–1231. doi: 10.1197/j.aem.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Challen K., Bentley A., Bright J., Walter D. Clinical review: mass casualty triage—pandemic influenza and critical care. Crit Care. 2007;11:212. doi: 10.1186/cc5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayton C., Ibrahim J., Augenbraun M., Brooks S., Mody K., Holford D. Integrated plan to augment surge capacity. Prehosp Disaster Med. 2008;23:113–119. doi: 10.1017/s1049023x00005719. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum R.A., Benyo J.S., O'Connor R.E., Passarello B.A., Williams D.R., Humphrey B.D. Use of a portable forced air system to convert existing hospital space into a mass casualty isolation area. Ann Emerg Med. 2004;44:628–634. doi: 10.1016/j.annemergmed.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson D.L., Lynch R.A., Mead K.R. Containment effectiveness of expedient patient isolation units. Am J Infect Control. 2009;37:94–100. doi: 10.1016/j.ajic.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Subhash S.S., Radonovich L.J. Hospital surge capacity. ASHRAE J. 2011;53:76. [Google Scholar]
- 18.NIOSH . Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Atlanta (GA): 2009. Environmental control for tuberculosis: basic upper-room ultraviolet germicidal irradiation guidelines for healthcare settings. DHHS (NIOSH) Publication No. 2009-105. [Google Scholar]
- 19.ACGIH . 7th ed. American Council of Governmental Industrial Hygienists; Cincinnati (OH): 2012. Ultraviolet radiation: TLV® physical agents. [Google Scholar]
- 20.Adams N.J., Johnson D.L., Lynch R.A. The effect of pressure differential and care provider movement on airborne infectious isolation room containment effectiveness. Am J Infect Control. 2011;39:91–97. doi: 10.1016/j.ajic.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Hyttinen M., Rautio A., Pasanen P., Reponen T., Earnest G.S., Streifel A. Airborne infection isolation rooms—a review of experimental studies. Indoor Built Environ. 2011;20:584–594. [Google Scholar]
- 22.Johnson D.L., Mead K.R., Lynch R.A., Hirst D.V.L. Lifting the lid on toilet plume aerosol: a literature review with suggestions for future research. Am J Infect Control. 2013;41:254–258. doi: 10.1016/j.ajic.2012.04.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorne C.D., Khozin S., McDiarmid M.A. Using the hierarchy of control technologies to improve healthcare facility infection control: lessons from severe acute respiratory syndrome. J Occup Environ Med. 2004;46:613–622. doi: 10.1097/01.jom.0000134191.92225.f2. [DOI] [PubMed] [Google Scholar]
- 24.Subhash S.S., Baracco G., Fennelly K.P., Hodgson M., Radonovich L.J. Isolation anterooms: important components of airborne infection control. Am J Infect Control. 2013;41:452–455. doi: 10.1016/j.ajic.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousavi E.S., Grosskopf K.R. Airflow patterns due to door motion and pressurization in hospital isolation rooms. Sci Technol Built Environ. 2016;22:379–384. [Google Scholar]
- 26.Grosskopf K., Mousavi E. Bioaerosols in health-care environments. ASHRAE J. 2014;56:22. [Google Scholar]
- 27.Tung Y.C., Hu S.C., Tsai T.I., Chang I.L. An experimental study on ventilation efficiency of isolation room. Build Environ. 2009;44:271–279. [Google Scholar]
- 28.Kalliomäki P., Saarinen P., Tang J.W., Koskela H. Airflow patterns through single hinged and sliding doors in hospital isolation rooms—effect of ventilation, flow differential and passage. Build Environ. 2016;107:154–168. doi: 10.1016/j.buildenv.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelen G.D., McCarthy M.L., Kraus C.K., Ding R., Hsu E.B., Li G. Creation of surge capacity by early discharge of hospitalized patients at low risk for untoward events. Disaster Med Public Health Prep. 2009;3(Suppl):S10–6. doi: 10.1097/DMP.0b013e3181a5e7cd. [DOI] [PubMed] [Google Scholar]
- 30.Soremekun O.A., Zane R.D., Walls A., Allen M.B., Seefeld K.J., Pallin D.J. Cancellation of scheduled procedures as a mechanism to generate hospital bed surge capacity—a pilot study. Prehosp Disaster Med. 2011;26:224–229. doi: 10.1017/S1049023X11006248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 and S2.