Abstract
During the last century, 3 influenza A pandemics have occurred, and pandemic influenza will inevitably occur in the future. Although the timing and severity of the next pandemic cannot be predicted, the probability that a pandemic will occur has increased based on the current outbreaks of A(H5N1) in Asia, Europe, and Africa. Because of these widespread outbreaks, the World Health Organization declared a phase 3 pandemic alert in the fall of 2005. Early detection is essential to prevent the spread of avian influenza. Planning now can be achieved by integrating interventions to ensure a prompt and effective response to a pandemic. This article provides an overview of the current status of A(H5N1) influenza worldwide and recommendations for the prevention and control of avian influenza should it emerge in humans in the United States.
Three types of influenza have been identified: A, B, and C. Influenza A and B cause seasonal human disease and epidemics; however, type B viruses are not categorized into subtypes and cannot cause pandemics. Influenza C is of minimal concern because it causes infrequent sporadic cases and minor outbreaks. It is type A influenza that causes pandemics.1
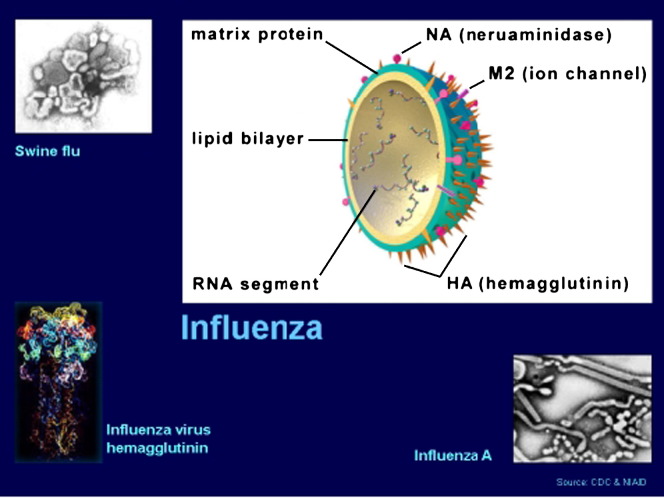
Influenza A viruses originate in birds and are categorized into subtypes on the basis of 2 surface antigens: hemagglutinin and neuraminidase. The influenza virus is similar to a membrane, and it contains 8 genes These genes give identity to the virus. The influenza virus is spherical in shape and has 2 protuberances projecting from its surface ( Fig 1). Barry describes the protuberances that resemble spikes as hemagglutinin (H) and the protuberances resembling trees as neuraminidase (N).1 It is the H of the virus that binds to the sialic acid receptors on the surface of cells in the respiratory tract. This binding is the beginning of a successful invasion by the virus. Because the virus has entered the cell rather than fusing with it on the surface membrane, it has effectively hidden from the immune system.
Fig 1.
Influenza virus structure. Source: National Science Foundation at: http://www.nsf.gov/news/speeches/colwell/rc02_hippocratic/sld016.htm. Accessed April 3, 2006.
In the meantime, N, the protuberance that juts out from the surface of the virus, is destroying the respiratory tract cell's ability to bind to the influenza virus. This is accomplished by N destroying any sialic acid remaining on the cell's surface. Thus, it is assured that new influenza viruses will be able to escape and invade other cells.1 Influenza A viruses have 16 H subtypes and 9 N subtypes. Only viruses of the H5 and H7 subtypes are known to cause the highly pathogenic form of the disease. New influenza virus variants result from frequent antigenic change (ie, antigenic drift) resulting from point mutations that occur during viral replication. It is these frequent antigenic changes through antigenic drift that require a new influenza vaccine be given each year.2
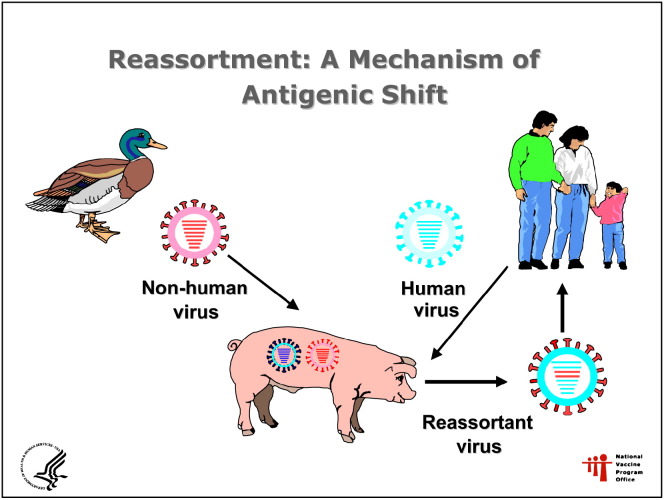
The H5N1 virus can improve its transmissibility among humans by 2 mechanisms. The first is antigenic shift, a “reassortment” event, in which genetic material is exchanged between human and avian viruses during coinfection of a human or pig. Reassortment could result in a fully transmissible pandemic virus, which could rapidly spread throughout the world ( Fig 2). The second mechanism is a more gradual process of antigenic drift, an adaptive mutation, whereby the capability of the virus to bind to human cells increases during subsequent infections of humans. Adaptive mutation, expressed initially as small clusters of human cases with some evidence of human-to-human transmission, would probably give the world some time to take defensive action.3
Fig 2.
Reassortment of avian influenza viruses. Source: Department of Health and Human Services National Vaccine Program Office, 2004.
Pandemics
During the past 100 years, 3 influenza A pandemics have been reported. The first was the 1918-1919, “Spanish flu,” [influenza A(H1N1)], which caused the highest number of known deaths because of influenza. In this pandemic, 20 to 40 million deaths occurred worldwide, with more than 500,000 deaths in the United States. The “Spanish flu” was unique because the causative agent was very deadly, and it spread across the globe in 6 months. Almost half of those who died were young, healthy adults between the ages of 20 and 40. Many individuals died within the first few days after infection, and others died of complications soon thereafter.4
The second pandemic, “Asian flu,” [influenza A(H2N2)], occurred in 1957-1958 and was first identified in China in late February 1957. It had spread to the United States by June. It caused approximately 70,000 deaths in the United States, with the highest mortality among the elderly population. The H2N2 influenza A virus in the “Asian flu” pandemic of 1957 disappeared from the human population 10 years later.4
The third pandemic, “Hong Kong flu” [influenza A(H3N2)], occurred in 1968-1969; the first cases were detected in Hong Kong in early 1968. The virus spread to the United States later that year, where it claimed approximately 34,000 lives.4 A(H3N2) viruses still circulate today.
Longitudinal virus surveillance studies that estimate the prevalence of avian H2N2 isolates among wild ducks and domestic poultry indicate that an antigenic shift in the hemagglutinins of human H2N2 influenza viruses began in 1962. As the prevalence of avian H2N2 influenza viruses increased among domestic turkeys and chickens, greater numbers of these avian viruses have come into closer proximity to susceptible human populations.5
Avian influenza
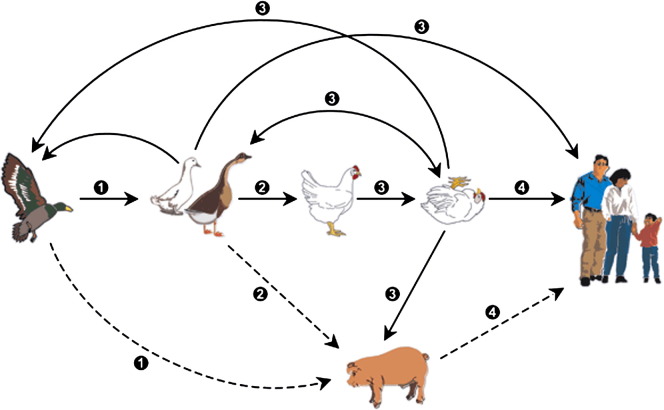
Avian influenza, which was identified over 100 years ago in Italy, differs from seasonal influenza in that it is an infection caused by bird (avian) influenza viruses.3 These influenza viruses occur naturally among wild birds worldwide. These birds carry the viruses in their intestines but, generally, do not show signs of infection. There is extensive evidence for transmission of influenza viruses between wild ducks and other species ( Fig 3, solid lines). Transmission of avian influenza has been demonstrated between pigs and humans, as well as between chickens and humans. However, transmission has not been seen between wild birds and humans (Fig 3, dotted lines).6
Fig 3.
Transmission of avian influenza. Source: Webster R, et al.6
Although type A strains of the influenza virus have 16 H subtypes and 9 N subtypes, only H5 and H7 are known to cause the highly pathogenic form of disease. Thus, finding these subtypes in birds is cause for concern. When allowed to circulate in poultry populations, the viruses mutate into the highly pathogenic form, usually within a few months.7 As seen with A(H5N1), avian influenza can be very contagious and can result in severe illness and death in some domesticated birds, including chickens, ducks, and turkeys.7
Avian influenza A(H5N1) outbreaks: 1997-2002
Until 1997, the risk of avian influenza was considered to be rare in humans. However, confirmed cases of human infection from several subtypes of avian influenza infection have been reported since 1997. Most of these human cases have resulted from contact with infected domestic poultry (eg, chicken, ducks, and turkeys) or surfaces contaminated with blood, and secretion/excretions from infected birds.7 The last epidemic of human infection with avian influenza A(H5N1) occurred in Hong Kong in 1997, with 18 confirmed cases, and six deaths. Serologic data obtained during an epidemiologic study supported the theory that persons with high levels of exposure to infected poultry may be at increased risk for infection with influenza A(H5N1) virus. In 2001, Hong Kong reported an outbreak of highly pathogenic avian influenza type A(H5N1) in its live-bird markets. As a result, all 1.2 million susceptible birds were destroyed as a preventive measure to stop the outbreak. The 2001 influenza A(H5N1) strain in Hong Kong did not affect humans.7
Avian influenza A(H5N1) outbreaks: 2003-2006
An outbreak of avian influenza A(H5N1), a highly pathogenic virus, spread among millions of birds (mostly chickens) across Southeast Asia in 2003. Although the H5N1 virus rarely infects humans, since December 2003, the World Health Organization (WHO) has reported at least 228 human cases, with 130 deaths (57%).8 Most of these cases have occurred as a result of people having direct or close contact with infected poultry or contaminated surfaces. These outbreaks of human H5N1 infections are the largest documented since H5N1 emergence in humans. In addition, this virus is more lethal than the 1997 virus. As more humans become infected with the H5N1 virus, transmissibilty to humans improves.9
The spread of avian influenza viruses from one ill person to another has been reported very rarely. Cases of probable person-to-person transmission of avian influenza were reported in a mother and aunt caring for an extremely ill patient in Thailand; all were positive for influenza A(H5N1) by polymerase chain reaction (RT-PCR). The mother had come from another city and had no contact with the infected chickens.10
As of early March 2006, human cases of influenza A(H5N1) infection have been reported in Cambodia, China, Indonesia, Thailand, Vietnam, Turkey, and Iraq. In addition, ongoing outbreaks of influenza H5N1 among migratory birds and poultry have been reported in 14 countries (Iraq, Nigeria, Azerbaijan, Bulgaria, Greece, Italy, Slovenia, Iran, Austria, Germany, Egypt, India, France, and Sweden). In February 2006, the first H5N1 bird influenza in Nigeria, Africa, was found in a large commercial farm.11 Soon thereafter, H5N1 influenza was confirmed in poultry in Niger, marking spread of the virus to a second country in sub-Saharan Africa. Discovery of the virus in Niger confirms fears that conditions in Africa, such as failure to recognize outbreaks, will increase the spread of the virus to additional countries.12
The outbreak of highly pathogenic avian H5N1 influenza is unprecedented in its geographic extent, and its transmission to humans is a threatening sign. Experts from around the world are watching the H5N1 situation very closely and are preparing for the possibility that the virus may begin to spread more easily and widely from person to person. The current ongoing spread of avian H5N1 influenza and a series of genetic reassortment events are traceable to the precursor of the H5N1 viruses that caused the initial human outbreak in 1997 and subsequent avian outbreaks in 2001 and 2002. Antigenic analysis of human isolates from 2005 provided evidence of antigenic drift among the most recently circulating H5N1 strains. Avian influenza A(H5N1) is now considered the most likely virus to ignite the next pandemic. International health experts indicate that 2 of the 3 conditions for an influenza pandemic have already been met. First, a strain of the virus, A(H5N1), has reemerged to which humans have little or no immunity; second, this strain can jump between species. The only remaining obstacle is that this strain of avian influenza has not yet mutated into a form that is easily transmitted from human to human.9
Certain parallels exist between the “Spanish flu” of 1918 and the H5N1 virus. Like the 1918 virus, H5N1 influenza has unusually high virulence. Scientists have found strong evidence the 1918 influenza virus was derived wholly from an ancestor that originally infected birds. The 1918 virus was not a reassortant virus, as were those of the 1957 and 1968 pandemics. In these outbreaks, human and avian influena viruses infected the same person at the same time, thus allowing their genes to mix (reassort). The 1918 virus more likely was an entirely avian-like virus that adapted to humans.13 Because of these concerns, the WHO declared a phase 3 pandemic alert in November 2005.14
Stages of a pandemic
The WHO has identified 3 periods and 6 stages of a pandemic. The 3 periods are as follows: the interpandemic period, the pandemic alert period, and the pandemic period. The interpandemic period has 2 phases. Phase 1 occurs when the virus subtype is present in animals, but the risk to humans is considered low. In phase 2, a circulating animal influenza virus subtype poses a substantial risk to humans.15
The pandemic alert period begins with phase 3, when human infections occur with a new virus subtype. In this phase, human-to-human spread is rare. Phases 4 and 5 occur during the pandemic alert period when (1) larger cluster(s) of influenza have occurred, but human-to-human spread is still localized; and (2) the virus is becoming increasingly better adapted to humans and is not yet fully transmissible but is a substantial pandemic risk. The pandemic period and phase 6 occur with increased and sustained transmission in the general population.15
Pandemic preparedness
Although the timing and severity of the next pandemic cannot be predicted, the probability that a pandemic will occur has increased based on current events. Planning now for a pandemic can be achieved by integrating interventions to ensure a prompt and effective response. Both the WHO and the US Department of Health and Human Services (DHHS) have developed pandemic preparedness and response plans.15, 16 The 2005 comprehensive DHHS Influenza Plan provides a blueprint for all pandemic influenza preparedness and response planning. Part 1, the Strategic Plan, describes a coordinated public health and medical care strategy to prepare for, and begin responding to, an influenza pandemic. Part 2, Public Health Guidance for State and Local Partners provides recommendations for specific aspects of pandemic influenza planning and response for the development of state and local preparedness plans.16
An influenza pandemic may require activation of the National Response Plan (NRP), especially if the first appearance of the disease in the United States occurs in 1 or a few isolated communities. The NRP combines the capabilities of federal government departments and agencies and the American Red Cross into emergency support functions (ESFs) to provide the resources and emergency services that are most likely to be needed. The DHHS will have primary responsibility for coordinated federal government assistance to supplement state, local, and tribal resources in response to public health and medical care needs, including veterinary and/or animal issues when appropriate.17
In January 2006, the WHO issued a draft protocol for rapid response and containment of pandemic influenza. The document calls for each country to be responsible for surveillance for a “novel” influenza virus, which has demonstrated the ability to be transmitted from person to person. It also calls for reporting of such an event to the WHO within 24 hours. The WHO will then provide immediate recommendations to the affected country. These recommendations can range from holding off rapid containment (if the evidence suggests that the pandemic threat is minimal) to taking other actions if the pandemic activity is too extensive to contain. When containment is recommended, the WHO will coordinate international support with its global partners. Together, they will work with the country to mobilize the necessary resources and initiate actions to contain the pandemic. These resources, among others, include antiviral drugs, antibiotics to treat secondary pneumonias, and other supplies such as personal protective equipment.18
Surveillance for pandemic influenza
Through surveillance, astute clinicians have identified emerging infections and have controlled/prevented further cases and/or outbreaks in the past. The most important warning signal of a pandemic strain comes when clusters of patients with clinical symptoms of influenza, closely related in time and place, are detected. This may be the first indication of human-to-human transmission of the virus. In addition, the detection of cases in health care workers caring for influenza patients would suggest human-to-human transmission. Detection of such cases should be followed up by immediate investigation to confirm the diagnosis, identify the source, and determine whether human-to-human transmission is occurring. Specialized WHO reference laboratories can corroborate these investigations by spotting genetic and other changes in the virus, which indicate the improved ability of the virus to infect humans. To this end, the WHO repeatedly asks affected countries to share viruses with the international research community.5
In the case of the recent human H5N1 outbreaks in Asia and Europe, the WHO has issued frequent updated cumulative surveillance data reports on the number of confirmed human cases and deaths of avian influenza. It is important to note the WHO reports only laboratory-confirmed cases; therefore, there are more human H5N1 influenza cases that are not reported by the WHO because of the lack of data to confirm the diagnosis.8
Early detection is essential to prevent the spread of avian influenza. The DHHS recommends enhanced surveillance by state and local health departments, health care settings, and clinicians to identify patients at risk for A(H5N1) and other novel pathogens (eg, severe acute respiratory syndrome [SARS]). The information needed is best determined through a travel and contact history during assessment. The patient should be asked the following questions19, 20:
-
•
Have you traveled to (a place where avian influenza reemerges) in the past 10 days or been in close contact with other ill persons who have? (Note: The incubation period for avian influenza is 2 to 4 days).
-
•
Have you had direct contact with poultry?
-
•
Are you employed as a health care worker with direct patient contact?
-
•
Do you have close contacts who have been told they have pneumonia?
(Note: The above are the same assessment questions to ask when SARS is suspected).
Avian influenza: Clinical signs and symptoms
The signs and symptoms of avian influenza include fever (>100.4°F), shortness of breath, and cough with sputum production. As the infection progresses, the respiratory rate becomes rapid, with respiratory distress and crackles on examination of the chest. Abnormal chest radiographs, with extensive bilateral infiltration, lobar collapse, focal consolidation, and air bronchograms are present.19 Because SARS and avian influenza symptoms are similar, patients with pneumonia or acute respiratory distress syndrome (ARDS) who have recently traveled to mainland China, Hong Kong, or Taiwan should have diagnostic testing for SARS performed immediately.
The Centers for Disease Control and Prevention (CDC) recommends the following diagnostic tests for patients who meet certain epidemiologic and clinical criteria. Patients with an abnormal chest x-ray should have a nasopharyngeal swab or respiratory aspirate taken to test for influenza A. Based on the patient's history, an acute- (within 1 week of onset of illness) and a convalescent-phase (3 weeks after illness onset) serum sample should also be collected and stored locally in case testing for antibodies to the avian influenza virus is needed. Cases of suspected avian influenza should be reported to the local and/or state health department.21
Prevention and control of avian influenza
Vaccines and antivirals
Vaccination is one of the most effective ways to minimize morbidity and mortality from influenza. However, there is currently no vaccine available to protect humans against avian influenza H5N1. In 2004, the National Institute of Allergy and Infectious Diseases (NIAID) awarded contracts to 2 companies to manufacture an inactivated vaccine from an H5N1 virus isolated in Southeast Asia. These trials are testing the vaccine's safety and ability to generate an immune response in 450 healthy adults aged 18 to 64 years at 3 sites. In October 2005, a second trial was undertaken to determine the dose-related safety of inactivated H5N1 vaccine in healthy elderly adults.22 In addition, the NIAID and MedImmune Inc. have a cooperative research and development agreement for the development of vaccines against avian influenza viruses that have the potential to cause pandemics.23 However, any vaccine that is developed must closely match the pandemic virus, and large-scale commercial production will not start until the new virus has emerged and a pandemic has been declared. In any event, current global production capacity falls far short of the demand expected during a pandemic.5
Four antiviral drugs are available to treat and/or prevent influenza. They are amantadine, rimantadine (Forest Pharmaceuticals, New York, NY), zanamivir (GlaxoSmithKline Inc, Research Triangle Park, NC), and oseltamivir (Hoffman-La Roche Inc, Nutley, NJ). Rimantadine and amantadine are effective only against type A influenza. Zanamivir and oseltamivir inhibit both influenza A and B viruses.24 However, because of evidence of growing influenza A virus resistance to rimantadine and amantadine, the CDC recommends that these drugs not be used to prevent and treat influenza during the flu season. During this period, the CDC recommends oseltamivir or zanamivir be prescribed if an antiviral medication is indicated for the treatment of influenza; oseltamivir should be prescribed for chemoprophylaxis against influenza.25
Prevention and control of avian influenza in the community
The WHO-recommended pandemic influenza interventions for the community during the pandemic alert period (phases 3-5) include isolation of patients and quarantine of contacts, accompanied by antiviral therapy. In the pandemic period (phase 6), the focus shifts to delaying spread and reducing risk through population-based measures. Nonpharmaceutic pandemic influenza interventions suggest that ill persons remain home at the first sign of symptoms of influenza but add that forced isolation and quarantine are ineffective and impractical. If the pandemic is severe, social gatherings and school closures should be considered. Nonessential domestic travel to affected areas also should be deferred. Hand and respiratory hygiene should be routine, along with disinfection of contaminated household surfaces. Mask use should be based on setting and risk.26
Prevention and control of avian influenza in health care facilities
To prevent the transmission of all respiratory infections in health care settings, including influenza, respiratory hygiene/cough etiquette measures should be implemented at the first point of contact with a potentially infected person and should be incorporated into standard precautions. They include covering the nose/mouth when coughing or sneezing, using tissues to contain respiratory secretions and disposing of them in the nearest waste receptacle after use, and hand hygiene (eg, handwashing with nonantimicrobial soap and water, alcohol-based handrub, or antiseptic handwash) after having contact with respiratory secretions and contaminated objects/materials.27
The CDC is revising its interim guidance for infection control precautions for avian influenza. The revised recommendations will be posted on its Web site when they are finalized. Until the revisions are available, the following enhanced precautions have been recommended by the CDC to protect health care providers in the United States who care for patients with known or suspected avian influenza. Patients with a history of travel within 10 days to a country with avian influenza activity and are hospitalized with a severe febrile respiratory illness, or are otherwise under evaluation for avian influenza, should be managed using isolation precautions identical to those recommended for patients with known SARS.28 These include the following:
Standard precautions:
-
•
Careful attention to hand hygiene before and after all patient contact or contact with items potentially contaminated with respiratory secretions.
Contact precautions:
-
•
Wear gloves and gown for all patient contact;
-
•
use dedicated equipment such as stethoscopes, disposable blood pressure cuffs, and others; and
-
•
wear eye protection (ie, goggles or face shields) when within 3 feet of the patient.
Airborne precautions:
-
•
Place the patient in an airborne isolation room. These rooms should have negative air pressure, with 6 to 12 air changes per hour, and exhaust air directly outside or have recirculated air filtered by a high-efficiency particulate air filter. If an airborne isolation room is unavailable, portable high-efficiency particulate air filters should be used in the patient's room.
-
•
Use a fit-tested respirator, at least as protective as a National Institute of Occupational Safety and Health (NIOSH)-approved N-95 disposable respirator, when entering the room.
These precautions should be continued for 14 days after onset of symptoms or until either an alternative diagnosis is established or diagnostic tests indicate that the patient is not infected with influenza A virus.28
In addition to the above precautions, the CDC also recommends vaccination of health care workers against seasonal influenza. Vaccination rates for health care workers are typically <40%. However, these rates must be increased because seasonal influenza vaccination not only provides protection against the predominant circulating influenza strains, it also reduces the risk of a health care worker being coinfected with human and avian influenza strains, during which time genetic reassortment could take place and lead to the emergence of a potential pandemic strain. Surveillance and monitoring of health care workers for exposure to avian influenza should be ongoing during an avian influenza outbreak. Health care workers who develop fever, respiratory symptoms, and/or conjunctivitis should be advised to stay home until 24 hours after resolution of fever, unless an alternative diagnosis is established or diagnostic tests are negative for influenza A virus.28
Summary
In summary, pandemic influenza will inevitably occur again sometime in the near future. Therefore, health care professionals should be aware of the following facts.
Pandemic influenza is different from avian influenza
An influenza pandemic occurs when a new virus subtype emerges that has not previously circulated in humans. Avian H5N1 is a strain with pandemic potential. It could ultimately adapt into a strain that can be transmitted from person to person. Once this adaptation occurs, it will no longer be a bird virus, and it will have mutated into a human influenza virus. Because the virus is new, the human immune system will have no preexisting immunity, making pandemic influenza a more serious disease than that caused by the usual seasonal influenza. The WHO has defined 6 distinct phases to facilitate pandemic preparedness planning.15 We are now in phase 3: pandemic alert.14
Influenza pandemics are recurring events
Three pandemics occurred in the previous century. A pandemic occurs when a new influenza virus emerges and starts spreading as easily as seasonal influenza. International health experts indicate that 2 of 3 conditions for a pandemic have already been met. First, a strain of the A(H5N1) virus has emerged to which humans have little or no immunity. Second, this strain can jump between species. The only remaining obstacle is that this strain of H5N1 virus has not yet mutated into a form that is easily transmitted from human to human. Should H5N1 evolve to a form as contagious as seasonal influenza, a pandemic could begin. As many as 400 million people could die if an influenza A pandemic similar to the one in 1918 occurs.9
All countries will be affected
Every country must be prepared. Countries might delay arrival of the virus through border closures and travel restrictions but cannot stop it. Pandemics of the previous century encircled the globe in 6 to 9 months when most international travel was by ship. Today, the virus could spread more rapidly, possibly reaching all continents in less than 3 months. Economic and social disruption will be great.9
Widespread illness will occur
Projections for the next pandemic estimate that a substantial percentage of the world's population will require some form of medical care. Medical supplies will be inadequate. Few countries have the staff, facilities, equipment, and hospital beds needed to cope with large numbers of people who suddenly become ill.9
Chatham, Massachusetts, and Pittsburgh, Pennsylvania
References
- 1.Barry J.M. The Penguin Group; New York: 2004. The great influenza. p. 101-4. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Influenza background. Available at: http://www.cdc.gov/flu/professionals/background.htm. Accessed February 7, 2006.
- 3.World Health Organization. Avian influenza frequently asked questions. Available at: http://www.who.int/csr/disease/avian_influenza/avian_faqs/en/index.html. Accessed February 1, 2006.
- 4.Centers for Disease Control and Prevention Influenza pandemics during the 20th century. Available at: http://www.cdc.gov/flu/pandemic/keyfacts.htm#history. Accessed February 9, 2006.
- 5.Schäfer J.R., Kawaoka Y., Bean W.J., Süss J., Senne D., Webster R.G. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology. 1993;194:781–788. doi: 10.1006/viro.1993.1319. [DOI] [PubMed] [Google Scholar]
- 6.Webster R., Peiris M., Chen H., Guan Y. H5N1 outbreaks and enzootic influenza. Emerg Infect Dis. 2006;12:1–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Avian influenza fact sheet. Available at: http://www.who.int/mediacentre/factsheets/avian_influenza/en/#history. Accessed February 6, 2006.
- 8.World Health Organization. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO [June 20, 2006]. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2006_06_20/en/index.html. Accessed June 29, 2006.
- 9.World Health Organization. Ten things you need to know about avian influenza. Available at: http://www.who.int/csr/disease/influenza/pandemic10things/en/index.html. Accessed February 4, 2006.
- 10.Ungchusak K., Auewarakul P., Dowell S.F., Kitphati R., Auwanit W., Puthavathana P. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Avian influenza–spread of the virus to new countries. Available at: http://www.who.int/csr/don/2006_02_21b/en/index.html. Accessed February 22, 2006.
- 12.World Health Organization. Avian influenza–situation (poultry) in Niger. Available at: http://www.who.int/csr/don/2006_02_28/en/index.html. Accessed March 1, 2006.
- 13.Taubenberger J.K., Reid A.H., Lourens R.M., Wang R., Jin G., Fanning T.G. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Current WHO phase of pandemic alert. Available at: http://www.who.int/csr/disease/avian_influenza/phase/en/index.html. Accessed February 4, 2006.
- 15.World Health Organization. WHO global influenza preparedness plan. Available at: http://www.who.int/csr/resources/publications/influenza/GIP_2005_5Eweb.pdf. Accessed February 4, 2006.
- 16.Department of Health and Human Services. HHS pandemic influenza plan. Available at: http://www.hhs.gov/pandemicflu/plan/overview.html#es. Accessed February 1, 2006.
- 17.Department of Health and Human Services. HHS pandemic influenza plan. Appendix A. Available at: http://www.hhs.gov/pandemicflu/plan/appendixa.html. Accessed February 1, 2006.
- 18.World Health Organization. WHO pandemic influenza draft protocol for rapid response and containment. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/RapidResponse_27%2001.pdf. Accessed February 1, 2006.
- 19.Centers for Disease Control and Prevention. Avian influenza in humans. Available at: http://www.cdc.gov/flu/avian/gen-info/avian-flu-humans.htm. Accessed February 1, 2006.
- 20.Department of Health and Human Services. HHS pandemic influenza plan. Appendix 2. Available at: http://www.hhs.gov/pandemicflu/plan/sup2.html#app2. Accessed February 1, 2006.
- 21.Department of Health and Human Services. HHS pandemic influenza plan. Supplement 2, laboratory diagnosis. Available at: http://www.hhs.gov/pandemicflu/plan/sup2.html#testavi. Accessed February 1, 2006.
- 22.National Institute of Allergies and Infectious Diseases. News Release, November 2005. Available at: http://www3.niaid.nih.gov/news/newsreleases/2005/H5N1QandA.htm. Accessed February 1, 2006.
- 23.National Institute of Allergies and Infectious Diseases. News Release, September 28, 2005. Available at: http://www3.niaid.nih.gov/news/newsreleases/2005/medimmune.htm. Accessed February 1, 2006.
- 24.Centers for Disease Control and Prevention Prevention and control of influenza. Recommendations of the advisory committee on immunization practices (ACIP) MMWR. 2005;54(RR08):1–40. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention High levels of amantadine resistance among influenza A(H3N2) viruses and interim guidelines for use of antiviral agents–United States, 2005-06 influenza season. MMWR. 2006;55(RR02):44–46. [PubMed] [Google Scholar]
- 26.World Health Organization Writing Group Nonpharmaceutical interventions for pandemic influenza, national and community measures. Emerg Infect Dis. 2006;12:88–94. doi: 10.3201/eid1201.051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Respiratory hygiene/cough etiquette in healthcare settings. Available at: http://www.cdc.gov/flu/professionals/infectioncontrol/resphygiene.htm. Accessed February 25, 2006.
- 28.Centers for Disease Control and Prevention. Interim recommendations for infection control in health-care facilities caring for patients with known or suspected avian influenza. Available at: http://www.cdc.gov/flu/avian/professional/infect-control.htm. Accessed March 4, 2006.