Abstract
Background
We performed an assessment of an independent rapid flu clinic service (RFCS) unit, which was set up outside the emergency department (ED) during the 2009 H1N1 pandemic season. The unit was able to relieve the crowding of regular ambulatory and emergency services.
Methods
Between August and December 2009, a total of 6,152 patients with influenza-like illness were enrolled in this observational retrospective study. Patients with positive influenza tests were interviewed to evaluate the efficiency of RFCS.
Results
The mean length of stay (LOS) for the RFCS was 50 minutes, which was shorter than the LOS for ambulatory services (1 hour) and regular ED services (3.5 hours). Overall, 88% of patients were satisfied with the RFCS. Of 6,152 patients receiving flu tests, 1,235 (20%) had a positive result. Fever (odds ratio [OR], 4.28, 95% confidence interval [CI]: 3.11-5.89), fever combined with cough and sore throat (OR, 2.52; 95% CI: 2.18-2.92), fever combined with sore throat (OR, 2.42; 95% CI: 2.13-2.75), history of contacting confirmed flu patients within 7 days (OR, 2.40; 95% CI: 2.07-2.78), fever combined with cough (OR, 2.19; 95% CI: 1.92-2.47), sore throat (OR, 2.03, 95% CI: 1.79-2.30); and cough (OR, 1.91, 95% CI: 1.69-2.17) were significantly associated with positive influenza tests.
Conclusion
Setting up the RFSC was beneficial to health care facilities during a pandemic flu season.
Key Words: Taiwan, H1N1, Pandemic flu, Emergency department, Rapid flu clinic services
In the spring of 2009, a novel H1N1 swine-origin influenza virus (SOIV) originated from North America and rapidly spread to other countries. On June 11, 2009, the World Health Organization (WHO) raised the worldwide flu pandemic alert to phase 6, characterized by community level outbreaks in at least 1 other country in a different WHO region.1 Although the mortality and morbidity of H1N1 SOIV were lower than those of the 1918 pandemic flu, the rapid spread of H1N1 SOIV still raised concerns for preparedness of health care systems.2
The 2009 H1N1 SOIV epidemic was the first opportunity to examine the preparedness plans of health care systems in the 21st century.2, 3 After the 2003 severe acute respiratory syndrome (SARS) epidemic, many hospitals set up protocols for contagious airborne diseases of high virulence.4 Most of these protocols focused on infection control measures for health care systems, but few considered an abruptly increased hospital and emergency department (ED) censuses during a pandemic period. Experiences of the SARS outbreak indicated that treating abrupt increases in contagious ambulatory patients in an independent area could alleviate ED crowding and avoid transmissions of nosocomial infections within the ED and other hospital areas.4, 5, 6 Therefore, a rapid flu clinic service (RFCS) was set up at our nearby hospital ED for 2009 H1N1 SOIV infections after it spread in the community. The protocols, based on experiences from the 2003 SARS epidemic and recommendations from the Center for Disease Control (CDC; Taiwan) and the Bureau of National Health Insurance (NHI) in Taiwan, included adequate health care workers and facilities, an independent area to treat patients, and relevant educational information for family and patients.7 The aim of this study was to examine the effects of the protocols for an abruptly increased hospital census on the ED during a pandemic period and analyze the responses from patients who went to our RFCS.
Materials and methods
A single-center observational clinical study was conducted from August 2009 to October 2009. The first case of H1N1 SOIV was reported in Taiwan in April 2009. Taiwanese hospitals and health care workers started a series of infection control measures to prevent hospital-acquired infection. The Taiwanese Department of Health, collaborating with the CDC and the Bureau of NHI, suggested rapid influenza diagnostic tests (RIDTs) as a preliminary diagnostic measure. Patients with positive RIDTs were eligible for a free 5-day dose of antiviral agent oseltamivir. Hospitals were encouraged to set up flu clinics to reduce social interactions between patients with influenza-like illness and other patients, especially from immunocompromised patients.7
Our hospital is a tertiary hospital located in southern Taiwan, equipped with 1,000 total beds, 100 of which are intensive care unit (ICU) beds. The annual census of the ED was approximately 750,000. The mean length of stay (LOS) at the ED during 2008 was 5.6 hours. The LOS for patients who left after ED management (ie, those who were not admitted after the ED visit) was 3.4 hours. To shorten the unnecessary LOS for flu patients and to follow the national policy, we established the RFCS to provide influenza tests and counseling for the H1N1 SOIV in August 2009.
RFCS at our hospital
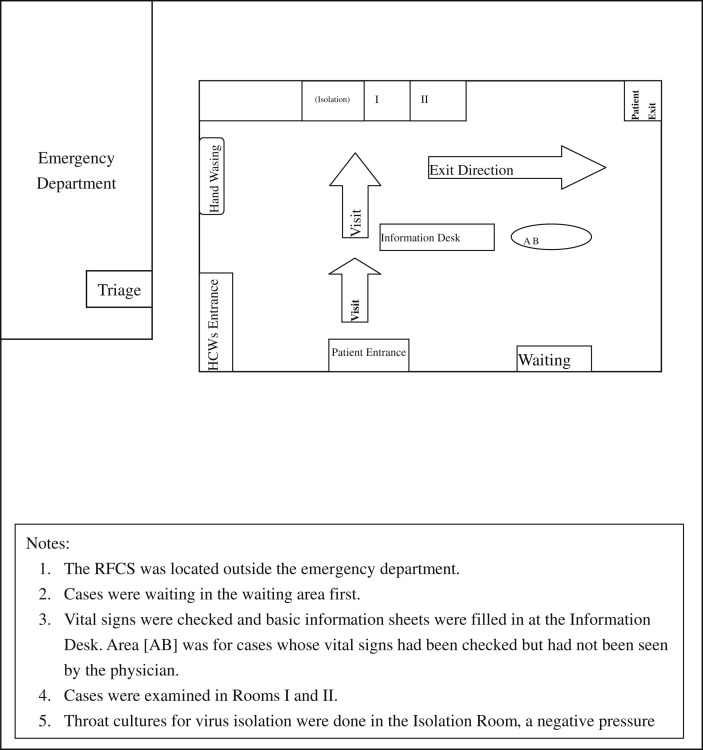
From August 2009 to December 2009, patients with influenza-like illnesses were centralized to the RFCS, an independent building with 1 negative-pressure isolation room near the ED, which was built after the 2003 SARS epidemic and was planned to treat large numbers of ambulatory patients with airborne contagious diseases during outbreaks (Fig 1 ). Ambulatory patients could stay in the RFCS and have their history taken and undergo physical examination, sputum induction, and nasopharyngeal or throat swab and receive oseltamivir treatment if indicated. Usually, the RFCS ran 24 hours per day and was staffed with 1 physician and 2 nurses. During day shifts (09:00-18:00), the RFCS was staffed with rotating physicians from all clinical departments. During night shifts, emergency physicians (EP) took over the work. Additional physicians were called to the RFCS from the department of internal medicine when the ED was overcrowded, and EPs could be freed from their clinical duties to manage ED patients.
Fig 1.
Design of the working area for the rapid flu clinic service (RFCS) during the 2009 pandemic flu season.
Each non-ED physician took a 3-hour shift evaluating patients who presented with influenza-like illness. A 3-page medical sheet, including basic demographic information, underlying diseases, possible modes of transmission, travel history, physical examination, and past history of flu vaccination, was used.
Following the recommendations from the NHI and the CDC of Taiwan,7 throat swabs for rapid influenza tests and/or viral cultures were performed by duty physicians. Samples from possible cases with cluster infection were sent for reverse-transcription polymerase chain reaction (RT-PCR) at the same time. Additional viral cultures were sent to the laboratory based on clinical judgments. After the history and physical examination, patients with negative RIDTs, or patients who did not have influenza-like symptoms, were asked to wear surgical masks and transferred to main ED for further evaluation and treatment. Patients with more severe symptoms such as persistent cough, high fever, dyspnea, or unstable vital signs were admitted to the ward or were sent to another independent isolation room of the ED if ward beds were unavailable.
The results of the RIDT and RT-PCR were obtained within 60 minutes and within 1 day, respectively. If their symptoms were stable, patients were discharged from the ED but were asked to leave contact phone numbers. If the RIDT or RT-PCR were positive, patients were called to return the hospital to receive the oseltamivir prescription, instructions for taking oseltamivir, health education information, and sheets for personal care and household contacts.
Laboratory tests for influenza
A sterile cotton swab was used to collect throat cultures from patients with influenza-like illness. The swabs were kept in transport medium and sent to the central virology laboratory for RIDT and RT-PCR testing, described below.
RIDT
Influenza antigens were detected using the Binax-NOW Influenza A & B Test (Inverness Medical, Scarborough, ME), a commercial, qualitative, visual-read kit, as previously described.8 Briefly, the cotton swabs were eluted with 0.5 to 3.0 mL transport media by vigorously rotating the swab to get a liquid sample. A liquid sample of 100 μL was required for each test. The test device was read after 15 minutes of incubation at room temperature. A positive test result was indicated by a pink to purple test line and a pink to purple control line on a white background. A negative test result was indicated by a pink control line only. The overall sensitivity and specificity versus virus culture are 83% (95% confidence interval [CI]: 73%-90%) and 93% (95% CI: 88%-96%), respectively, according to the manufacturer.
Real-time RT-PCR for pandemic H1N1 2009
Two pandemic 2009 H1N1 viral genes (common matrix protein 2 gene M2 for influenza A and hemagglutinin gene H1 specific for SOIV) were detected with 1-step RT-PCR. Using the RealTime ready Influenza A/H1N1 Detection Set and the RealTime ready RNA Virus Master on the LightCycler 480 System (Roche Diagnostics GmbH, Mannheim, Germany), test results were reported as negative (M2[−], H1 [−]), positive for influenza A (M2[+], H1[−]) or positive for 2009 H1N1 pandemic influenza (M2[+], H1 [+]).
Data were collected from patients who presented with influenza-like illness, including symptoms such as fever, cough, and malaise. The study period was from August 1 to December 31, 2009.
Patients with flu were confirmed by positive results from the RIDT, influenza viral cultures, or influenza RT-PCR tests. Fever over 38°C (100.4°F) measured at triage was defined as tympanic temperature. Underlying diseases included for consideration were diabetes mellitus, hypertension, coronary artery disease, heart failure, asthma, chronic obstructive pulmonary disease, cancer, hematologic malignancies, and history of treatment with immunosuppressant agents.
Clinical characteristics for influenza-like illness
The primary end point for this study was information regarding clinical symptoms, underlying diseases, and possible influenza exposure history obtained from the patients with influenza-like illness through the 3-page rapid influenza illness medical records from those patients enrolled in this study. The results of influenza laboratory tests and LOS at the ED were subsequently collected from information systems of the hospital.
Satisfaction questionnaires from patients taking oseltamivir
Those patients who were prescribed oseltamivir received telephone interviews after RFCS visits because previous studies noted severe neuropsychiatric adverse effects after oseltamivir, especially in Asian children. The interview included 7 questions regarding the efficacy and possible adverse effects of the oseltamivir treatment, health education information related to H1N1 SOIV, and satisfaction with the RFCS. Interviews with patients aged less than 18 years were assisted by parents. All data collection and analysis were approved by the Institutional Review Board of the hospital.
Statistical analysis
Proportions were calculated from categorical data. The proportions were compared using the χ2 test or Fisher exact test. Logistic regression modeling with backward elimination was used for multivariate analysis and was used to calculate odds ratios (ORs) with 95% CIs. All tests of significance were 2-tailed, and a P value of .05 or less was considered to be statistically significant. Data were analyzed by a commercially available software package (SAS, version 9.13, Cary, NC).
Results
Rapid flu clinical services
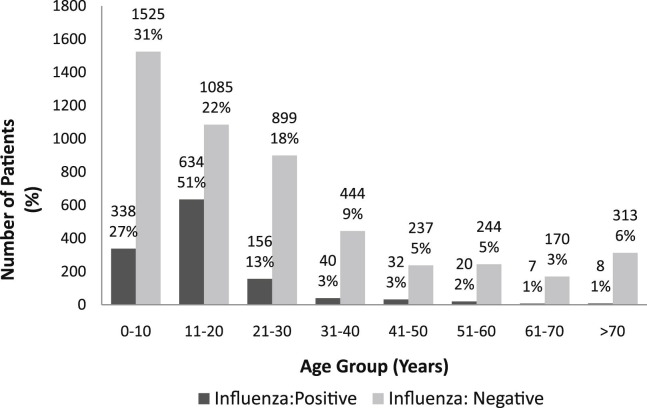
From August 2009 to October 2009, 33,868 patients visited our ED for nontraumatic problems. Patients who visited the ED with influenza-like illness during the 3-month period are illustrated in Figure 2 according to age groups. Of the 6,152 patients receiving influenza tests such as RIDTs, viral cultures, and the RT-PCR test, 3,326 (54%) were male. Most of the patients were young; 1,863 (30%) were less than 10 years of age; 1,719 (28%) were between the ages of 10 and 20 years; 1,055 (17%) were between 20 and 30 years of age.
Fig 2.
Patients who visited the emergency department with influenza-like illness by age group, from August 2009 to December 2009.
RFCS shortened the waiting time at the ED. The LOS for the RFCS, the LOS to the regular preregistered ambulatory services, and the LOS for nontraumatic patients who left after ED management were 50 minutes, 1 hour, and 3.5 hours during the study period, respectively. Non-ED physicians who were responsible for the RFCS only had an additional 6 hours of service per month. Patients with influenza-like illness symptoms were isolated from ambulatory clinic services, where immunocompromised, multicomorbidity, and elderly patients stayed for management.
Presentations at RFCS
Of the 6,152 patients undergoing RIDTs, 1,235 cases (20%) were positive. Demographic and clinical characteristics of the 6152 patients with influenza-like illness who visited our RFCS during the study period are summarized in Table 1 . By univariate analysis, fever (OR, 4.28; 95% CI: 3.11-5.89), fever combined with cough and sore throat (OR, 2.52; 95% CI: 2.18-2.92), fever combined with sore throat (OR, 2.42; 95% CI: 2.13-2.75), history of contacting flu-positive individuals within 7 days (OR, 2.40; 95% CI: 2.07-2.78), fever combined with cough (OR, 2.19; 95% CI: 1.92-2.47), sore throat (OR, 2.03; 95% CI: 1.79-2.30), and cough (OR, 1.91; 95% CI: 1.69-2.17) were significantly associated with a positive influenza test. The logistic regression with backward elimination revealed fever (OR, 4.52; 95% CI: 3.27-6.24), history of contact with flu-positive individuals within 7 days (OR, 2.19; 95% CI: 1.88-2.55), sore throat (OR, 1.93; 95% CI: 1.70-2.19), and cough (OR, 1.76; 95% CI: 1.55-2.00) were significantly associated with a positive influenza test.
Table 1.
Demographic and clinical characteristics of 6,152 patients who visited the ED because of influenza-like illness during the pandemic flu season
| Cases, n (%) |
|||||
|---|---|---|---|---|---|
| Characteristics | Flu positive 1,235 (20) | Flu negative 4,917 (80) | All cases 6,152 (100) | Odd ratio (95% confidence interval) | P values |
| Male-to-female ratio | 1.36:1 | 1.13:1 | 1.12:1 | 1.2012 (1.06-1.36) | .0047 |
| Fever | 1,193 (97) | 4,273 (87) | 5,466 (89) | 4.28 (3.11-5.89) | <.001∗ |
| Cough | 657 (53) | 1,834 (37) | 2,491 (41) | 1.91 (1.69-2.17) | <.001∗ |
| Sore throat | 697 (56) | 1,917 (39) | 2,614 (42) | 2.03 (1.79-2.30) | <.001∗ |
| Malaise | 246 (20) | 918 (19) | 1,164 (19) | 1.08 (0.93-1.27) | .3194 |
| Fever and sore throat | 673 (54) | 1,628 (33) | 2,301 (37) | 2.42 (2.13-2.75) | <.001∗ |
| Fever and cough | 637 (52) | 1,613 (33) | 2,250 (37) | 2.19 (1.92-2.48) | <.001∗ |
| Fever and cough and sore throat | 352 (29) | 671 (14) | 1,023 (17) | 2.52 (2.18-2.92) | <.001∗ |
| Health professionals | 10 (0.81) | 62 (1) | 72 (1) | 0.63 (0.33-1.25) | .1875 |
| Contacted flu–positive case within 7 days | 352 (29) | 701 (14) | 1,053 (17) | 2.40 (2.07-2.78) | <.001∗ |
| Flu vaccine last year | 37 (3) | 176 (4) | 213 (3) | 0.83 (0.58-1.19) | .3161 |
| Underlying diseases | 146 (12) | 1,229 (25) | 1,375 (22) | 0.40 (0.33-0.48) | <.001∗ |
P < .05.
Patients’ satisfactions with RFCS
Of the 1,235 cases of a positive influenza test, 300 patients were contacted by telephone survey for follow-up on their RFCS visit. Of the 111 patients who responded to interviews, 62 (56%) were less than 20 years of age. Most of the patients (96, 86%) followed instructions for taking oseltamivir and completed the 5-day treatment course. After treatment, most of the patients reported relief of flu symptoms (within 2 days [60 (49%)] and within 4 days [99 (90%)], respectively). The most common adverse effects after taking oseltamivir included dizziness (18, 16%), nausea (10, 9%), vomiting (9, 8%), and dyspnea (8, 7%). Overall, 88% were satisfied with the RFCS.
Discussion
Setting up RFCSs was beneficial to health care facilities during pandemic flu seasons, efficiently separating airborne communicable patients from ambulatory patients and inpatients to avoid possible nosocomial transmissions at the ED. The mean LOS at the hospital was shorter, few additional clinical loading shifts were incurred by physicians, and most of the positive influenza patients were satisfied with their visits to the RFCSs.
Treating contagious ambulatory patients in an isolated area separated them from the regular ED or hospital services to avoid transmissions of nosocomial infections during outbreak periods.4, 6, 9 During the 2003 SARS outbreak, an ED was closed because of nosocomial infection at the overcrowded space.4, 5, 6 Overwhelming of health care systems because of heavy clinical burdens and high censuses at health care facilities was observed for the 2009 H1N1 pandemic flu in the southern hemisphere.10, 11, 12, 13 High-volume ambulatory influenza-like illness patients visited the ambulatory clinic and ED of hospitals after August 2009 since the first H1N1 SOIV cases were identified in Taiwan as well.14 The mean LOS of the RFCS at our ED was 50 minutes, which was shorter than the mean LOS at the ambulatory clinic or ED. The National Emergency Department Overcrowding Scale (NEDOCS) was used to further assess our ED during August to November 2009. The NEDOCS is a tool developed in the United States that uses a linear regression model using 5 variables and 6 items including total patients, total admissions, the longest admissions, hospital beds, ventilators, and last bed time at the hospital to quantify emergency department and hospital crowding. Scores over 100 were regarded as overcrowded.15 During the study period, our ED frequently reached the highest overcrowding level of dangerous or disaster level (NEDOCS > 180). However, no nosocomial outbreaks or health care worker cluster infections occurred at the ED in our study.
Young patients made up the largest population of visitors the RFCS. Many studies indicated that school age children were more susceptible to infection by H1N1 SOIV.2, 3, 16, 17, 18, 19, 20 The virus surveillance data from laboratories of the CDC of Taiwan indicated that H1N1 SOIV was isolated from 80% of flu cases from August 2009 to December 2009 in Taiwan.21 Based on the epidemiologic data from the CDC of Taiwan, although most of the patients at our hospital were tested with the RIDT only as recommended, we could still infer that patients having positive RIDTs were infected by H1N1 SOIV, and children or previously healthy individuals were more vulnerable to influenza like-illness or infection.
Symptoms in influenza-positive patients in our study were not significantly different from those in patients with previous seasonal flu strains. Previous studies reported fever, cough, malaise, or sore throat as the most common symptoms of seasonal flu.3 In our study, fever, cough, and/or sore throat and close contact with flu-positive individuals within 7 days were important risk factors for positive influenza tests during the 2009 pandemic flu season.
The administration of oseltamivir seemed beneficial to adults and children. Most of our patients who took oseltamivir found their flu symptoms relieved within 4 days and thought that oseltamivir was helpful. Previous studies reported that oseltamivir might have adverse effects such as dizziness or nausea.22, 23, 24 Studies in Japan suggested that children who took oseltamivir might have higher possibilities of delirium or abnormal behaviors.23, 25, 26, 27 In our study, no neuropsychiatric adverse effects were reported during interviews.
The major limitations for our study included wide variations of sensitivity (83%, 95% CI: 73%-90% by the manufacturer) for RIDTs, skill varieties for RIDT administrations among physicians, limited coverage for telephone interviews, and cooperation between physicians and patients during sampling.28, 29, 30 Under these circumstances, the number of influenza cases could be underestimated. Furthermore, our telephone interviews only covered positive influenza cases; the lack of interviews for negative influenza cases limited the reliability of our RFCS satisfaction surveys. The lack of patients with neuropsychiatric adverse effects in our study is probably due to the low response rate for questionnaires. During our study period, no obvious neuropsychiatric adverse effects were reported to our hospital’s drug adverse effect system nor were they reported from phone interviews (even though nearly 56% of cases enrolled in this study were young patients). Many patients did not answer their phones after ED visits, even though those patients provided telephone numbers to receive notifications of their influenza test results. Those patients who answered our phone survey might only reflect part of the status results from oseltamivir administration. Whether or not oseltamivir only causes neuropsychiatric adverse effects in the Japanese population still needs further studies.
In conclusion, RFCSs may be helpful for high-volume medical services during pandemic flu seasons or airborne disease outbreaks. Hospitals or EDs may adapt them as part of preparedness plans for high-volume medical demand situations during pandemic flu seasons.
Footnotes
Conflicts of interest: None to report.
References
- 1.World Health Organization . World Health Organization; Geneva: 2009. World now at the start of 2009 influenza pandemic. [Google Scholar]
- 2.Scalera N.M., Mossad S.B. The first pandemic of the 21st century: a review of the 2009 pandemic variant influenza A (H1N1) virus. Postgrad Med. 2009;121:43–47. doi: 10.3810/pgm.2009.09.2051. [DOI] [PubMed] [Google Scholar]
- 3.Bautista E., Chotpitayasunondh T., Gao Z., Harper S.A., Shaw M., Uyeki T.M. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.C., Chang S.C., Tsai K.S., Lin F.Y. Certainties and uncertainties facing emerging respiratory infectious diseases: lessons from SARS. J Formos Med Assoc. 2008;107:432–442. doi: 10.1016/S0929-6646(08)60150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y.C., Chen M.F., Liu S.Z., Romeis J.C., Lee Y.T. SARS in teaching hospital. Taiwan. Emerg Infect Dis. 2004;10:1886–1887. doi: 10.3201/eid1010.040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y.C., Huang L.M., Chan C.C., Su C.P., Chang S.C., Chang Y.Y. SARS in hospital emergency room. Emerg Infect Dis. 2004;10:782–788. doi: 10.3201/eid1005.030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center for Disease Control, Taiwan . Center for Disease Control; Taipei: 2009. Oseltamivir usage is covered by National Health Insurance during 2009 H1N1 pandemic. [Google Scholar]
- 8.Smit M., Beynon K.A., Murdoch D.R., Jennings L.C. Comparison of the NOW Influenza A & B, NOW Flu A, NOW Flu B, and Directigen Flu A+B assays, and immunofluorescence with viral culture for the detection of influenza A and B viruses. Diagn Microbiol Infect Dis. 2007;57:67–70. doi: 10.1016/j.diagmicrobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Tsai M.C., Arnold J.L., Chuang C.C., Chi C.H., Liu C.C., Yang Y.J. Impact of an outbreak of severe acute respiratory syndrome on a hospital in Taiwan, ROC. Emerg Med J. 2004;21:311–316. doi: 10.1136/emj.2003.011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotsimbos T., Waterer G., Jenkins C., Kelly P.M., Cheng A., Hancox R.J. Influenza A/H1N1 2009: Australia and New Zealand’s winter of discontent. Am J Respir Crit Care Med. 2010;181:300–306. doi: 10.1164/rccm.200912-1878CP. [DOI] [PubMed] [Google Scholar]
- 11.Lum M.E., McMillan A.J., Brook C.W., Lester R., Piers L.S. Impact of pandemic (H1N1) 2009 influenza on critical care capacity in Victoria. Med J Aust. 2009;191:502–506. doi: 10.5694/j.1326-5377.2009.tb02914.x. [DOI] [PubMed] [Google Scholar]
- 12.Bishop J.F., Murnane M.P., Owen R. Australia’s winter with the 2009 pandemic influenza A (H1N1) virus. N Engl J Med. 2009;361:2591–2594. doi: 10.1056/NEJMp0910445. [DOI] [PubMed] [Google Scholar]
- 13.Waterer G.W., Hui D.S., Jenkins C.R. Public health management of pandemic (H1N1) 2009 infection in Australia: a failure! Respirology. 2010;15:51–56. doi: 10.1111/j.1440-1843.2009.01675.x. [DOI] [PubMed] [Google Scholar]
- 14.Center for Disease Control (Taiwan) Center for Disease Control, Taiwan; Taipei: 2010. Influenza-like illness surveillance. [Google Scholar]
- 15.Weiss S.J., Ernst A.A., Derlet R., King R., Bair A., Nick T.G. Relationship between the National ED Overcrowding Scale and the number of patients who leave without being seen in an academic ED. Am J Emerg Med. 2005;23:288–294. doi: 10.1016/j.ajem.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 16.CDC Patients hospitalized with 2009 pandemic influenza A (H1N1)—New York City, May 2009. MMWR Morb Mortal Wkly Rep. 2010;58:1436–1440. [PubMed] [Google Scholar]
- 17.Gerrard J., Keijzers G., Zhang P., Vossen C., Macbeth D. Clinical diagnostic criteria for isolating patients admitted to hospital with suspected pandemic influenza. Lancet. 2009;374:1673. doi: 10.1016/S0140-6736(09)61983-8. [DOI] [PubMed] [Google Scholar]
- 18.Mermel L.A. Swine-origin influenza virus in young age groups. Lancet. 2009;373:2108–2109. doi: 10.1016/S0140-6736(09)61145-4. [DOI] [PubMed] [Google Scholar]
- 19.Miller E., Hoschler K., Hardelid P., Stanford E., Andrews N., Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 20.CDC Update: influenza activity—United States, August 30, 2009-January 9, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:38–43. [PubMed] [Google Scholar]
- 21.Center for Disease Control (Taiwan) Center for Disease Control, Taiwan; Taipei: 2009. Flu weekly report. [Google Scholar]
- 22.Strong M., Burrows J., Stedman E., Redgrave P. Adverse drug effects following oseltamivir mass treatment and prophylaxis in a school outbreak of 2009 pandemic influenza A (H1N1) in June 2009, Sheffield, United Kingdom. Euro Surveill. 2010;15 pii/19565. [PubMed] [Google Scholar]
- 23.Blumentals W.A., Song X. The safety of oseltamivir in patients with influenza: analysis of healthcare claims data from six influenza seasons. Med Gen Med. 2007;9:23. [PMC free article] [PubMed] [Google Scholar]
- 24.Roche. Product information of oseltamivir, 2010. Available from: http://www.rocheusa.com/products/tamiflu/pi.pdf. Accessed April 9, 2011.
- 25.Toovey S., Rayner C., Prinssen E., Chu T., Donner B., Thakrar B. Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: a comprehensive review. Drug Saf. 2008;31:1097–1114. doi: 10.2165/0002018-200831120-00006. [DOI] [PubMed] [Google Scholar]
- 26.Casscells S.W., Granger E., Kress A.M., Linton A. The association between oseltamivir use and adverse neuropsychiatric outcomes among TRICARE beneficiaries, ages 1 through 21 years diagnosed with influenza. Int J Adolesc Med Health. 2009;21:79–89. doi: 10.1515/ijamh.2009.21.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Yorifuji T., Suzuki E., Tsuda T. Oseltamivir and abnormal behaviors: true or not? Epidemiology. 2009;20:619–621. doi: 10.1097/EDE.0b013e3181a3d3f6. [DOI] [PubMed] [Google Scholar]
- 28.CDC Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:826–829. [PubMed] [Google Scholar]
- 29.Faix D.J., Sherman S.S., Waterman S.H. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;361:728–729. doi: 10.1056/NEJMc0904264. [DOI] [PubMed] [Google Scholar]
- 30.Kok J., Blyth C.C., Foo H., Patterson J., Taylor J., McPhie K. Comparison of a rapid antigen test with nucleic acid testing during cocirculation of pandemic influenza A/H1N1 2009 and seasonal influenza A/H3N2. J Clin Microbiol. 2010;48:290–291. doi: 10.1128/JCM.01465-09. [DOI] [PMC free article] [PubMed] [Google Scholar]