Highlights
-
•
B-IC used in conjunction with A-IC may reduce the risk of pathogen spread to HCWs.
-
•
Full personal protective equipment use for advanced airway management in ED.
-
•
Hospital epidemic and ED contingency plan implementation.
-
•
On-site MERS-CoV real time polymerase chain reaction (PCR) testing capability.
Key Words: MERS-CoV, Infection prevention, Health care worker, Occupational exposure
Abstract
Background
Transmission of Middle East respiratory syndrome-coronavirus (MERS-CoV) among health care workers (HCWs) and patients has been documented with mortality rate approximating 36%. We propose advanced infection control measures (A-IC) used in conjunction with basic infection control measures (B-IC) help reduce pathogen transmission. B-IC include standard and transmission-based precautions. A-IC are initiatives implemented within our center to enhance effectiveness of B-IC.
Objective
Study effectiveness of combining B-IC and A-IC to prevent transmission of MERS-CoV to HCWs.
Methods
A retrospective observational study was undertaken. A-IC measures include administrative support with daily rounds; infection control risk assessment; timely screening, isolation, and specimen analysis; collaboration; epidemic planning; stockpiling; implementation of contingency plans; full personal protective equipment use for advanced airway management; use of a real-time electronic isolation flagging system; infection prevention and control team on-call protocols; pretransfer MERS-CoV testing; and education.
Results
A total of 874 real-time polymerase chain reaction MERS-CoV tests were performed during the period beginning July 1, 2013, and ending January 31, 2015. Six hundred ninety-four non-HCWs were tested, of these 16 tested positive for MERS-CoV and their infection was community acquired. Sixty-nine percent of the confirmed MERS-CoV-positive cases were men, with an average age of 56 years (range, 19-84 years). Of the total tested for MERS-CoV, 180 individuals were HCWs with zero positivity.
Conclusions
Adhering to a combination of B-IC and A-IC reduces the risk of MERS-CoV transmission to HCWs.
Transmission of Middle East respiratory syndrome-coronavirus (MERS-CoV) among health care workers (HCWs) and patients in hospitals within Saudi Arabia has been documented.1 Of the HCWs who have acquired this infection, more than 63% acquired the infection within Saudi Arabia, where the majority of MERS-CoV cases have been reported.2 As of June 20, 2015, 1,338 cases have occurred worldwide, and of these 77.5% (n = 1,038) were identified within Saudi Arabia.3, 4 The main contributing factor for health care-associated transmission and causality of outbreaks among Saudi hospitals is due to emergency department (ED) overcrowding and poor ventilation.5 In turn, this can be a reflection of institutional overcrowding, inpatient bed occupancy reaching or exceeding full capacity, and/or a lack of compliance to and understanding of the importance of implementing infection control and prevention (ICP) measures.
This belief is supported by internationally recognized agencies, including the World Health Organization (WHO) and the US Centers for Disease Control and Prevention. The organizations support the practice of adhering to ICP measures. Basic infection control measures (B-IC), defined as standard and transmission-based precautions, play a major role in preventing and controlling pathogen spread, including adherence to hand hygiene, environment and equipment cleanliness, use of personal protective equipment (PPE) such as high-efficiency particulate respirators (eg, N-95 or R-95), and adhering to respiratory/cough etiquette.6 We propose that the spread of MERS-CoV among HCWs is preventable when B-IC are used in combination with institution-specific advanced infection control measures (A-IC). In this article, A-IC implemented in a tertiary care hospital during a time of epidemic is described.
During the initial and intermediate period after discovering this novel virus during June 2012 it was noted that symptoms and complexity of patients presenting with infection were variable.7 This resulted in a case definition that required modification over time to allow for broader screening of patients with flu-like illness. The initial case definition mainly encouraged screening of patients with severe acute respiratory illness requiring intensive care unit (ICU) admission.3
In the authors' experience, in dealing with MERS-CoV cases in the early stages of disease recognition there were poorly established systems in place for identification of suspected/confirmed cases. We believe that this was due to an unclear incubation period, poor rapid implementation of isolation precautions and use of PPE, and the unavailability of diagnostic testing among regional health care facilities. These health care facilities relied on an inconsistent availability of appropriate packaging and transportation of specimens to approved Ministry of Health (MOH) reference laboratories for testing of samples. Consequently, there were delays in communication and appropriate isolation of positive cases resulting in spread of infection to HCWs.1
Interventions
B-IC are well-recognized preventative measures considered the minimum requirement for infection prevention and control.6, 8 A-IC are institution-specific measures that enhance B-IC to further reduce the risk of transmission.
B-IC
B-IC are based on key components of standard precautions and recommended transmission-based precautions for prevention and control of transmissible diseases. This includes appropriate hand hygiene practices; proper cleaning of the environment and equipment; prompt initiation of transmission-based precautions for suspected/confirmed cases until noninfectious; segregation of confirmed/suspected cases in waiting areas; use of single hospital patient rooms; and proper availability, quality, type, and use of PPE6, 9 (Table 1 ).
Table 1.
Basic and advanced infection control measures
| Basic infection control measures | Advanced infection control measures |
|---|---|
|
|
A-IC
A-IC is a group of institution-specific measures that go above and beyond B-IC and enhance their effectiveness. It is vital that administrative support is sought to approve and fund the necessary initiatives. The following outlines the A-IC measures adopted at our institution (Table 1).
-
1.
Interdepartmental collaborative meetings: As part of the established institutional epidemic plan, using both interdepartmental collaboration and institutional expertise, daily morning meetings are held. These meetings are undertaken at a time of high incidence within the community, other regional institutions, or when an alert has been issued by the MOH. Key members include hospital administration, senior staff from the infectious diseases department, ICP, ED, ICU, nursing, microbiology, case management, and others as deemed necessary. Their objective is to assess, monitor, and recommend risk mitigation strategies. Current regional and international information regarding MERS-CoV disease activity, institutional preparedness, and its capabilities are discussed. During these meetings initiatives are formulated, implemented if applicable, and evaluated for success and/or modification. Measures to reduce risks referred to in this article as A-IC were discussed in these meetings and implemented institution-wide.
-
2.
Infection Control Risk Assessment: Multidisciplinary institution-wide infection control risk assessment to address areas of concern was undertaken. Areas of deficiencies addressed at the organization level include insufficient number of staff in high-risk areas, fit testing for high-efficiency particulate respirators (especially for ED, ICU, and direct patient care providers), overcrowding in the ED, ventilation systems in the ED, extended turnaround time of MERS-CoV test results, and awareness of the importance of early identification and isolation of suspected cases. With each automated telephone text message reminder of upcoming clinic or admission came an additional reminder to inform staff of any flu-like illness upon arrival. This is in conjunction with a prescreening process established in the ED and clinic receptions.
-
3.
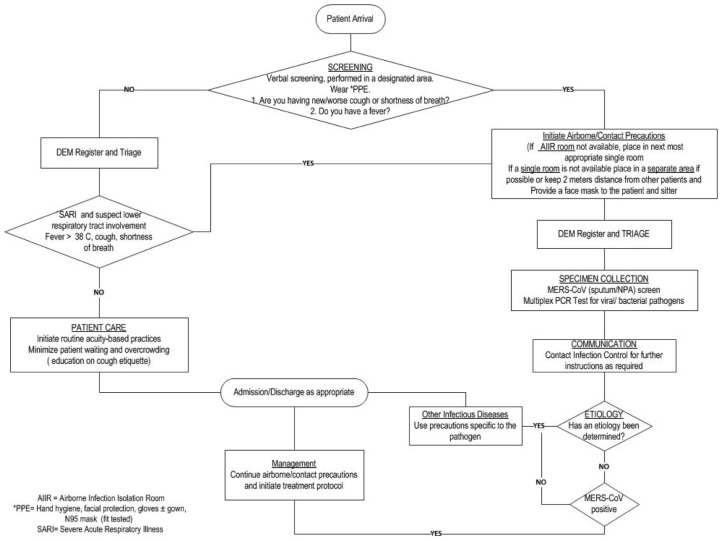
MERS-CoV Epidemic Plan: A MERS-CoV epidemic plan was implemented as an extension of the existing institutional epidemic plan (Table 2 , Fig 1 ).
-
4.
Flagging Electronic Charts: Real-time isolation supported by an electronic integrated clinical information system (ICIS) where transmission-based precautions are activated upon MERS-CoV order entry. Discontinuation of isolation precautions per ICP team protocol.
-
5.
ICP Team On Call: Newly implemented permanent service to assist in bed use and ICP issues.
-
6.
Interhospital Patient Acceptance and Transfer Criteria: Referrals from outside centers require documented MERS-CoV testing before acceptance. Documentation of travel and exposure history has been established as a new routine practice for each admission, clinic appointment, or ED visit.
-
7.
Ordering, Collection, and Transportation of MERS-CoV Specimens: The criteria for MERS-CoV testing is based on currently approved MOH case definitions posted on the MOH Web site.3 Staff education and compliance monitoring of appropriate ordering, collection technique, and transportation of MERS-CoV specimens to optimize yield and reduce risk of false results.
-
8.
Real-Time Polymerase Chain Reaction (PCR) MERS-CoV Testing Capability: To improve test turnaround time, patient management, bed/resource use, and decrease reliance on the MOH reference in-house MERS-CoV PCR testing capabilities were implemented. Turnaround time was reduced from 1 week or more to <6 hours, 7 days per week.
-
9.
Stockpiling of Essential Supplies: On activation of the MERS-CoV epidemic plan stockpiling of essential supplies is initiated. An increase from the normal 6-month availability to an 8-month supply is authorized. Additional stockpiling is considered based on demand and/or anticipated market unavailability.
-
10.
High-Efficiency Particulate Respirator Fit Testing: Qualitative fit testing was undertaken for high-efficiency particulate respirators (eg, N-95 and R-95) as a major interdisciplinary performance improvement project. Of the hospital staff members who require fit testing (approximately 8,000 individuals) 50% were fit tested over a 12-month period. A permanent process was implemented to fit test the remaining staff and new hires. Fit testing results are documented in the employee's medical file on ICIS.
-
11.
Advanced Airway Management: Use of indirect video laryngoscopy (eg, glidescope [Verathon, Bothell, WA]) for intubation and use of a high-efficiency particulate respirator for all intubations involving patients with suspected/confirmed airborne or droplet diseases and/or emergency respiratory failure due to respiratory illness was introduced as a standard of care. Powered air purifying respirators were purchased. Approved use includes during tracheostomy and other procedures (eg, lung biopsy for suspected/confirmed MERS-CoV cases) or when a high-particulate respirator mask cannot be worn (eg, HCW failed fit testing).
-
12.ED Contingency Plan:
-
a.Reduce overcrowding: Administrative approval for institutional occupancy decrease by 20% with the aim to conversely decrease ED boarding of admitted patients.
-
b.External prescreening capabilities: In anticipation of a patient influx, specialized outdoor tents that can be converted into negative pressure were purchased.
-
c.Reduce patient wait time (door-to-doctor time): The institutional standard median waiting time from presentation to the ED (door) and until examined by a physician (doctor) for all category patients (per the Canadian Triage Acuity Scale) is kept below 1 hour. To maintain and where possible reduce door-to-doctor time below 1 hour a lean methodology was used for all ED processes. A sustained reduction in waiting time was achieved for category 2 patients to around 30 minutes by implementing improved communication between ED staff.
-
d.ED early isolation and patient placement: Airborne infection isolation room are available in the ED for patients with airborne diseases. This was expanded to use for all patients requiring aerosol-generating procedures to reduce the risk of pathogen spread.10
-
e.Patient transportation: Isolation transportation pods were purchased to be used for transportation of any patient suspected or confirmed positive for an airborne infectious disease, including MERS-CoV.
-
a.
-
13.
Patient, Family, and Visitor Education and Communication: During epidemic plan activation a disease-specific education campaign is implemented. Posters, pamphlets, and the intrahospital television system are used for communication (Fig 2).
-
14.
Staff Education and Communication: Staff preparation training and specific education to care for patients with/suspected MERS-CoV was undertaken.
-
15.
Transparency: While maintaining confidentiality, transparency is important to enhance dissemination of accurate information to all staff. This can create an atmosphere of trust, loyalty, and support; therefore, HCWs are more likely to adhere to recommendations and reduce their absenteeism.
Table 2.
Middle East respiratory syndrome-coronavirus (MERS-CoV) epidemic plan
| Part I |
| Phase 1: Low level of alertness for MERS-CoV epidemic plan: No cases or expected influx of MERS-CoV—usual practice |
| • Process for admission/discharge, inpatient management, and assessment in emergency department per policy |
| • Infection control measures per policy |
| Phase 2: Moderate level of alertness for MERS-CoV—1-2 confirmed cases within the institution |
| • Administration announce escalation of alertness for potential of transmission |
| • Increase stockpiles of essential supplies by 6 times the average monthly usage (ie, surgical and N95 masks, gown, gloves, goggles, and hand hygiene products) |
| • Focused hospitalwide education campaign to improve compliance with infection control measures related to MERS-CoV |
| • Emergency department prescreening for early identification and isolation of flu-like illness (Fig 1) |
| • Assigned unit with airborne infection isolation rooms for admission and cohort of confirmed MERS-COV cases |
| Phase 3: High level of alertness for MERS-CoV epidemic plan—greater than 3 cases within the institution, outbreak risk, and/or expected influx due to Ministry of Health directive |
| • In addition to phase 2 |
| • Decrease hospital occupancy by 15% |
| • Limit admission of referrals as applicable; that is, refer noncritical patients to other medical centers |
| De-escalation of alertness |
| • Decrease in the number of confirmed MERS-CoV cases within the institution |
| • Ministry of Health situational reports and directive |
| Part II |
| Measures to decrease inpatient occupancy |
| • Daily assessment of service specific bed use by department chairman and case management |
| • Cancellation of elective and nonessential surgeries, procedures, and admissions |
Fig 1.
Prescreening for flu-like illness in the emergency department. AIIR, airborne infection isolation room; DEM, Department of Emergency Medicine; MERS-CoV, Middle East respiratory syndrome-coronavirus; NPA, nasopharyngeal aspirate; PCR, polymerase chain reaction; PPE, personal protective equipment; SARI, severe acute respiratory illness. *Including hand hygiene, facial protection, gloves with or without gown, and N-95 mask (if fit tested).
Fig 2.

Education posters/rollups.
Institutional MERS-CoV cases and HCW exposure
Method
ICIS was used to retrieve patient and HCW data. For the purpose of this observational study, data were retrieved retrospectively and collected for a continuous period of 19 months. Those tested for MERS-CoV included patients with suspected/confirmed cases who met the case definition and/or HCWs who were tested during an outbreak investigation for protected/unprotected exposure. Duplicate tests were removed if testing was performed during the same episode of illness or exposure. All patient and HCW confidentiality and rights were maintained. Microsoft Office (Redmond, WA) and Visio (Microsoft, Redmond, WA) were used to plot results and Research Advisory Committee approval (RAC# 2151-194) was obtained to use the data for this publication.
Results
A total of 874 cases were tested for MERS-CoV from the period beginning July 1, 2013, and ending January 31, 2015. Of these, 16 (1.8%) were MERS-CoV positive. They were all non-HCWs (Fig 3 ). During this time period the ED served more than 80,000 patients and institutional inpatient bed capacity was >700 with an occupancy rate consistently >90%. Institution employee numbers exceeded 11,000.
Fig 3.
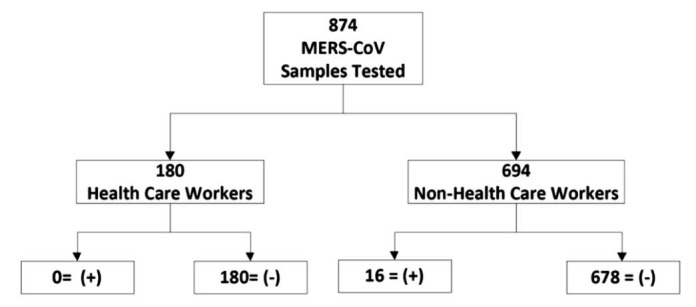
Test results of the study period beginning July 1, 2013, and ending January 31, 2015.
Of the 874 MERS-CoV samples tested, 180 (21%) were from HCWs. HCW testing was due to meeting the case criteria or to protected/unprotected exposure. Exposure occurred in 3 of the confirmed cases as a result of a delay in the initiation of isolation precautions due to lack of early recognition of symptoms or incubation period. Testing of the non-HCWs (n = 694) was due to case criteria being met during admission or upon presentation to the institution through the ED or clinics, or as part of a screening requirement at the time of admission; that is, direct admission from another hospital per the epidemic plan.
The majority of patients with positive cases were men (69%) with a mean age of 56 years (range, 19-84 years). All case patients except 1 had comorbidities and/or immunocompromised status. Ten of the 16 patients with confirmed cases died (62%). Mortality was directly or indirectly attributed to complications that developed as a result of MERS-CoV infection. All case patients had respiratory symptoms on initial presentation except for 1 patient who was admitted for elective surgery and developed respiratory symptoms with fever postoperatively on day 7. This was considered community acquired because the incubation period overlapped with the preadmission period. No cases were considered health care-associated infections (Table 3 ).
Table 3.
Patient demographic characteristics
| Case | Age, y | Gender | Comorbidities | Presenting symptoms | Date of admission | Date first sample taken and positive | Date negative or died | Potential HCW exposure days | Outcome | Death attributed to MERS-CoV Yes/No |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | Female | DM/HTN/lymphoma/cardiomyopathy | Respiratory | 24/07/2013 | 15/08/2013 | 28/08/2013 | 33 | Discharged 05/09/2013 | N/A |
| 2 | 52 | Male | Retroperitoneal sarcoma | Respiratory | 24/07/2013 | 27/07/2013 | 08/08/2013 | 16 | Died | Yes |
| 3 | 54 | Male | HTN/DM/ESRD/CAD | Respiratory | 28/07/2013 | 14/08/2013 | 18/08/2013 | 14 | Died | Yes |
| 4 | 50 | Male | DM | Respiratory/ARDS/known MERS-CoV positive | 06/08/2013 | 27/08/2013 | 29/08/2013 | 23 | Discharged 09/09/2013 | N/A |
| 5 | 54 | Female | HTN/treated parotid carcinoma | Respiratory | 24/09/2013 | 24/09/2013 | 06/10/2013 | 13 | Discharged 18/10/2013 | N/A |
| 6 | 73 | Male | DM/HTN/CAD | Respiratory | 12/10/2013 | 15/10/2013 | 08/12/2013 | 57 | Died | Yes |
| 7 | 73 | Male | CKD/HTN/RHD | Respiratory | 12/12/2013 | 12/12/2013 | 18/12/2013 | 7 | Died | Yes |
| 8 | 84 | Male | HTN/right BBB | Respiratory | 01/03/2014 | 10/03/2014 | 23/03/2014 | 24 | Died | Yes |
| 9 | 19 | Male | Nil | Respiratory | 13/03/2014 | 15/03/2014 | 30/03/2014 | 18 | Discharged 02/04/2014 | N/A |
| 10 | 56 | Female | DM/HTN | Respiratory | 02/05/2014 | 03/05/2014 | 05/05/2014 | 4 | Discharged 25/06/2014 | N/A |
| 11 | 49 | Male | HTN/ESRD on PD/HBV | Respiratory | 02/05/2014 | 02/05/2014 | 09/06/2014 | 39 | Discharged 08/07/2014 | N/A |
| 12 | 72 | Male | NHL/HBV/cardiomyopathy | Respiratory | 06/08/2014 | 09/08/2014 | 17/08/2014 | 12 | Died | Yes |
| 13 | 32 | Female | NHL on CTX | Respiratory | 23/12/2014 | 24/12/2014 | 18/01/2015 | 27 | Died | Yes |
| 14 | 52 | Male | DM/HTN/ renal cell Ca | Respiratory | 22/02/2015 | 22/02/2015 | 17/03/2015 | 24 | Died | Yes |
| 15 | 60 | Male | Metastatic pancreatic Ca | Respiratory | 24/02/2015 | 26/02/2015 | 02/03/2015 | 7 | Died | Yes |
| 16 | 64 | Male | Postrenal transplant | Respiratory | 28/02/2015 | 01/03/2015 | 19/03/2015 | 20 | Died | Yes |
ARDS, adult respiratory distress syndrome; BBB, bundle branch block; Ca, cancer; CAD, coronary heart disease; CKD, chronic kidney disease; CTX, chemotherapy; DM, diabetes mellitus; ESRD, end-stage renal disease; HBV, hepatitis B virus; HTN, hypertension; MERS-CoV, Middle East respiratory syndrome-coronavirus; NHL, non-Hodgkin's lymphoma; PD, peritonitis dialysis; RHD, rheumatic heart disease.
Potential HCW exposure days are calculated from the date of MERS-CoV symptom onset while in hospital until either the date of negative screening result(s) or death while positive. During the period of study the potential HCW exposure days totaled 338. The mean duration of potential infectivity per case was 21 days (range, 4-57 days).
Discussion
Due to the nature of their work, HCWs remain at high risk of acquiring communicable diseases from occupational exposure. Experience and data collected during the severe acute respiratory syndrome epidemic in Hong Kong suggests an infection rate of 22% among HCWs.11 During April-August 2014 a number of hospital outbreaks were reported within Saudi Arabia increasing the risk of HCW exposure. This risk to HCWs is supported by ongoing findings reported by the WHO. The MERS-CoV Situation Update Report—15 June 201512 states that 10%-29% of the total MERS-COV cases identified in Saudi Arabia were in HCWs. Front-line HCWs caring for undifferentiated and severely ill patients who do not adhere to ICP measures are at the highest risk.10
Upon the discovery of the novel coronavirus and in anticipation of a local and regional threat, a taskforce was formed to evaluate our institution's readiness to deal with the developing situation and to initiate proactive measures to protect staff, patients, and visitors. This taskforce included representatives from experts from the infectious disease department (adult and pediatric), ICP, ED, laboratory, nursing, research, supply chain, and other relevant stakeholders. This article reflects the work undertaken by the taskforce members who recommended and implemented measures to mitigate the risk of viral spread.
Although institutions aim to protect and safeguard their HCWs against communicable diseases, success is not always attainable without a culture of vigilance. The approval by executive management to implement the recommended ICP measures was primarily due to recognition of a true potential threat, and in support of recommendations by the taskforce. This prompt action and support is reflective of the mission, vision, and core values of our institution, which supports a culture of safety. Due to urgency, time for a prescheduled mock drill to test proposed interventions and make the necessary adjustments based on the results of the simulation was not possible at time of initial implementation. Changes or improvements to ICP measures occurred in real time with immediate effect. Ongoing monitoring for effectiveness occurred through observational HCW compliance monitoring, noting frequency of unprotected exposure events, and screening during outbreak investigations. Those working in our institution—especially within high-risk areas (eg, ED and ICU) observed an increased level of anxiety reflected by an increased use of masks while not performing direct patient care. In particular, in the use of high-particulate respirators. In our opinion this anxiety was as a result of rumors and misconceptions of MERS-CoV transmission and number of actual cases within our institution, documented human-to-human and animal-to-human transmission, a high mortality rate (36%) that includes HCWs, limited treatment options, and the unavailability of vaccines.4 Communication and transparency helped to reduce levels of anxiety and rumor. Our reported MERS-CoV mortality rate of 62% is higher than that reported nationally and most likely due to the complexity and comorbidities of the patient population served in our major city tertiary care institution.
There were 16 confirmed MERS-CoV cases over a 2-year period with a collective potential infectivity duration of 338 days. None of the confirmed cases were health care-associated infections or HCWs from this institution. As reflected in Table 3, cases identified in 2013 from date of admission to date of first MERS-CoV sample took longer than what was observed in subsequent years. This reflects the successful work of the taskforce. B-IC were already in place but required reinforcement. Standard and transmission-based precautions, and hand hygiene are ICP key performance indicators for continual compliance monitoring and reporting.13, 14 A-IC were introduced and reinforced to support the B-IC measures. Many of the A-IC measures implemented during this period have now become standard-of-care practices within the institution. Without administrative and leadership support, targeted resource allocation, and high HCW compliance rates, ICP measures would not be effective. This includes timely information dissemination, investment by the institution on education, ongoing awareness and staff development, adequate and high-quality supplies, and meticulous screening processes. Clear and open communication channels are vital between all parties and are a prerequisite for success.
Close contact with the MOH was maintained both in reporting of cases and receiving directives for case identification and management. In addition, updates posted by the WHO and Centers for Disease Control and Prevention were reviewed on a daily basis to remain current and monitor for changes to guidelines, and in the understanding of the disease processes. Adjustments were made accordingly to institutional case definitions, management guidelines, and ICP measures. No outside consultation was undertaken to develop and refine the A-IC measures.
Limitations
This was a single-center study; therefore, results may not be generalized to other institutions. The actual level of compliance and benefits of implemented ICP measures were not systematically measured because a randomized control study would be unethical. Seroconversion rates for MERS-CoV were not undertaken among HCWs because this test is currently unavailable within our institution.
Conclusions
Our institution successfully reduced the transmission risk of MERS-CoV among HCWs and patients. We believe that our success is multifactorial, including a proactive and visionary response by leadership, collaborative efforts by all departments, and staff adherence to both B-IC and A-IC measures. We suggest that other institutions under similar circumstances conduct an internal review as soon as possible and implement appropriate measures accordingly. To our knowledge, a similar experience of preventing the spread of MERS-CoV through the implementation of both B-IC and A-IC has not been published.
Acknowledgment
The authors thank Elenette Prado, Department of Emergency Medicine, for providing secretarial assistance.
Footnotes
Conflicts of Interest: None to report.
References
- 1.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A. KSA MERS-CoV Investigation team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2014. Severe respiratory disease associated with Middle East respiratory syndrome coronavirus (MERS-CoV)—tenth update, 31 May 2014. Stockholm, Sweden: European Centre for Disease Prevention and Control. [Google Scholar]
- 3.Sau, di Ministry of Health Command and Control Center Infection Prevention and Control Guidelines for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection. http://www.moh.gov.sa/en/CCC/StaffRegulations/Corona/Documents/IPC%20Guidelines%20for%20MERS-coV%20Infection.pdf Available from: Accessed October 25, 2015.
- 4.CoronaMap Realtime Tracking of MERS Corona virus on world map. 2015. http://coronamap.com/ As of June 20; Available from. Accessed October 25, 2015.
- 5.Zumla A., Hui D. Infection control and MERS-CoV in health-care workers. Lancet. 2014;383:1869–1871. doi: 10.1016/S0140-6736(14)60852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel D., Rhinehart E., Jackson M., Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare setting. The healthcare infection control practices advisory committee. 2007. http://www.cdc.gov/hicpac/pdf/isolation/isolation2007.pdf Available from. Accessed October 25, 2015. [DOI] [PMC free article] [PubMed]
- 7.The World Health Organization (WHO) MERS-CoV Research Group State of knowledge and data gaps of middle east respiratory syndrome coronavirus (MERS-CoV) in humans. 2013. http://currents.plos.org/outbreaks/article/state-of-knowledge-and-data-gaps-of-middle-east-respiratory-syndrome-coronavirus-mers-cov-in-humans-2/ Available from. Accessed October 25, 2015. [DOI] [PMC free article] [PubMed]
- 8.WHO Infection prevention and control during health care for probable or confirmed cases of novel coronavirus (nCoV) infection Interim guidance 6 May 2013. http://www.who.int/csr/disease/coronavirus_infections/IPCnCoVguidance_06May13.pdf Available from: Accessed October 25, 2015.
- 9.Centers for Disease Control (CDC) Interim infection prevention and control recommendations for hospitalized patients with Middle East respiratory syndrome (MERS-CoV) 2014. http://www.cdc.gov/coronavirus/mers/infection-prevention-control.htmlhttp://www.cdc.gov/coronavirus/mers/downloads/MERS-Infection-Control-Guidance-051414.pdf Available from. Accessed October 25, 2015.
- 10.Marshall C., Kelso A., McBryde E., Barr I.G., Eisen D.P., Sasadeusz J. Pandemic (H1N1) 2009 risk for frontline health care workers. Emerg Infect Dis. 2011;17:1000–1006. doi: 10.3201/eid1706.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng V., Chan J., To K., Yuen K.Y. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 2013;100:407–419. doi: 10.1016/j.antiviral.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization MERS-CoV situation. http://www.emro.who.int/surveillance-forecasting-response/surveillance-news/mers-cov-update-15-june-2015.html Available from. Accessed October 25, 2015.
- 13.World Health Organization. Clean Care is Safer Care http://who.int/gpsc/tools/Five_moments/en/ Available from. Accessed October 25, 2015.
- 14.Brosseau L.M., Jones R. Commentary: protecting health workers from airborne MERS-CoV—learning from SARS, centers for infectious disease research and policy. 2014. http://www.cidrap.umn.edu/news-perspective/2014/05/commentary-protecting-health-workers-airborne-mers-cov-learning-sars May 19; Available from. Accessed October 25, 2015.