Abstract
Background
Respiratory protection relies heavily on user compliance to be effective, but compliance among health care personnel is less than ideal.
Methods
In 2008, the Department of Veterans Affairs formed the Project Better Respiratory Equipment using Advanced Technologies for Healthcare Employees (BREATHE) Working Group, composed of a variety of federal stakeholders, to discuss strategies for improving respirator compliance, including the need for more comfortable respirators.
Results
The Working Group developed 28 desirable performance characteristics that can be grouped into 4 key themes: (1) respirators should perform their intended function safely and effectively; (2) respirators should support, not interfere, with occupational activities; (3) respirators should be comfortable and tolerable for the duration of wear; and (4) respiratory protective programs should comply with federal/state standards and guidelines and local policies. As a necessary next step, the Working Group identified the need for a new class of respirators, to be called “B95,” which would better address the unique needs of health care personnel.
Conclusion
This article summarizes the outputs of the Project BREATHE Working Group and provides a national strategy to develop clinically validated respirator test methods, to promulgate B95 respirator standards, and to invent novel design features, which together will lead to commercialized B95 respirators.
Key Words: Respiratory protection, Infection control, Occupational health, N95, Personal protective equipment
Preventing health care-acquired infections (HAI) has become a major infection control platform, leading to increased efforts and resources in the reduction and elimination of such events. Although there has been significant positive change in the culture of patient safety in hospitals,1 extension of the same protective measures to those who provide the care lags behind. Health care personnel (HCP) not only face a risk of acquiring respiratory infection in the community but also in the hospital environment, where the likelihood of coming in close contact with an infectious patient is high.2, 3 To reduce worker exposure to a myriad of hazards, including respiratory hazards, a hierarchy of controls has been developed. This systematic approach has been used to implement the most effective and practical means of protecting workers.4 In health care, the patient is often the source of the exposure but requires medical care. In this case, elimination, substitution, and administrative controls (eg, shorter work times) are often not possible to implement. Engineering controls (eg, isolation rooms, upper room ultraviolet germicidal irradiation) can be an effective option but are typically only utilized after infection is suspected, are expensive, and often need to be factored in during the hospital design phase. Personal protective equipment (PPE) is the least desirable choice because it relies heavily on user compliance but can be implemented widely, quickly (eg, during a pandemic), and seamlessly in a health care setting compared with the other techniques for reducing worker exposure.
History of respiratory protection in health care
Respirators have been used to protect workers from inhaling dangerous substances for over 2,000 years, with these hazards including dusts, fumes, and vapors.5 Not surprisingly, the use of respiratory protection in industry and manufacturing is more common than in health care. According to a 2001 survey, respirator use as a percentage of private sector establishments was less in health care (3.2%) than in manufacturing (12.8%), mining (11.7%), construction (9.6%), or agriculture (9.4%).6, 7
Although HCP face a variety of potential respiratory hazards (eg, ethylene oxide and formaldehyde), respiratory protection in health care did not receive much attention until the late 1980s.8 A change did not occur until the number of Mycobacterium tuberculosis (TB) cases in the United States was observed to be steadily increasing, including outbreaks of multidrug-resistant TB.9 After the deaths of 8 HCP who acquired TB in the workplace, the Centers for Disease Control and Prevention (CDC) began recommending the use of respiratory protection among all HCP who cared for patients with known or suspected TB infection.10 Surgical masks had been commonly used for respiratory protection in TB isolation rooms until this time11; this policy change was the first major guidance document specifically recommending the use of respirators for HCP exposed to an infectious aerosol. In 1997, the Occupational Safety and Health Administration (OSHA) published a proposed rule for occupational exposure to TB, which included respiratory protection. This proposed rule, which was later rescinded, demonstrated an expansion of the use of respirators into new types of workplaces, which were not always familiar with all of the requirements for the proper use of respirators, including fit testing. In 1998 and 2006, OSHA published updates to its respiratory protection standard (29 CFR Part 1910.134), consolidating a number of substance-specific regulations. Through this standard, OSHA enforces the proper use of respiratory protection in workplaces where respirators are needed to reduce worker exposures to acceptable levels, including health care settings.
While CDC and OSHA were expanding the role of respiratory protection into health care settings, the National Institute for Occupational Safety and Health (NIOSH) was revising the federal regulations governing how respirators are certified and labeled in the United States. In 1995, NIOSH published a new regulation, 42 CFR Part 84, replacing 30 CFR Part 11, which gave NIOSH primary authority over certification of respiratory protective devices. These new regulations also created new tests and terminology for particulate respirators, which enabled users to select from a broader range of devices to meet performance criteria recommended by the CDC for protection against TB exposure. By the late 1990s, the N95 class of disposable (single use) filtering face piece respirators (also known informally as an “N95,” “N95 respirator,” or “N95 Mask”) became the standard of practice for HCP providing care to patients with known or suspected TB. In 2002, the Food and Drug Administration (FDA) and NIOSH began issuing approvals for “Surgical N95 respirators,” which are NIOSH-approved N95 filtering face piece respirators that also meet the FDA requirements to be labeled as a surgical mask. These devices are often recommended in cases in which a respirator that provides fluid protection and maintaining a sterile surgical field are important.12, 13, 14
More recently, N95 respirators have been recommended by many public health organizations as a means of reducing exposure to a variety of airborne infectious diseases, such as TB, measles, and varicella (chickenpox).15, 16 N95 respirators also serve as the foundation for preparations for emerging infectious disease threats where aerosol transmission is considered possible. When severe acute respiratory syndrome (SARS) emerged in 2003, N95 respirators became widely used to protect against this pathogen because little was known about modes of transmission during the early outbreak phase.17 The emergence of H5N1 influenza in 2005, and the novel H1N1 influenza pandemic of 2009, led to a resurgence of appropriate usage-related questions regarding respirators. For example, during the initial stages of the 2009 novel H1N1 pandemic, the CDC issued guidance calling for the use of N95 respirators, instead of surgical masks, for HCP protection.18 This decision differed from recommendations by the World Health Organization18 and was considered controversial by some,19 thus leading the Institute of Medicine (IOM) to review the science behind this recommendation and develop a better understanding of PPE necessary for a novel influenza pandemic. The IOM committee concluded that properly used N95 respirators should be better at reducing exposures and protecting against 2009 pandemic influenza than surgical masks.18 Currently, N95 respirators remain the recommended level of PPE for highly aerosol-generating procedures with seasonal influenza patients.
Current state of respiratory protection in health care
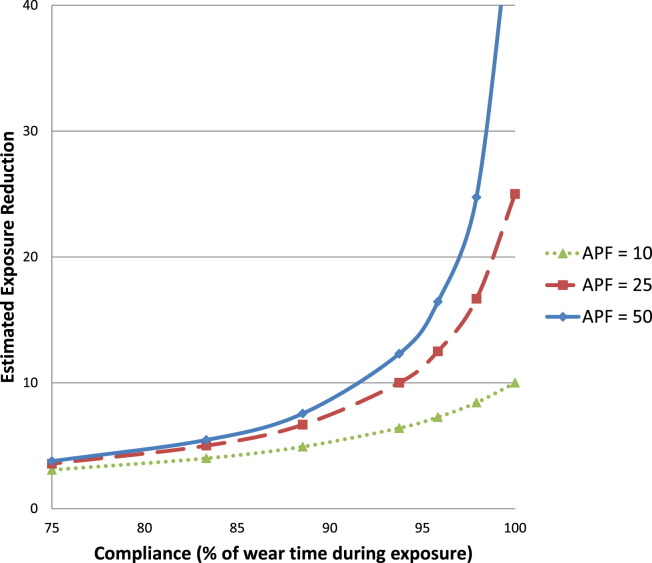
Although it is understood that HCP assume some level of personal occupational risk when caring for contagious patients,20 and numerous policies and regulations call for respiratory protection in the health care environment,5, 15, 16 noncompliance is unfortunately quite common.21 As noted above, one of the limitations of PPE as a tool for exposure reduction is its reliance on the wearer to use the device correctly at all times during the entire period of exposure. Figure 1 graphically illustrates the impact of compliance on exposure reduction using a model described previously by the by the American Industrial Hygiene Association respiratory protection committee.22 In Figure 1, the different lines represent different types of respirators with different levels of potential exposure reduction. A disposable N95 respirator, such as those used in health care, has an assigned protection factor rating of 10.23 This indicates that the wearer of an N95 respirator, when properly fitted and used correctly, could expect to inhale no more than one-tenth of an airborne contaminant(s) present. Accordingly, wear time needs to be > ∼75% to begin seeing a significant difference in exposure reduction, even for better performing respirators with higher assigned protection factor ratings. Ensuring that HCP wear respiratory protection in compliance with guidelines is vital to the effectiveness of the respirator; if the device is not worn during exposure, it is not providing appropriate protection.
Fig 1.
Effect of compliance on exposure reduction. Notes: Assigned protection factor (APF) is an estimate of the exposure reduction that a type of respirator is expected to provide when used correctly. Higher APF levels are assigned to respirators types that are expected to provide better levels of exposure reduction. APFs of 10, 25, and 50 are assigned to disposable N95 respirators, loose-fitting powered air-purifying respirators, and full facepiece elastomeric respirators, respectively.
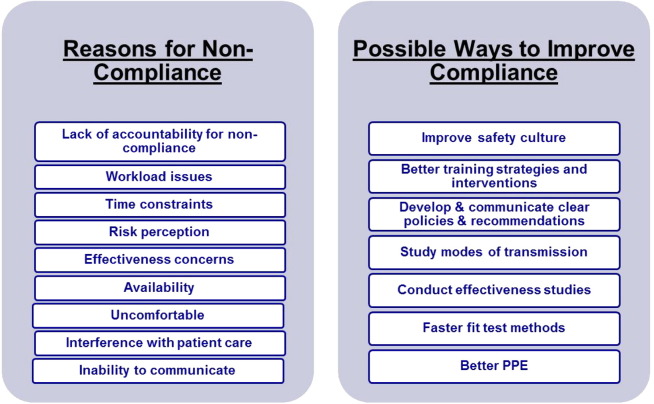
Figure 2 graphically illustrates some of the reasons for poor compliance, as identified in the peer-reviewed literature,24 as well as some possible solutions to increase compliance. The solutions listed are only possible solutions; much work has been done to identify the issue, and there is still more to be done to remedy these issues. Some HCP do not believe that the risks of exposure to airborne diseases warrant donning a respirator,21, 25 perhaps because they do not believe in the necessity and/or effectiveness of these devices21 or because they are uncomfortable26, 27, 28 and tend to interfere with occupational activities.26 Among the many causes of poor compliance, several of them are unique, or of heightened importance, in health care settings. These issues (discomfort, communication, interference, time constraints) are summarized below:
-
•
Discomfort experienced by HCP who wear respirators is often associated with the tight-fitting N95 respirator models.27 Discomfort was routinely raised as a key factor limiting the practicality of the CDC and OSHA recommendations during the 2009 novel H1N1 influenza pandemic. HCP routinely use surgical masks to protect their face from splashes and sprays and, depending on the hazards, may switch several times throughout the course of their work shift between a surgical mask and a respirator. In general, surgical masks are viewed as more comfortable than respirators. Most HCP are more accustomed to prolonged use of surgical masks29; thus, small differences in comfort between the 2 types of devices are heightened, further leading to the perception that respirators are uncomfortable. In fact, discomfort is the most typical reason HCP cite for improper use of respirators, but this may encompass a variety of sensations and experiences, most commonly facial pressure, facial heat, facial pain, labored movement of facial muscles, or skin itchiness.21, 30, 31 Psychologic manifestations of respirator wear, such as claustrophobia, may also be considered forms of discomfort,32 and improper usage of these devices is relatively common,26, 33 such that it may lead to discomfort.
-
•
Interference with occupational duties in the field of health care is a common problem as well.21 Nearly half of the HCP surveyed by Baig et al reported that an N95 respirator, at least occasionally, interfered with their ability to care for patients,26 which is their primary concern.
-
•
Poor communication has been shown to be a concern with existing N95 respirators. Speech intelligibility may be diminished in some settings when a respirator is worn, especially in noisy environments such as emergency departments, intensive care units, and prehospital environments.34, 35 Speech intelligibility issues are of particular concern, given the potential for miscommunication leading to critical treatment mistakes.35, 36
-
•
Time constraints are often raised as factors leading to noncompliance, with 2 principle areas of deficit: the OSHA requirement to be fit tested upon being hired and annually thereafter and the time required for proper donning and doffing of respirators. There is a cost—in terms of time and money—to utilize respiratory protection, which competes for the limited resources of HCP and hospitals.37, 38
Fig 2.
Problems and possible solutions related to respiratory protection usage in health care.
The numerous issues expressed by HCP demonstrate the need for respiratory protection that is designed to specifically meet their needs. Furthermore, many policy experts believe that the science to drive health care respiratory protection policies is still missing. In 2007, NIOSH’s National Personal Protective Technology Laboratory charged an IOM committee to examine research directions, certification and establishment of standards, and risk assessment issues related to PPE for HCP during an influenza pandemic. One of the recommendations issued by IOM in its final report21 called for respirator developers to revisit the entire respirator design and development process with attention to the unique needs of HCP.
Project Better RESPIRATORY Equipment using Advanced Technologies for Healthcare Employees: Project BREATHE
Based on recommendations issued by the IOM21 and findings from its own respirator research in 2007 and 2008,26, 30, 39 the Department of Veterans Affairs (VA) was prompted to improve the PPE practices used by its employees. VA is the largest integrated health care system in the United States and employs or oversees 180,000 HCP who use approximately 1.6 million respirators per year. In 2008, VA formed the Project Better Respiratory Equipment using Advanced Technologies for Healthcare Employees (BREATHE) Working Group, composed of a variety of federal stakeholders (Table 1 ) to discuss strategies for improving compliance with respiratory protective devices used in health care. The Working Group produced a report40 featuring 28 recommended features and performance characteristics (Table 2 ) for the next generation of respirators in health care. The goal was to provide the respirator research, standards development, and manufacturing communities with a reasoned list of ideal characteristics.
Table 1.
Project BREATHE Working Group participants
| Project BREATHE Working Group participants |
|---|
| • The National Personal Protective Technology Laboratory in the National Institute for Occupational Safety and Health in the Centers for Disease Control and Prevention (Department of Health and Human Services) |
| • Office for Infection Control, Division of Healthcare Quality Promotion in the Centers for Disease Control and Prevention (Department of Health and Human Services) |
| • National Center for HIV, STD, and TB Prevention, Division of Healthcare Quality Promotion in the Centers for Disease Control and Prevention (Department of Health and Human Services) |
| • The US Army Edgewood Chemical Biological Center (Department of Defense) |
| • The Occupational Safety and Health Administration (Department of Labor) |
| • The National Institute of Standards and Technology (Department of Commerce) |
| • The National Aeronautics and Space Administration |
| • Biomedical Advanced Research and Development Authority (Department of Health and Human Services) |
| • Office of Public Health and Environmental Hazards in the Veterans Health Administration (Department of Veterans Affairs) |
Table 2.
Project BREATHE recommendations
| Feature/characteristic | B95 recommendations∗ |
|---|---|
| 1. Safety and effectiveness | Respirators should meet all current NIOSH (eg, 42 CFR Part 84) and FDA standards (eg, 510(k) process for class II medical devices) and be used within an OSHA respiratory protection program, including fit testing. |
| 2. Self-contamination† | Users need to be able to easily and reproducibly don and doff respirators without self-contamination in a clinical environment. |
| 3. Fomite transmission† | Respirators should not be a conduit for fomite transmission of pathogens between persons. |
| 4. Respirator fit† | Respirators (available in 1 or few sizes) should be well fitting and capable of passing an OSHA-accepted fit test on a majority (∼90%) of US health care workers. |
| 5. Blood and body fluids | Respirators should serve as a barrier to protect the wearer from blood and body fluids. |
| 6. Reuse† | Respirators should be durable enough for the respirator to provide expected levels of protection (eg, protection factor of 10 or greater for a half-mask respirator) after multiple brief worker-patient encounters, if necessary, during a crisis. |
| 7. Repeated disinfection durability† | Respirators should be durable enough to provide expected levels of protection after 50 disinfections, each taking < 60 seconds to complete. |
| 8. Shelf-life durability† | Respirators should be durable enough to provide expected levels of protection after being stored in air-conditioned space for 10 years at 21°C-23°C (69°F-73°F) and 45%-55% relative humidity. |
| 9. Gauging fit† | Respirators should have a manufacturer-specified fit assessment technique (eg, a user seal check) that is capable of detecting inadequate fit (which would result in less than expected protection) with at least 75% accuracy during work activities. |
| 10. Hearing integrity† | Respirators should not impede, and preferably improve, the wearer’s ability to hear in a hospital environment. |
| 11. Speech intelligibility† | Respirators should not impede, and preferably improve, the ability of others to hear the wearer’s spoken words. |
| 12. Visual field† | Respirators should cause minimal obstruction of the wearer’s visual field. |
| 13. Facial visualization† | Respirators should be transparent, to the extent feasible, allowing visualization of the wearer’s face. |
| 14. Equipment compatibility | Respirators should not interfere with other equipment (eg, stethoscope) used in health care. |
| 15. Breathing resistance | Respirators should have a breathing resistance (eg, filter air flow resistance) low enough that it does not impact tolerance (eg, should be < 10-mm water pressure drop on average at 85 liters per minute). |
| 16. Facial irritation | Respirators should not cause facial irritation. |
| 17. Allergenicity | Respirators should not cause allergic reactions. |
| 18. Facial pressure† | Respirators should be constructed such that they cause minimal discomfort from pressure on the face (eg, facial pressure should be low enough to be comfortable and tolerable for (1) >2 hours of uninterrupted wear and (2) >8 hours with 15-minute break periods every 2 hours). |
| 19. Facial heat† | Respirators should be constructed such the level of facial heat rise is low enough to be comfortable for (1) >2 hours of uninterrupted wear and (2) >8 hours with 15-minute break periods every 2 hours. |
| 20. Air exchange† | Respirators should be constructed such that they have adequate air exchange from the environment and do not cause unnecessary buildup of respiratory gases (eg, CO2 dead space retention should be low enough to be comfortable for (1) >2 hours of uninterrupted wear and (2) >8 hours with 15-minute break periods every 2 hours. |
| 21. Moisture management† | Respirators should be constructed such that they have adequate air exchange from the environment and do not cause unnecessary buildup of humidity in the dead space (eg, respirator dead space humidity levels should be maintained at levels perceived as comfortable for (1) >2 hours of uninterrupted wear and (2) >8 hours with 15-minute break periods every 2 hours. |
| 22. Mass features† | Respirators should be positioned on the face in a fashion that is comfortable and tolerable for (1) >2 hours of uninterrupted wear and (2) >8 hours with 15-minute break periods every 2 hours. Respirator weight and mass distribution should be evaluated with a standardized and validated practical performance test for which performance criteria are developed. |
| 23. Odor† | Respirators should be non-malodorous. |
| 24. Prolonged tolerability† | Respirators should be comfortable enough to be worn for a prolonged period of time during a crisis (eg, for 10 consecutive days under the following circumstances: (1) >2 hours of uninterrupted wear and (2) >8 hours with 15-minute break periods every 2 hours). |
| 25. Employer desirability† | Respirators should be viewed by employers as an important and desirable component of their worker safety and infection control programs. |
| 26. Employee desirability† | Respirators should be viewed by employees as an important and desirable component of their workplace safety and infection control programs. |
| 27. Patient desirability† | Respirators should be viewed by patients/visitors as an important and desirable component of workplace safety and infection control programs. |
| 28. Cost-effective for employers† | Respirator usage should be cost-effective. |
Adapted from the Project BREATHE final report by editing for clarity, combining objectives and recommendations, and removing the column with current standards.
Not a current NIOSH/FDA Surgical N95 requirement.
Four key themes emerged from the Project BREATHE Working Group: (1) respirators should perform their intended function safely and effectively; (2) respirators should support, not interfere, with occupational activities; (3) respirators should be comfortable and tolerable for the duration of wear; and (4) respiratory protective programs should comply with federal standards and guidelines, state regulations, and local policies. The characteristics in Table 2 largely follow this framework, including additional recommendations related to health care policies and procedures.
Table 2 also identifies which of these requirements are currently used in the certification and testing of Surgical N95 respirators that have approval by NIOSH as an N95 respirator and are cleared by the FDA for sale as a surgical mask. Only 6 of the 28 Project BREATHE characteristics are currently evaluated with Surgical N95 respirators, suggesting that adoption of these characteristics in future respirator designs should help improve respirator compliance among HCP. It should be noted that some of the Project BREATHE characteristics in Table 2 can be met today with other respirator types. For example, loose-fitting, hooded, powered air-purifying respirators (PAPR) do not require fit testing and thus meet the desirable characteristics related to fit and gauging fit, whereas elastomeric half-mask (EHM) respirators with a particulate filter cartridge would readily meet characteristics 6 through 8. Whereas some respirator types meet a subset of the Project BREATHE characteristics, no single device today meets all of them nor could all of the recommendations be met by a single device. The health care environment is not only unique from other industries, but there are many niche settings that require different features for an idealized product. The Working Group recognized that meeting all 28 characteristics simultaneously was improbable because some of the recommendations were conflicting when applied concurrently but decided to publish all of them to provide maximum flexibility for subsequent users of the report to balance trade-offs and to not appear to favor any one type of respirator (eg, Surgical N95, PAPR, EHM, and others).
The Working Group attempted to specify how these requirements might be tested and what level of performance might be acceptable to HCP but recognized that many of the proposed test methods still needed to be developed. In general, the Working Group agreed that there is a preference for clinical assessment methods over methods performed solely in a laboratory. In such cases in which this is not practical, laboratory tools that have been validated against clinical outcomes would be acceptable. Further discussion about test methods and performance requirements will be the subjects of future reports.
B95 respirator
An overarching recommendation from the Project BREATHE Working Group was that a new class of respirator should be developed to address the unique needs of the health care community. The health care environment differs greatly from many other settings that require the usage of respiratory protection. The next generation of devices used should thoroughly reflect this difference. For example, the filtered particulates in health care settings are mainly infectious biologic aerosols, rather than nonbiologic aerosols. These biologic aerosols present unique challenges to ensure that the respirator does not contribute to disease transmission. Furthermore, current methods of testing respirators may not represent how respirators are used in health care. For example, a controlled environment, such as a hospital, does not have the levels of airborne dust and particulate matter typically seen at a manufacturing plant. Any respirator designed specifically for HCP should focus on diminishing infection from exposures to biologic hazards as its primary goal and focus less on particulate load capacity or other enhancing features, such as exhalation valves that could potentially contaminate the surgical field and are not compatible with many health care settings. With approximately 13 million HCP in the United States, many of whom will need to use respiratory protection during public health emergencies, the market for health care respirators should be large enough to warrant devices meeting their unique needs.
To correspond with the widely used N-P-R classification scheme used for NIOSH respirator certification,41 the Working Group believed that the term “Biological 95” or “B95” should be used to characterize this new class of respirators developed specifically for health care. The term “B95” will also serve as a reminder of its historical lineage to Project BREATHE. In the future, respirators developed, marketed, and recognized as meeting the requirements of a B95 respirator could enable hospitals to purchase respirators that are more likely to be used correctly. We believe that wider acceptance and improved compliance resulting from B95 respirator use will ultimately lead to better patient care, reduced HAIs, and fewer workers inhaling infectious biologic aerosols.
Because of the many new desirable characteristics of a B95 respirator, manufacturers may choose to combine features from one or more existing types of respirators. One B95 respirator option discussed by the Working Group to satisfy conflicting desirable characteristics would be a “hybrid” respirator combining features of Surgical N95 and EHM respirators, such that it would be disposable for routine use but reusable during a public health emergency (eg, pandemic). B95 respirator options that are “scalable” would be desirable under these circumstances. This might include a lightweight, relatively simple Surgical N95 respirator, equipped with a small fan to encourage air exchange and facial cooling, which could be temporarily added when necessary.
Path forward
Unfortunately, the global development of respiratory protection devices has not evolved enough to support the mass production of a “B95” respirator meeting many of the desired characteristics. The 28 recommendations from the Project BREATHE Working Group would serve well as framework for a national strategy of research and standards development, leading toward commercialization of B95 respirators designed specifically for health care. To implement the recommendation, 3 inter-related efforts are needed:
-
1.
Develop clinically validated B95 test methods: Research and development organizations such as respirator manufacturers, university researchers, and federal agencies, including the Department of Defense, National Institute for Standards and Technology, and NIOSH, are encouraged to develop test methods to measure and quantify the generic Project BREATHE characteristics in Table 2. To know which respirators are most comfortable or achieve the best fit, validated test methods are necessary. In particular, the science that underlies the understanding of factors affecting respirator comfort and tolerability is not well defined. Ultimately, laboratory-based test methods will need to be validated against clinical outcomes. Recent advances from NIOSH32, 42, 43, 44 and VA27, 30 in assessing respirator comfort and tolerability are promising, and Department of Defense45, 46 and VA47 scientists have developed unique approaches to assess hearing and speech intelligibility.
-
2.
B95 standards development: Purchasing and procurement decisions for PPE are typically based on voluntary consensus standards, such as those developed by the American Society for Testing and Materials, National Fire Protection Association, International Organization for Standardization, American National Standards Institute, and the Association for the Advancement of Medical Instrumentation or government regulations (eg, NIOSH, FDA, OSHA).48 As noted in the 2010 IOM report on Certifying Personal Protective Technologies, many parties benefit from a well-written standard. In the short-term (within the next 5 years), VA and its partners should work with voluntary consensus standards development organizations to develop a B95 respirator standard that incorporates clinically validated test methods, wherever possible. There is precedent for dual certification of respirators that allows the employer to meet OSHA requirements to use a NIOSH-certified respirator while obtaining additional features desired by the end user. For example, in other occupational settings such as firefighting, it is not uncommon to set voluntary consensus standards that exceed minimal performance standards set by government agencies. A good example of this is the National Fire Protection Association 1981 standard for self-contained breathing devices,49 which requires NIOSH certification as the baseline. The Federal government sets the minimum set of general requirements for devices used by any worker in any type of workplace setting, but the standards development organizations set special, additional requirements that benefit workers in unique workplace settings. The health care setting is one of those unique workplace environments. Obtaining a B95 standard will not be mandatory for manufacturers but, instead, will be optional. B95 respirators will be the best in class in terms of comfort and fit and tailored for HCP, and thus it will be desirable for manufacturers to have this designation for their products. Hospitals will benefit from more certainty in respirator fit, higher compliance, and less absenteeism. A more long-term effort (>5 years) will include work by OSHA, NIOSH, and FDA to reduce barriers to better performing respirators in health care caused by any outdated, unnecessary, or burdensome federal regulations.
-
3.
B95 prototype development: Respirator manufacturers and other research organizations are urged to conduct research and develop prototype devices that incorporate innovative design features such as more breathable filter media, adhesives for improving respirator fit, and devices for cooling and air management that will meet the desirable characteristics in Table 2. Manufacturers, in particular, are encouraged to work with HCP to better understand the challenges faced when wearing respirators while providing patient care and to develop novel solutions. To expedite this effort, US respirator manufacturers were recruited via a Federal Register notice to partner with VA to design and build new prototype B95 respirators, using the Project BREATHE recommendations as guidance. Cooperative Research and Development Agreements were signed in 2012 with 2 manufacturers, and the first prototypes produced by these collaborations are expected in 2013. As a first step, VA plans to test the prototypes in a health care simulator laboratory for usability, communication, and comfort, whereas NIOSH has agreed to conduct laboratory testing involving human test subjects to assess fit and comfort. If fully successful, Project BREATHE will eventually result in the production of at least 1 respirator that addresses some or many of the specific needs of the health care community. Performing B95 prototype development with a select group of leading manufacturers, in parallel with test method and standards development, should help bring new and emerging respirator technologies to the marketplace. End-user feedback obtained by VA and NIOSH on comfort and tolerability will be essential before these devices are commercialized, ensuring that the subjective nature of comfort and tolerability will be thoroughly assessed. Involving end users in the early stages of conceptualization tends to decrease both time and money required to move a product from an idea to the marketplace.50
Conclusion
Respirators commonly used in the US health care setting were not originally developed with HCP in mind; rather, they were borrowed from other industries, such as manufacturing and construction. A health care-specific B95 respirator should address the unique needs of this environment and meet the desires of HCP. Collaborative efforts involving technology-leading US manufacturers and forward-looking health care organizations are needed to optimize proficient development of clinically validated test methods, promulgation of B95 respirator standards, and invention of novel design features, which together should lead the B95 to commercialization. Hopefully, the collaborative application of science, policy, and workplace practices will synergize development of a new generation of B95 respirators specifically designed for health care, leading to increased compliance and reduced risk for occupationally acquired respiratory infections.
Footnotes
The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the US government. Mention of trade names or commercial products does not constitute their endorsement by the US government.
Conflicts of interest: None to report.
References
- 1.Sammer C.E., Lykens K., Singh K.P., Mains D.A., Lackan N.A. What is patient safety culture? A review of the literature. J Nurs Scholarsh. 2010;42:156–165. doi: 10.1111/j.1547-5069.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuster S.P., Shah P.S., Coleman B.L., Lam P.-P., Tong A., Wormsbecker A. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS ONE. 2011;6:e26239. doi: 10.1371/journal.pone.0026239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise M.E., De Perio M., Halpin J., Jhung M., Magill S., Black S.R. Transmission of pandemic (H1N1) 2009 influenza to healthcare personnel. Clin Infect Dis. 2011;52:S198–S204. doi: 10.1093/cid/ciq038. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society Respiratory protection guidelines. Am J Respir Crit Care Med. 1996;154:1153–1165. doi: 10.1164/ajrccm.154.4.8887621. [DOI] [PubMed] [Google Scholar]
- 5.Birkner J.S., Colton C.E. Respiratory protective equipment. Patty's Industrial Hygiene. 2011:1169–1233. [Google Scholar]
- 6.Doney B.C., Groce D., Campbell D.L., Greskevitch M.F., Hoffman W.A., Middendorf P.J. A survey of private sector respirator use in the United States: an overview of findings. J Occup Environ Hyg. 2005;2:267–276. doi: 10.1080/15459620590949020. [DOI] [PubMed] [Google Scholar]
- 7.Doney B., Greskevitch M., Groce D., Syamlal G., Moon B.K., Masurek J. Improving respiratory protection programs in healthcare to reduce and control infection. Infect Control Today. 2009 http://www.infectioncontroltoday.com/articles/2009/06/improving-respiratory-protection-programs-in-heal.aspx Available from: Accessed October 15, 2012. [Google Scholar]
- 8.Krishnan U., Janicak C.A. Compliance with OSHA’s respiratory protection standard in hospitals. Am Ind Hyg Assoc J. 1999;60:228–236. doi: 10.1080/00028899908984440. [DOI] [PubMed] [Google Scholar]
- 9.CDC Tuberculosis elimination revisited: obstacles, opportunities, and a renewed commitment. Advisory Council for the Elimination of Tuberculosis (ACET) MMWR Morb Mortal Wkly Rep. 1999;48:1–13. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4809a1.htm Available from: Accessed October 15, 2012. [PubMed] [Google Scholar]
- 10.CDC Guidelines for preventing the transmission of Mycobacterium tuberculosis in health care facilities. MMWR Morb Mortal Wkly Rep. 1994;43:1–132. http://www.cdc.gov/mmwr/preview/mmwrhtml/00035909.htm Available from: Accessed October 16, 2012. [PubMed] [Google Scholar]
- 11.Fennelly K.P. Personal respiratory protection against Mycobacterium tuberculosis. Clin Chest Med. 1997;18:1–17. doi: 10.1016/s0272-5231(05)70352-x. [DOI] [PubMed] [Google Scholar]
- 12.Spruce L., Braswell M. Implementing AORN recommended practices for electrosurgery. AORN J. 2012;95:373–387. doi: 10.1016/j.aorn.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 13.National Personal Protective Technology Laboratory. Respirator trusted-source information. Available from: http://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/RespSource3healthcare.html. Accessed January 30, 2013.
- 14.Occupational Safety and Health Administration. Pandemic influenza preparedness and response guidance for healthcare workers and healthcare employers. OSHA 3328–05R. 2009. Available from: http://www.osha.gov/Publications/OSHA_pandemic_health.pdf. Accessed January 30, 2013.
- 15.Siegal J.D., Rhinehart E., Jackson M., Chiarello L., Health Care Infection Control Practices Advisory Committee 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Am J Infect Control. 2007;35(10 Suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.California Division of Occupational Safety and Health. California Code of Regulations, Title 8; Section 5199. Aerosol Transmissible Disease (ATD) Standard. April 15, 2010.
- 17.CDC Cluster of severe acute respiratory syndrome cases among protected health care workers: Toronto, Canada, April 2003. MMWR Morb Mortal Wkly Rep. 2003;52:433–436. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5219a1.htm Available from: Accessed October 18, 2012. [PubMed] [Google Scholar]
- 18.Institute of Medicine . National Academies Press; Washington [DC]: 2009. Respiratory protection for healthcare workers in the workplace against novel H1N1 influenza A: a letter report. [PubMed] [Google Scholar]
- 19.SHEA, IDSA, APIC. Letter to President Barack Obama. November 5, 2009. Available from: www.idsociety.org/WorkArea/DownloadAsset.aspx?id=15676. Accessed January 30, 2013.
- 20.Martin S.D. Nurses’ ability and willingness to work during pandemic flu. J Nurs Manage. 2011;19:98–108. doi: 10.1111/j.1365-2834.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine . National Academies Press; Washington [DC]: 2008. Preparing for an influenza pandemic: personal protective equipment for healthcare workers. [Google Scholar]
- 22.American Industrial Hygiene Association Respiratory Protection Committee. Respirator performance terminology. Available from: http://aiha.org/insideaiha/volunteergroups/RPC/Documents/rpc-terms.pdf. Accessed October 18, 2012.
- 23.Occupational Health and Safety Administration. Assigned protection factors for the revised respiratory protection standard. OSHA 3352-02. 2009. Available from: http://www.osha.gov/Publications/3352-APF-respirators.pdf. Accessed January 30, 2013.
- 24.Mitchell R., Ogunremi T., Astrakianakis G. Impact of the 2009 influenza A (H1N1) pandemic on Canadian health care workers: a survey on vaccination, illness, absenteeism, and personal protective equipment. Am J Infect Control. 2012;40:611–616. doi: 10.1016/j.ajic.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine . National Academies Press; Washington [DC]: 2011. Preventing transmission of pandemic influenza and other viral respiratory diseased. [PubMed] [Google Scholar]
- 26.Baig A.S., Knapp C., Eagan A.E., Radonovich L.J., Jr. Health care workers’ views about respirator use and features that should be included in the next generation of respirators. Am J Infect Control. 2010;38:18–25. doi: 10.1016/j.ajic.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radonovich L.J., Jr., Cheng J., Shenal B.V., Hodgson M., Bender B.S. Respirator tolerance in health care workers. JAMA. 2009;301:36–38. doi: 10.1001/jama.2008.894. [DOI] [PubMed] [Google Scholar]
- 28.Moore D.M., Gilbert M., Saunders S., Bryce E., Yassi A. Occupational health and infection control practices related to severe acute respiratory syndrome: health care worker perceptions. AAOHN J. 2005;53:257–266. [PubMed] [Google Scholar]
- 29.Radonovich L.J., Jr., Perl T.M., Davey V., Cohen H. Preventing the soldiers of health care from becoming victims on the pandemic battlefield: respirators or surgical masks as the armor of choice. Disaster Med Public Health Prep. 2009;3:S203–S210. doi: 10.1097/DMP.0b013e3181be830c. [DOI] [PubMed] [Google Scholar]
- 30.Shenal B.V., Radonovich L.J., Jr., Cheng J., Hodgson M., Bender B.S. Discomfort and exertion associated with prolonged wear of respiratory protection in a health care setting. J Occup Environ Hyg. 2012;9:59–64. doi: 10.1080/15459624.2012.635133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Tojura H., Guo Y.P. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int Arch Occup Environ Health. 2005;78:501–509. doi: 10.1007/s00420-004-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberge R.J., Coca A., Williams W.J., Powell J.B., Palmiero A.J. Physiological impact of the N95 filtering facepiece respirator on healthcare workers. Respir Care. 2010;55:569–577. [PubMed] [Google Scholar]
- 33.Fukakusa J., Rosenblat J., Jang B., Ribeiro M., Tarlo S.M. Factors influencing respirator use at work in respiratory patients. Occup Med. 2011;61:576–582. doi: 10.1093/occmed/kqr132. [DOI] [PubMed] [Google Scholar]
- 34.Thomas F., Butts A.C., Rhoades W., Brandon C., Handrahan D.L. Does wearing a surgical facemask or N95-respirator impair radio communication? Air Med J. 2011;30:97–102. doi: 10.1016/j.amj.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Coates M.J., Jundi A.S., James M.R. Chemical protective clothing; a study into the ability of staff to perform lifesaving procedures. J Accid Emerg Med. 2000;17:115–118. doi: 10.1136/emj.17.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng P.W., Wong D.T., Bevan D., Gardam M. Infection control and anesthesia: lessons learned from the Toronto SARS outbreak. Can J Anesth. 2003;50:989–997. doi: 10.1007/BF03018361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMahon E., Wada K., Dufresne A. Implementing fit testing for N95 filtering facepiece respirators: practical information from a large cohort of hospital workers. Am J Infect Control. 2008;36:298–300. doi: 10.1016/j.ajic.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee K., Slavcev A., Nicas M. Respiratory protection against Mycobacterium tuberculosis: quantitative fit test outcomes for five type N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1:22–28. doi: 10.1080/15459620490250026. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhury H., Mahmood A., Valente M. The effect of environmental design on reducing nursing errors and increasing efficiency in acute care settings. Environ Behav. 2009;41:755–786. [Google Scholar]
- 40.Department of Veterans Affairs. Better respiratory equipment using advanced technologies for healthcare employees (Project B.R.E.A.T.H.E.). 2012. Available from: http://www.publichealth.va.gov/docs/cohic/project-breathe-report-2009.pdf. Accessed October 16, 2012.
- 41.Code of Federal Regulations Respirator protection devices. Title 42, CFR, Part 84. Washington [DC]: Office of the Federal Register. 1995;60:30337–30404. [Google Scholar]
- 42.Roberge R.J., Kim J.H., Benson S. N95 filtering facepiece respirator dead space temperature and humidity. J Occup Environ Hyg. 2012;9:166–171. doi: 10.1080/15459624.2012.660428. [DOI] [PubMed] [Google Scholar]
- 43.Roberge R.J. Are exhalation valves on N95 filtering facepiece respirators beneficial at low-moderate work rates: an overview. J Occup Environ Hyg. 2012;9:617–623. doi: 10.1080/15459624.2012.715066. [DOI] [PubMed] [Google Scholar]
- 44.Roberge R.J., Kim J.H., Coca A. Protective facemask impact on human thermoregulation: an overview. Ann Occup Hyg. 2012;56:102–112. doi: 10.1093/annhyg/mer069. [DOI] [PubMed] [Google Scholar]
- 45.Coyne K.M., Barker D.J. ECBC-TR-779. US Army Edgewood Chemical and Biological Center Aberdeen Proving Ground Report; Aberdeen [MD]: June 2010. Speech intelligibility while wearing civilian full-facepiece air-purifying respirators. [Google Scholar]
- 46.Caretti D.M., Coyne K.M. ECBC-TR-605. US Army Edgewood Chemical and Biological Center Aberdeen Proving Ground Report; Aberdeen [MD]: January 2008. Reassessment of human performance parameter estimates for respiratory protection design and development. [Google Scholar]
- 47.Radonovich L.J., Jr., Yanke R., Chang J., Bender B. Diminished speech intelligibility associated with certain types of respirators worn by healthcare workers. J Occup Environ Hyg. 2010;7:63–70. doi: 10.1080/15459620903404803. [DOI] [PubMed] [Google Scholar]
- 48.Institute of Medicine . National Academies Press; Washington [DC]: 2010. Certifying personal protective technologies: improving worker safety. [PubMed] [Google Scholar]
- 49.National Fire Protection Association. NFPA, 1981 . 2007 Ed. Batterymarch Park; Quincy [MA]: 2007. Standard on open-circuit self-contained breathing apparatus (SCBA) for fire and emergency services. [Google Scholar]
- 50.Saiedian H., Dale R. Requirements engineering: making the connection between software developer and customer. Inform Software Tech. 2000;42:419–428. [Google Scholar]