Abstract
Objective
To describe an outbreak of influenza A in an oncology unit, highlighting infection control methods implemented, and examining reasons health care workers (HCWs) present to work with influenza-like illness (ILI).
Methods
Confirmed cases were defined by the presence of ILI and a positive nasopharyngeal polymerase chain reaction swab for influenza A H3. Probable cases were defined as exposed HCWs with ILI who were unavailable for polymerase chain reaction testing. Infection prevention measures included closing the ward for new admissions, oseltamivir prophylaxis for all exposed groups, and dismissal from work of HCWs with ILI until resolution of symptoms. An anonymous survey of the cases in our HCWs was conducted to better elucidate reasons behind presenteeism.
Results
Over the course of 8 days (November 16, 2017, to November 22, 2017), influenza was diagnosed in 7 of 10 inpatients on the oncology ward, 16 HCWs (14 confirmed, 2 probable), and 2 visitors. The suspected index case was an HCW. Of the surveyed HCWs, 64% presented to work despite feeling ill (ie, presenteeism). The most common reason was “sense of duty as a health care worker.”
Conclusions
This nosocomial outbreak of influenza highlights the challenges of protecting inpatients from viral respiratory tract infections. HCWs and patient visitors with ILI should avoid work or visiting until resolution of peak respiratory symptoms and adhere to strict respiratory etiquette.
Key Words: Hospital epidemiology, Presenteeism
Seasonal influenza epidemics in the United States result in 140,000-710,000 hospitalizations and 12,000-56,000 deaths annually, with the highest morbidity occurring among persons of extreme age and those who are immunocompromised.1 The 2017-2018 influenza season in the United States saw record-high laboratory-confirmed cases, as well as high rates of both hospitalizations and deaths following infection.1 Exacerbating this was that the overall vaccine efficacy was estimated to be 36%, and the efficacy against influenza A H3N2 (the predominant circulating strain) was around 25%.2
It is important to note that nosocomial acquisition of influenza is a significant contributor to annual rates, accounting for up to 17% of influenza cases in the United States.3 Nosocomial influenza outbreaks are challenging to contain, as there is considerable temporal overlap of inpatient stays within the facility.4 In addition, visitors and health care workers (HCWs) can continuously introduce respiratory viruses to inpatients, particularly if proper hand hygiene and respiratory etiquette are not followed.1 The transmission of influenza from HCWs to patients is well described and is an important source for targeted prevention efforts.5
Outbreaks among groups of immunocompromised persons create unique challenges due to prolonged viral shedding and atypical presentations.4, 6 In fact, a previously described outbreak of influenza H3N2 in an ambulatory stem cell transplant center found that only 7 of 31 patients (23%) diagnosed with influenza A H3N2 met the Centers for Disease Control and Prevention (CDC) influenza-like illness (ILI) case definition.6
Unfortunately, published investigations involving nosocomial outbreaks among adult immunocompromised groups remain limited. We report an influenza A H3N2 outbreak (influenza A H3) among inpatients, visitors, and employees on our oncology ward that is postulated to have been introduced by an HCW attending work while ill. We also describe aggressive containment efforts instituted by our team of infection preventionists to limit the spread of disease.
Methods
Outbreak setting
On November 19, 2017, the infection control team was notified of a potential influenza outbreak, as 3 oncology inpatients admitted for 5 days were diagnosed with influenza A H3 between November 16, 2017, and November 18, 2017. The oncology ward contains 14 single patient rooms and an open access multipurpose room where patients and visitors can prepare meals and socialize. There is a single point of entry without direct access to other units so as to prevent through traffic. Transportation of patients to and from other locations in the hospital is limited. However, HCWs often disperse to other locations in the hospital (ie, other units, outpatient clinics, and administrative offices), and patient visitors are not limited. At the time an outbreak was identified, 10 of 14 beds on the ward were occupied. Although the exact number of all HCWs with some exposure to the ward remains uncertain, we were able to confirm 39 nurses were assigned to the unit during the outbreak period of interest, November 14, 2017, to November 27, 2017 (2 days prior to the first patient case until the last day admissions were diverted).
Case definitions and identification
The population screened for influenza in our outbreak investigation consisted of inpatients, visitors, and HCWs, including physicians, nurses, patient care technicians and corpsman, clinical pharmacists, social workers, and hospital administrative personnel. Information was collected both via an anonymous staff survey and medical record review.
Confirmed cases were defined as any inpatient, visitor, or HCW with an ILI and a positive multiplex polymerase chain reaction (PCR) test for influenza A H3. The definition of ILI used by the US Outpatient Influenza-Like Illness Surveillance Network is a fever ≥100°F and a cough or sore throat.7 However, knowing persons with influenza often present without fever, we defined ILI as 1 or more symptoms of cough, fever, sore throat, myalgias, or shaking chills in an effort to capture more cases.8
Testing for influenza was collected via flocked nasal swab from the nares using the FilmArray Respiratory Panel (RP) 1.0 (BioFire Diagnostics, Salt Lake City, UT), which evaluates for 21 common community-acquired respiratory pathogens, including influenza A, influenza A H1, influenza A H1 2009, influenza A H3, and influenza B. The designation of nosocomial acquisition of influenza A H3 required patients be admitted for at least 5 days prior to diagnosis.
Nasopharyngeal specimens were transported to the microbiology laboratory in universal transport media (UTM; Quidel, San Diego, CA). In accordance with FilmArray RP 1.0 protocols and validated procedures, specimens were subjected to a nested PCR and a melting curve analysis to determine the presence or absence of influenza A, influenza A H3, or influenza A H1 2009.9 Probable cases were defined as exposed persons who had ILI at the time of the outbreak but were not available for testing with the FilmArray RP 1.0 PCR panel.
Exposed persons were defined as those who had been working on the oncology ward within 2 days prior to when the first patient developed symptoms or persons who had direct exposure to a confirmed or probable influenza case. Direct exposure was defined as unprotected contact within 3-6 feet for more than 1 hour and was derived from the CDC definition of close contacts.10
Infection control and prevention interventions
The day that an outbreak was identified, November 19, 2017, all inpatients on the oncology ward were placed on droplet isolation and further admissions to the ward were diverted until November 27, 2017, 8 days (2 influenza incubation periods) after the last patient case was identified on the oncology ward (November 19, 2017). All inpatients who were housed on the oncology ward were tested for influenza using FilmArray RP 1.0. Inpatients with confirmed influenza A were treated with 75 mg of oseltamivir orally twice daily for 10 days, owing to the potential for prolonged viral shedding in immunocompromised patients.11, 12 Inpatients who were asymptomatic and tested negative for influenza were administered a standard prophylactic course of 75 mg of oseltamivir orally for 10 days.13
Beginning November 19, 2017, HCWs who were assigned to the oncology ward were screened for ILI symptoms prior to starting their shift. During the investigation period, November 19, 2017, to November 27, 2017, symptomatic HCWs were tested for influenza using FilmArray RP 1.0, if available. Symptomatic HCWs with ILI and either confirmed or probable influenza infection were treated with 75 mg of oseltamivir orally twice a day for 5 days and excluded from work until symptom resolution. Symptom resolution was defined as resolution of fevers for 24 hours without the use of antipyretics or resolution of peak respiratory symptoms for 24 hours. Once HCWs returned to work, they were advised to wear a surgical mask until their respiratory symptoms were completely resolved (derived from CDC guidance).14 Exposed symptomatic HCWs with negative influenza testing and exposed asymptomatic HCWs were offered a prophylactic course of oseltamivir (75 mg by mouth daily for 10 days).
Other groups of HCWs who are not necessarily directly assigned to the oncology ward (physicians, ancillary medical staff, social workers, and hospital administrative staff) were notified through their supervisors of the outbreak and asked to report for testing in the occupational health department or the infectious diseases clinic if symptomatic with ILI. Active surveillance for ILI in HCWs assigned to the oncology ward continued throughout the influenza season; however, we did not administer prophylaxis after November 27, 2017.
Family members who self-reported ILI were instructed to report to their primary care providers for influenza testing and to avoid visiting until symptom resolution as defined above. Those who were asymptomatic were recommended to consider oseltamivir prophylaxis. To increase awareness among family members and visitors and prompt self-reporting, the entrance to the ward contained information about influenza symptoms, guidance for symptomatic visitors, surgical face masks, and hand sanitizer.
Additionally, every room on the hematology oncology unit was sanitized with Virex II 256 (Diversey, Charlotte, NC), followed by ultraviolet light disinfection with Tru-D (Tru-D SmartUVC, Memphis, TN). Housekeeping was advised to focus daily attention to “high-touch surfaces” in the rooms of patients, and the nurses’ station (including phones and keyboards) was cleaned every shift with CaviWipes (Metrex Research, Orange, CA).
Over the course of the outbreak, multidisciplinary leadership meetings were conducted with infection control, preventive medicine, occupational medicine, emergency management, nursing and physician leadership, pharmacy, and laboratory services to ensure a coordination of efforts.
Staff survey
An anonymous survey was conducted of all staff who had confirmed or probable influenza to characterize symptoms, timeline of illness, and to describe the reasons why ill HCWs may have presented to work despite their symptoms (prior to the implementation of screening staff for symptoms). The information collected included: sex, age, type of employment (active duty vs contractor vs general contractor civilian), job title, influenza vaccination status, date of symptom onset, duration of symptoms, whether any work was missed, specific symptoms (eg, fever, muscle aches, cough, sore throat, runny nose, nasal congestion, chest congestion, headache, vomiting, and diarrhea), primary place of work, dates working or visiting on the oncology ward, and if direct patient care was provided. The survey also investigated whether the staff reported to work despite feeling ill and the reasons for working while ill. The reasons for working while ill included: sense of duty as an HCW, viewed illness as too minor to pose risk to others, did not want to incur repercussions from leadership or coworkers, did not want to consume sick leave, and a free text option.
Results
Ultimately, 7 of 10 inpatients on the oncology ward were diagnosed with influenza A H3 by FilmArray RP 1.0 (attack rate of 70%). The last diagnosis of influenza A in an inpatient was on November 19, 2017. Unfortunately, an attack rate among the staff cannot be reliably calculated, because the exact number of all exposed HCWs in this outbreak is difficult to quantify owing to the frequency of visits from outside personnel (ie, pharmacists, social workers, chaplains, and consultants). Identification of influenza cases in HCWs who work in various locations throughout the hospital resulted in an exponential number of staff with potential exposure (ie, office mates and outpatient clinic staff), all of whom could not be accurately accounted for. Prophylactic oseltamivir was prescribed to 108 persons during the outbreak period, which we suspect exceeds the number of truly exposed persons, but also reflects the diversity of the staff involved. However, we were able to confirm 39 nurses were assigned to the ward between the dates of November 14, 2017 and November 27, 2017 (2 days prior to the first inpatient case through the end of the outbreak). The attack rate for nurses specifically was 8 of 39 or 21%.
Of the 50 HCWs for whom symptom data are available and exposure was confirmed, 16 of 50 (32%) tested positive for influenza (14 confirmed and 2 probable), 7 of 50 (14%) tested positive for an alternative organism by FilmArray RP 1.0 (6 rhinovirus/enterovirus and 1 coronavirus), and 26 of 50 (52%) tested negative for any organism (24 by FilmArray RP 1.0 and 2 self-reported by unknown tests). One of the symptomatic staff declined testing. In addition, 2 patient visitors (both spouses of influenza inpatients) were confirmed to be infected with influenza A H3 by FilmArray RP 1.0. These are the only 2 visitors for whom symptoms were self-reported during the outbreak period.
Word of the outbreak spread quickly, and numerous symptomatic and asymptomatic staff with questionable exposures presented to request evaluation or testing. We suspect that inappropriate testing may have been performed on some HCWs who were asymptomatic (or did not quite meet the criteria for exposure or for ILI) due in large part to a fear of the outbreak. Although 50 symptomatic staff with confirmed exposure are described above, the total number of FilmArray panels performed at our institution during the outbreak period far exceeded this number at 101.
Of the 22 virus samples that were available for culture and sequencing, all were identical, consistent with an outbreak scenario. Sequencing demonstrated a newly identified virus strain, which had 6 amino acid differences relative to the hemagglutinin of the A/Hong Kong/4801/2014 strain used in the 2017-2018 vaccine. The vaccine strain for 2017-2018 was a quadrivalent formulation comprised of an A/Michigan/45/2015 (H1N1), an A/Hong Kong/4801/2014 (H3N2), a B/Brisbane/60/2008-like virus (similar to the B/Victoria/2/87 lineage), and a B/Phuket/3073/2013-like virus (of the B/Yamagata/16/88 lineage).15 A total of 15 of 16 infected staff members with confirmed or probable disease had been immunized for influenza, whereas only 2 of 7 patients were vaccinated in 2017.
Of the 16 HCWs diagnosed with influenza, there were 8 nurses, 3 physicians, 1 corpsman (ie, medical technician), 1 pharmacist, 1 chaplain, 1 social worker, and 1 stem cell transplant coordinator. Of these HCWs, 14 completed the anonymous survey (13 with confirmed influenza, 1 with probable disease). The HCWs were all healthy and immunocompetent, although 1 was 11 weeks pregnant. The average duration of illness in the HCWs was 7.5 days, ranging from 3-15 days. The most frequently reported symptom was sore throat (in 12 of 14), followed by cough and myalgias (both in 11 of 14). Notably, fever was reported in only 50% of the HCWs (7 of 14 respondents).
Of the responses we received from the surveyed staff, 64% (9 of 14) continued to work despite feeling ill. The reasons for working included sense of duty, viewing illness as too minor to impose risk to others, and desire not to incur repercussions from leadership or coworkers. No one reported coming to work ill owing to a desire to not consume sick leave or issues with pay (Table 1 ). Of note, influenza was not the only respiratory virus brought into the ward by the HCWs, as 6 tested positive for rhinovirus/enterovirus and 1 tested positive for coronavirus.
Table 1.
Reasons for presenteeism (9 of 14 respondents)
| Why did you come to work feeling ill? (N (%) | |
| Sense of duty as a health care worker | 5/9 (56) |
| Viewed illness as too minor to pose risk to others | 4/9 (44) |
| Did not want to incur repercussions from leadership or coworkers | 2/9 (20) |
| Other (paperwork, results) | 2/9 (20) |
| Did not want to consume sick leave | 0 (0%) |
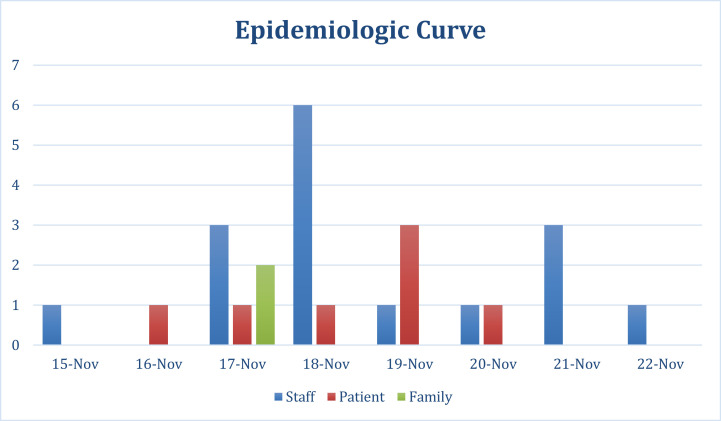
Because most patients and staff were tested within a 3-day span, our epidemiologic curve was based on reported or documented start of symptoms. This curve demonstrates there was an ill staff member who was symptomatic while working on the floor prior to the first ill patient. He was also working on the ward for 2 days preceding his symptom onset, suggesting this HCW may have been the index case (Fig 1 ). Most staff reported onset of symptoms prior to identification of an outbreak on November 19, 2017, which further supports HCW contribution to nosocomial spread. The suspected index case HCW had the longest duration of illness at 15 days, which has been shown to correlate with infectivity.16, 17
Fig 1.
Epidemiologic curve based on start of symptoms (family, green bar; patients, red bars; staff, blue bars). Nov, November.
No adverse outcomes from influenza illness were noted. One inpatient who tested positive for influenza A H3 died owing to his underlying end-stage malignancy. Death was imminent in this patient prior to his diagnosis of influenza, and testing was performed for outbreak investigation purposes alone. The remainder of the patients, staff, and family members recovered uneventfully. Additionally, no exposed HCW or visitor who was placed on prophylaxis with oseltamivir developed ILI, although surveillance was limited to self-reporting after the outbreak period.
Discussion
Acute viral respiratory tract infections, including influenza, have been associated with considerable morbidity and mortality following outbreaks in health care settings.4, 18 , 19 We describe an outbreak of influenza among patients, staff, and visitors on an oncology unit and demonstrate that HCWs presenting to work while ill contributed to the introduction and nosocomial spread of illness.
In 1 published account of an outbreak of influenza A H1N1 among hematology patients, a much lower attack rate was noted (30% vs 70% described here) with higher mortality (13% vs no attributable deaths described here).4 However, outbreaks of influenza A H3N2 described in other settings involving largely immunocompetent persons have reported widely variable attack rates (24%-80%) and clinical outcomes.5, 20 , 21 Disparity in attack rates and mortality is a reflection of the variation in influenza strain virulence and the baseline immune function of inpatients.22 Certainly, environmental factors and HCW behaviors also play a role in limiting or exacerbating spread of influenza in these circumstances.
The outbreak that we describe was short compared to other examples in the literature.4, 5, 6 , 23 We suspect the early identification, removal of ill HCWs, and universal use of oseltamivir prophylaxis, as recommended by the CDC, helped to reduce secondary cases.1, 24
Nosocomial outbreaks of influenza have been shown to consume considerable amounts of hospital resources.4 Ours was no different as we consumed $9,799 worth of oseltamivir, in treating the 23 cases, and prophylaxis of 108 people to contain the outbreak. Additionally, we spent an estimated $14,430 on laboratory costs for the FilmArray RP 1.0 (10 patients, 101 HCWs, and 2 visitors). This is an underestimate, based only on the cost of the FilmArray RP 1.0 pouch, excluding the cost of the swab, and the cost of time and labor both in the clinic and in the lab.
Furthermore, HCWs with influenza were sent home from work until their symptoms resolved, resulting in an incalculable loss of manpower hours over the course of the outbreak. This loss of highly specialized oncology nurses limited our ability to provide care for patients needing chemotherapy.
Presenteeism, defined as the practice of coming to work despite illness, in HCWs with ILI is a known concern.25, 26 We found that 64% of the HCWs in our outbreak continued to work despite having ILI and based on timing of symptoms, we suspect this outbreak was related to, or at least accelerated by, high rates of presenteeism. The suspected index patient in this outbreak was an HCW. It is possible that the nosocomial spread of influenza could have been prevented if the ill HCW either did not come to work or wore a mask. To limit nosocomial outbreaks of ILI in the future, it is important for health care facilities to re-educate all employees annually about the importance of avoiding work during peak respiratory symptoms and strict adherence to respiratory etiquette and hand hygiene.
The primary reasons the HCWs with ILI presented to work in this outbreak were “sense of duty as a health care worker” and “a feeling that their illness was too minor to be a problem,” and yet they contributed to an outbreak that caused significant cost and morbidity. Additionally, the HCWs were also found to be circulating other respiratory viruses in addition to influenza A. This further emphasizes a need for a culture change across the health care industry to limit the risk that ill HCWs pose to inpatients if, and when, they present for duty with an ILI.26
Banach et al27 describe the potential for patient visitors to contribute to nosocomial spread of influenza. Although the 2 family members who became infected in this outbreak did not cause the outbreak, this situation demonstrates the challenges in establishing practical visitation policies that limit the potential for visitors to contribute to nosocomial spread of ILI during influenza season.
CDC guidelines recommend HCWs with influenza can return to work 24 hours after their fevers resolved without the use of antipyretics.28 However, as fevers were not consistently reliable for detecting influenza in HCWs, more specific guidance is needed to help form policy.23 In addition to following the CDC guidance with regards to using fever resolution as a measure for returning to work, we also recommended any HCWs with ILI stay home until 24 hours after the peak of their respiratory symptoms. On return, we recommend wearing a surgical mask until the respiratory symptoms are completely resolved, as is standard.
This outbreak occurred because all but 1 of our staff had been vaccinated against influenza, consistent with the fact that this influenza A H3N2 was 6 amino acids different from that used in the vaccine. A recent study demonstrated an egg-adapted mutation at a key hemagglutinin glycosylation site, which led to poor neutralizing antibodies in humans and ferrets.29 This highlights the need to develop a “universal” influenza vaccine, which could protect against seasonal influenza drift variants as well as pandemic strains.30, 31
Our study has a few limitations. First, all symptoms documented by the HCWs in the anonymous survey were self-reported after their illness, which leaves them susceptible to some degree of recall bias. Second, we did not routinely test reportedly asymptomatic HCWs; therefore, we may have missed asymptomatic individuals who contributed to the outbreak. Third, our ability to detect symptoms in visitors was significantly limited as we relied on self-reporting. Furthermore, due to limited sample size, the external validity of our findings is restricted.
Finally, more than one-half of our HCWs with influenza were military members, which may have played a role in continuing to work despite feeling ill. If military personnel are ill, they are required to obtain written documentation from a medical professional prior to the start of their shift for “SIQ” or “sick in quarters.” In the absence of severe symptoms, this added administrative task may have contributed to working while ill, although this was not included in our survey.
Current CDC guidance allows HCWs with ILI to return to work 24 hours after resolution of fevers, yet many HCWs with influenza are never febrile. Moving forward, more definitive guidance is needed to clarify when it is safe for providers to resume patient care activities. Although resolution of fevers remains an important requirement, added verbiage focusing on resolution of peak respiratory symptoms may be beneficial. Our hospital policy in preparation for the 2018 influenza season now requires both the absence of fever as well as the resolution of peak respiratory symptoms, as detailed above, for return to work.
Conclusions
This nosocomial outbreak of influenza A H3N2 on an oncology ward was perhaps caused by, and at least accelerated by, HCWs presenting to work with ILI. It required significant resources to contain. Hospitals should maintain vigilance with regards to the local epidemiology of influenza in the community and educate HCWs and their supervisors about their capacity to contribute to nosocomial infections.
Acknowledgments
We would like to thank the charge nurses and the staff members of our oncology unit for their leadership, spirit of volunteerism in completing the survey, and adherence with infection prevention measures to curtail the outbreak. We would also like to thank our preventative medicine team, department of nursing, emergency management, and occupational health colleagues for their rapid response to the outbreak.
Footnotes
Conflicts of interest: None to report.
REFERENCES
- 1.Centers for Disease Control and Prevention. Influenza (flu). Available from: https://www.cdc.gov/flu/index.htm. Accessed December 12, 2017.
- 2.Flannery B, Chung JR, Belongia EA, McLean HQ, Gaglani M, Murthy K, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness—United States, February 2018. Am J Transplant. 2018;18:1020–1025. [Google Scholar]
- 3.Taylor G, Mitchell R, McGeer A, Frenette C, Suh KN, Wong A, et al. Healthcare-associated influenza in Canadian hospitals from 2006 to 2012. Infect Control Hosp Epidemiol. 2014;35:169–175. doi: 10.1086/674858. [DOI] [PubMed] [Google Scholar]
- 4.Pollara CP, Piccinelli G, Rossi G, Cattaneo C, Perandin F, Corbellini S, et al. Nosocomial outbreak of the pandemic influenza A (H1N1) 2009 in critical hematologic patients during seasonal influenza 2010-2011: detection of oseltamivir resistant variant viruses. BMC Infect Dis. 2013;13:127. doi: 10.1186/1471-2334-13-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eibach D, Casalegno JS, Bouscambert M, Bénet T, Regis C, Comte B, et al. Routes of transmission during a nosocomial influenza A(H3N2) outbreak among geriatric patients and healthcare workers. J Hosp Infect. 2014;86:188–193. doi: 10.1016/j.jhin.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Apewokin S, Vyas K, Lester LK, Haselow DT, Wolfe F, Roberts M, et al. Influenza A outbreak in an ambulatory stem cell transplant center. Open Forum Infect Dis. 2014;1 doi: 10.1093/ofid/ofu050. ofu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Influenza. Chapter 6. In: Manual for the surveillance of vaccine-preventable diseases. Atlanta (GA): Centers for Disease Control and Prevention; 2011. p. 6.1-6.12.
- 8.Chughtai AA, Wang Q, Dung TC, Macintyre CR. The presence of fever in adults with influenza and other viral respiratory infections. Epidemiol Infect. 2017;145:148–155. doi: 10.1017/S0950268816002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byington CL, Ampofo K, Stockmann C, Adler FR, Herbener A, Miller T, et al. Community surveillance of respiratory viruses among families in the Utah Better Identification of Germs-Longitudinal Viral Epidemiology (BIG-LoVE) study. Clin Infect Dis. 2015;61:1217–1224. doi: 10.1093/cid/civ486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Interim guidance on case definitions to be used for investigations of influenza A (H3N2) virus cases. Available from: https://www.cdc.gov/flu/swineflu/case-definitions.htm. Accessed October 15, 2018.
- 11.Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J Infect Dis. 2009;199:1435–1441. doi: 10.1086/598684. [DOI] [PubMed] [Google Scholar]
- 12.Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003;348:867–868. doi: 10.1056/NEJM200302273480923. [DOI] [PubMed] [Google Scholar]
- 13.Tamiflu (oseltamivir phosphate) [package insert]. Foster City (CA): Gilead Sciences; 2011.
- 14.Centers for Disease Control and Prevention. Interim guidance for the use of masks to control seasonal influenza virus transmission. Available from: https://www.cdc.gov/flu/professionals/infectioncontrol/maskguidance.htm. Accessed October 20, 2018.
- 15.Sullivan SG, Chilver MB, Carville KS, Deng YM, Grant KA, Higgins G, et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.43.17-00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau LL, Cowling BJ, Fang VJ, Chan KH, Lau EH, Lipsitch M, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201:1509–1516. doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bischoff WE, Swett K, Leng I, Peters TR. Exposure to influenza virus aerosols during routine patient care. J Infect Dis. 2013;207:1037–1046. doi: 10.1093/infdis/jis773. [DOI] [PubMed] [Google Scholar]
- 18.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkhy HH, Alenazi TH, Alshamrani MM, Baffoe-Bonnie H, Arabi Y, Hijazi R, et al. Description of a hospital outbreak of Middle East respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2016;37:1147–1155. doi: 10.1017/ice.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayers G, Igoe D, Carr M, Cosgrave M, Duffy M, Crowley B, et al. High morbidity and mortality associated with an outbreak of influenza A(H3N2) in a psycho-geriatric facility. Epidemiol Infect. 2013;141:357–365. doi: 10.1017/S0950268812000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosby MT, Pimentel G, Nevin RL, Fouad Ahmed S, Klena JD, Amir E, et al. Outbreak of H3N2 influenza at a US military base in Djibouti during the H1N1 pandemic of 2009. PLoS One. 2013;8:e82089. doi: 10.1371/journal.pone.0082089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117:5050–5056. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridgway JP, Bartlett AH, Garcia-Houchins S, Cariño S, Enriquez A, Marrs R, et al. Influenza among afebrile and vaccinated healthcare workers. Clin Infect Dis. 2015;60:1591–1595. doi: 10.1093/cid/civ163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Interim guidance for influenza outbreak management in long-term care facilities. Available from: https://www.cdc.gov/flu/professionals/infectioncontrol/ltc-facility-guidance.htm. Accessed March 14, 2017.
- 25.Mossad SB, Deshpande A, Schramm S, Liu X, Rothberg MB. Working despite having influenza-like illness: results of an anonymous survey of healthcare providers who care for transplant recipients. Infect Control Hosp Epidemiol. 2017;38:966–969. doi: 10.1017/ice.2017.91. [DOI] [PubMed] [Google Scholar]
- 26.Chiu S, Black CL, Yue X, Greby SM, Laney AS, Campbell AP, et al. Working with influenza-like illness: presenteeism among US health care personnel during the 2014-2015 influenza season. Am J Infect Control. 2017;45:1254–1258. doi: 10.1016/j.ajic.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banach DB, Bearman GM, Morgan DJ, Munoz-Price LS. Infection control precautions for visitors to healthcare facilities. Expert Rev Anti Infect Ther. 2015;13:1047–1050. doi: 10.1586/14787210.2015.1068119. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Prevention strategies for seasonal influenza in healthcare settings. Available from: https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed March 14, 2018.
- 29.Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114:12578–12583. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS. The pathway to a universal influenza vaccine. Immunity. 2017;47:599–603. doi: 10.1016/j.immuni.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med. 2018;378:7–9. doi: 10.1056/NEJMp1714916. [DOI] [PubMed] [Google Scholar]