Abstract
Hospital staff and all other human or veterinary health care workers, including laboratory, research, emergency service, or cleaning personnel are exposed to the risk of occupational infection following accidental exposure to blood or body fluids (BBF) contaminated with a virus, a bacteria, a parasite, or a yeast. The human immunodeficiency virus (HIV) or those of hepatitis B (HBV) or C (HCV) account for most of this risk in France and worldwide. Many other pathogens, however, have been responsible for occupational infections in health care workers following exposure to BBF, some with unfavorable prognosis. In developed countries, a growing number of workers are referred to clinicians responsible for the evaluation of occupational infection risks following accidental exposure. Although their principal task remains the evaluation of the risks of HIV, HBV, or HCV transmission and the possible usefulness of postexposure prophylaxis, these experts are also responsible for evaluating risks of occupational infection with other emergent or more rare pathogens and their possible timely prevention. The determinants of the risks of infection and the characteristics of described cases are discussed in this article.
The risk posed by “septic wounds” is well documented by pathologists since the 19th century. The realization of the risks linked with exposure to blood and body fluids (BBF) in hospitals, however, only became widespread with the advent of the human immunodeficiency virus (HIV)/AIDS pandemic. Any patient with viremia,1, 2 parasitemia,3 bacteriemia,4 or fungemia5 may potentially transmit a pathogen to a health care worker (HCW) either during a sharps injury (needlestick or cut by a scalpel blade or any other sharp object contaminated by BBF) or by mucocutaneous contact (MCC; BBF contact with nonintact skin or the mucosa of the eyes or mouth). We were repeatedly faced with challenging requests for postexposure expertise at Bichat-Claude Bernard University hospital in Paris, France. Although other pathogens (eg, monkeypox6) have been shown to be transmissible via needlestick in experimental animals, we found it useful to limit our efforts to compiling an updated and exhaustive list of BBP whose transmission was documented following occupational exposure in humans.
Methods
We proceeded to a complete review of the literature referenced since 1966 using Medline, Webspirs, CAB Abstracts, and Embase without language restrictions. The MeSH terms used were “stab wound,” “injury,” “health personnel,” “accidental blood disease,” “occupational disease,” “occupational exposure,” and “blood-borne pathogen.” Reference handbooks were consulted in extenso. The bibliographic references for each article, review, or book chapter were systematically researched. The inocula transmitted by blood transfusion, in the clinical laboratory, during accidental sharps injuries in the health care setting, or during dissection represent situations with rising transmissibility profiles ( Fig 1). When several cases of occupational infection of a given bloodborne pathogen were associated with various types of exposure, we described the case associated with the exposure with the lowest theoretic inoculum order to approximate the pathogen's transmissibility. Cases that occurred following exposure to aerosolized BBF were not included because it was not possible to differentiate between respiratory and mucocutaneous routes for transmission.
Fig 1.
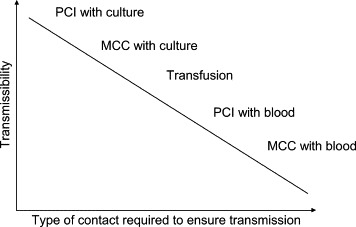
Schematic drawing showing the relationship between the transmissibility of a pathogen and the importance of the blood inoculum needed to ensure transmission following exposure in a HCW. PCI, percutaneous injury; MCC, mucocutaneous contact; culture, viral or bacterial culture concentrates in the laboratory setting.
Results
Three viruses alone (HBV, HCV, and HIV) account for most cases of occupational infection described in the literature because of their prevalence among patients and the severity of the infections they cause. Published case reports were found for a total of 60 pathogens or species: 26 viruses, 18 bacteria/rickettsia, 13 parasites, and 3 yeasts.
Viruses
The 26 viruses that have caused documented occupational infections following exposure to BBF are shown in Table 1.
Table 1.
Viruses that have caused documented occupational infection following exposure to BBF in HCW or laboratory personnel
| Pathogen | Exposure | Setting | Source |
|---|---|---|---|
| Argentinian VHF (Junin virus) | Nonintact skin | Contact with rodents' blood | 73 |
| Bolivian VHF (Machupo virus) | Needlestick, nonintact skin | Health care | 74, 75 |
| Brazilian VHF (Sabia virus) | Needlestick | Research laboratory | 18 |
| Crimean Congo VHF | Nonintact skin | Health care | 14 |
| Dengue | Needlestick | Health care | 1 |
| Ebola VHF | Nonintact skin | Health care | 76 |
| Hendra virus | Nonintact skin | Veterinary care | 77 |
| Hepatitis B virus | Needlestick, nonintact skin | Health care | 78 |
| Hepatitis C virus | Needlestick, nonintact skin | Health care | 2 |
| Hepatitis D virus | Needlestick | Health care | 8 |
| Hepatitis G virus | Needlestick | Health care | 10 |
| Herpes simplex 1 | Needlestick, nonintact skin | Health care | 23, 24 |
| Human immunodeficiency virus 1 (HIV 1) | Needlestick, nonintact skin | Health care | 11, 79 |
| Kyasanur virus | Needlestick | Research laboratory | 80 |
| Lassa VHF | Nonintact skin | Health care | 15 |
| Marburg VHF | Needlestick, nonintact skin | Health care | 81, 82 |
| Rift Valley Fever virus | Nonintact skin | Veterinary care | 82 |
| Simian Foamy virus | Nonintact skin | Animal handling | 83 |
| Simian immunodeficiency virus (SIV) | Splash to eyes | Research laboratory | 84 |
| Epizootic vesicular stomatitis | Nonintact skin | Veterinary care laboratory | 85 |
| Vaccine virus (recombinant) | Needlestick | Research laboratory | 30 |
| Varicella zoster virus (VZV) | Needlestick | Health care | 25 |
| Venezuelan VHF (Guanarito virus) | Nonintact skin (suspected) | Health care | 86 |
| Virus B (Herpes 1) | Splash to eyes | Research laboratory | 26, 28 |
| West Nile virus | Scalpel cut, needlestick | Research laboratory | 22 |
| Yellow Fever virus | Nonintact skin (suspected) | Hospital laboratory | 87 |
VHF, viral hemorrhagic fever.
The hepatitis viruses
In 1987, the Centers for Disease Control and Prevention (CDC) estimated that approximately 12,000 HCW were becoming occupationally infected in the United States each year.7 According to the CDC, 700 to 1200 would become chronic carriers, and 200 to 300 deaths a year were due to occupationally acquired HBV infection in HCW. In France, the yearly number of notified, occupationally acquired viral hepatitis by HCW to the social security system went from around 600 in the mid-1970s to less than 50 in recent years, thanks to the compulsory anti-HBV immunization in HCW and improvements in hygiene procedures. One case of occupational coinfection with the virus responsible for hepatitis D has been described in a surgeon.8 Immunization against HBV would have protected him against infection with virus Delta, which cannot develop unless there is concurrent HBV infection. It became clear in the mid-1970s that HCW were also exposed to the risk of becoming infected with “non-A, non-B” hepatitis. Most of these were due to HCV, which was identified in 1991. In France, 43 occupational seroconversions for HCV were documented in HCW following exposure to BBF as of June 30, 2001, including 32 cases in which the source patient was known to be infected with HCV.9 Finally, a case of transmission of hepatitis G following BBF exposure was proven at the molecular level in a nurse with chronic HCV infection, who was an asymptomatic carrier of HGV RNA without any other biologic sign.10
HIV
The first case of transmission of HIV from a patient to an HCW was described in 1984.11 In total, 334 occupational seroconversions have been documented or considered likely as of December 2002 (106 documented and 238 possible cases).12 None of these cases were documented in HIV-burdened sub-Saharan Africa, with the exception of 2 cases in South Africa and 1 in Zambia. This suggests that the number of HCW occupationally infected with HIV is underestimated. In France, 42 cases of probable or documented occupational HIV infections were documented in the health care setting as of June 30, 2001, including 13 documented seroconversions.9 The successes achieved in the control of exposures to BBF and the advent of potent treatments against HIV that allowed many HIV-infected patients to avoid being hospitalized have reduced HCWs' risk of being injured with HIV-contaminated hollow-bore needles in France as in other European countries such as Italy (SIROH, unpublished data). No new case of occupational infection with HIV has been documented in France since 1997.9
Viral hemorrhagic fever viruses
The most feared risk is that of being exposed to the viruses that cause viral hemorrhagic fever (VHF): viruses Machupo, Lassa, Hantavirus, Ebola, Marburg, Crimean-Congo, Sabia, Dengue, Yellow Fever, Junin, Guanarito, Kyasanur as well as Chikungunya and Omsk Fever.13 These pathogens have caused limited but sometimes devastating outbreaks in African or Asian countries. Although some are known to be endemic (Lassa), these sudden, high-visibility outbreaks are mainly health care-related. Hospital referral of patients with febrile hemorrhagic syndromes caused by Lassa, Ebola, Crimean-Congo, or, more recently in Angola, Marburg virus, viruses was followed by grouped cases of secondary transmission and spillover into the community in some cases.14, 15 Most documented VHF victims are frontline HCW with little or no access to means of protection while exposed to the BBF of patients. One case of suspected interhuman transmission of Hantavirus following exposure of nonintact skin to BBF in an Argentinian HCW has been described but remains arguable.16, 17 Cases of transmission to laboratory personnel have been described for Sabia virus in Brazil18 and Ebola in the United Kingdom19 and Russia. Patients infected with other VHF viruses have been admitted in hospitals of industrialized countries20, 21 with no documented case of secondary transmission. The recent emergence of West Nile virus in the Northern Hemisphere has led to cases of occupational infection in laboratory personnel.22
Viruses of the herpes group
Localized lesions of the hand have been reported in personnel who sustained documented accidental inoculation of herpes virus 1, some of which simulated whitlow.23, 24 One case of virus varicella-zoster inoculation may have led to zoster in a physician after he had sampled a vesicle in a patient.25
Zoonotic viruses
Zoonotic viruses have also been transmitted across the interspecies barrier by exposure to BBF. Such exposures are frequent and usually benign, but they can also lead to death.26 This is a risk mainly for veterinary personnel or animal handlers, as well as research laboratory staff. Students in human or animal biology working in tropical zones may also be affected, and their medium-term medical follow-up is often lacking. Exposure is probably very common in animal keepers or research staff. In the United States in 1990, 26 of 266 (9.8%) of quarantine staff and 16 of 284 (5.6%) of those who had contact with primates had measurable titers of antibodies against Ebola virus or a strain of Marburg virus.27 Exposure to the saliva of infected monkeys by bites or scratches or accidental exposure to infected blood or tissues have led to over 25 infections by virus B (Herpesvirus simiae) since the 1930s, nearly two thirds of which have been fatal in the infected person.28 A laboratory technician who received a splash of virus B-infected biologic fluid became infected and died.26 To date, there are no cases of occupational infection by Severe Acute Respiratory Syndrome (SARS) following a needlestick or cut or contact with nonintact skin.
Viral vectors
An emerging and heretofore little-known risk is that of exposure to viral vectors used in gene therapy.29 This issue was illustrated recently at Bichat University Hospital in Paris when a researcher was referred to the emergency room after accidental exposure to a recombinant adenovirus–with no apparent consequence at 1-month follow-up. A case of accidental inoculation of recombinant vaccine virus in a laboratory technician has been published.30 Progression was benign because this technician had been vaccinated against smallpox some years earlier, and no seroconversion was noted. The detailed analysis of lymphocytic response markers showed, however, that the virus had been transmitted. The uses of this new technique may become more widespread in the years to come. The risk of occupational infection will therefore increase in a growing number of research laboratory personnel in cases of ex vivo handling as well as health care personnel in cases of in vivo gene therapy.31 The potential consequences of infection in health care or laboratory personnel by genetically modified vectors should be systematically documented, and primary and secondary prevention methods must be known to all.
Bacteria, parasites, and yeasts
Bacteria
Bacteria caused some of the first cases of occupational infection described in the dissection room and the laboratory, whether in medical or in literary texts.32, 33 The risk of occupation transmission of bacteria and rickettsia in the laboratory has been greatly reduced by the observance of hygiene rules. Cases that used to be common among pathologists have now become rare.34 Most cases of bacterial inoculation give rise to a local, nodular lesion at the site of injury, such as Mycobacterium species, Neisseria gonorrhoeae, Treponema pallidum, or Streptococcus pyogenes (see Table 2). In some rare instances, local infections became extensive (tenosynovitis, bacteriemia).
Table 2.
Bacteria and rickettsia that have caused documented occupational infection following exposure to BBF in HCW or laboratory personnel
| Pathogen | Exposure | Setting | Source |
|---|---|---|---|
| Brucella abortus | Needlestick | Research laboratory | 88 |
| Veterinary care | 89 | ||
| Burkholderia mallei | Nonintact skin | Research laboratory | 90 |
| Corynebacterium diphteriae | Needlestick | Hospital laboratorys | 91 |
| Corynebacterium striatum | Scalpel cut | Health care | 92 |
| Leptospira icterohaemorragiae | Needlestick | Research laboratory | 93 |
| Mycobacterium leprae | Needlestick | Health care | 94 |
| Mycobacterium marinum | Needlestick | Hospital laboratory | 95 |
| Mycobacterium tuberculosis | Needlestick | Health care | 96 |
| Mycoplasma caviae | Needlestick | Research laboratory | 97 |
| Neisseria gonorrhoeae | Cut | Research laboratory | 98 |
| Orientia (or Rickettsia) tsutsugamuchi | Cut | Research laboratory | 99 |
| Pasteurella multocida | Needlestick | Veterinary care | 100 |
| Rickettsia rickettsii (Rocky Mountain Spotted Fever) | Needlestick | Health care | 101 |
| Rickettsia typhi (typhus) | Needlestick | Research laboratory | 102 |
| Staphylococcus aureus | Needlestick | Housekeeping | 103 |
| β-hemolytic streptococcus (S pyogenes) | Scalpel cut | Autopsy | 104 |
| Streptococcus A (necrotizing fasciitis) | Nonintact skin | Health care | 105 |
| Treponema pallidum | Needlestick | Research laboratory | 106, 107 |
Parasites
Parasites that caused occupational infections are shown in Table 3. Most of these cases are due to Plasmodium species, usually Plasmodium falciparum. A case was described recently in France in a nurse who sustained a needlestick.35 An extensive review of the literature found 20 other published cases (21 in total).36, 37 Fifteen of the 21 cases (71%) described occurred in Europe (8 in France). Seven (33%) cases occurred in physicians or biologists/researchers. In 5 cases (24%), infection was caused by mucocutaneous contact. This percentage of transmissions occurring after a low-volume exposure such as MCC is high as compared with those found for documented cases of HIV or HCV infection, at 8.5% and 2.3%, respectively.9 Although the transmission rate cannot be evaluated precisely for lack of denominator data, this may reflect a high rate of transmission.12 Diagnosis was excessively delayed in some cases. Such delay may have severe consequences, such as occurred with the death of an Italian physician.38
Table 3.
Parasites that have caused documented occupational infection following exposure to BBF in HCW or laboratory personnel
| Pathogen | Exposure | Setting | Source |
|---|---|---|---|
| Leishmania species (6 species) | Needlestick, nonintact skin | Hospital laboratory | 39, 108 |
| Plasmodium cynomolgi | Needlestick | Research laboratory | 109 |
| Plasmodium falciparum | Nonintact skin | Health care | 3 |
| Plasmodium malariae | Needlestick, nonintact skin | Health care | 110, 111 |
| Plasmodium vivax | Needlestick | Health care | 109 |
| Undetermined | Autopsy | 112 | |
| Toxoplasma gondii | Splash to eyes, needlestick | Research laboratory | 39, 113 |
| Trypanosoma brucei | Needlestick | Hospital laboratory | 39 |
| Trypanosoma cruzi | Projection | Hospital laboratory | 39 |
Approximately 60 cases of occupational infection by Trypanosoma cruzii (the agent of Chagas disease) following exposure to BBF have been described, mainly in Latin America.39 Cases of T brucei are more rare. Most of these occurred in research laboratories in Europe. Laboratory technicians working on Toxoplasmosis have also been infected following exposure to BBF.39
Yeasts
Reports of occupational infection by yeasts are rare. They are shown in Table 4.
Table 4.
Yeasts that have caused documented occupational infection following exposure to BBF in HCW or laboratory personnel
Other risks linked with BBF
BBF exposure and transfusion
There are no documented cases of occupational infection for several pathogens that are ubiquitous and known to be bloodborne. Some, however, have caused documented cases of infection following transfusion in patients (see Table 5) and may–at least in theory–be transferred by BBF exposure. The massive inoculum caused by blood transfusion is far greater than that associated with occupational exposures. Pathogens such as cytomegalovirus (CMV), parvovirus, or TT virus–one that is often found in hemophiliacs or patients who underwent dialysis or multiple transfusions40–were transmitted in this way.41 In a recent and dramatic incident, 3 patients died of rabies following organ transplantation from a deceased donor in the United States.42
Table 5.
Other pathogens for which there are no documented occupational infection following exposure to BBF in HCW or laboratory personnel but that are transmitted through blood transfusion or organ transplantation
BBF exposure and prion disease
Animal experiments have shown that the agent of Creutzfeldt-Jakob disease (CJD) and new variant CJD can be transmitted by transfusion43, 44, 45 or following the intracerebral injection of white blood cells in the guinea pig46 or subcutaneous and intramuscular injection in monkeys.47 Human cases have been described following direct cerebral inoculation (dura mater grafts) or intramuscular injection of growth hormones.48, 49 Cases of CJD have been described in Australian patients who received transfusion, although the origin of the blood could not clearly be established. In Canada, a patient who received albumin elaborated using blood products from a patient who developed CJD was found to have CJD at autopsy.48 One case of CJD was described in a neurosurgeon,50 one in a pathologist,51 and 24 others in laboratory personnel.52, 53 Finally, a debatable case occurred in a surgeon who worked with animals.54 A suspected case of vCJD transmission in a blood recipient has been described recently.55 HCWs, however, are not overrepresented among CJD/vCJD cases in the United Kingdom, and there is no definitive proof of transmission by transfusion in humans (Dr. Peter Smith, London, personal communication, October 12, 2002).56, 57 Studies are based on a small number of cases and depend on relatives' recall because this disease is rare and has an extremely long incubation period.48, 56 In view of available sources, we may conclude that, if the risk of prion transmission following occupational exposure to BBF actually exists, then it is extremely low.
Discussion
Antiretroviral postexposure prophylaxis (PEP) is leading a growing number of HCW to seek medical expertise and assistance following exposure to BBF. In most cases, the risk is due to HIV, HCV, or HBV, and management guidelines are readily accessible in most countries. Efforts are being made by some European countries to standardize management guidelines.58
Exposure to some of the rare pathogens described here may, however, pose a real challenge for the PEP expert. First, HCW may be unaware that they have been exposed to a rare pathogen.39 Second, injured personnel do not always seek assistance immediately. Third, clinicians responsible for postexposure management (often in the emergency room) may be at a loss because the progression of these infections is undocumented when their “natural” route is bypassed by direct inoculation.26 It may then become difficult to define a postexposure management strategy or to decide whether prophylaxis is indicated. There are no validated recommendations based on scientific proof for preventing infection by pathogens other than HIV and HBV, even when well-established treatments are available, such as is the case for Plasmodium species or Lassa or CCHF viruses. Fourth, HCW may be referred to a hospital other than the one in which the injury occurred, and there is no document to guide follow-up, a vital resource, especially if the infection causes neurologic symptoms preventing them from telling of their injury, as occurred with an occupational P falciparum infection in France or a lethal case in Italy.
HCW and the clinicians responsible for postexposure evaluation must bear in mind that many pathogens other than HBV, HCV, or HIV may be transmitted, not to mention the anecdotal case of the transmission of tumoral cells during an occupational exposure in the laboratory setting.59 Prospective follow-up in exposed HCW with denominator data is available only for HBV, HCV, and HIV. For other pathogens, the transmission risk cannot be rigorously assessed because of the small number of documented cases. It is nevertheless reflected to some extent by the mode of transmission: the lower the volume of the inoculum that causes infection by a given pathogen, the higher the theoretic risk of transmission for that pathogen. A low transmissibility and rare pathogen may be transmitted only by contact with cultures in the research laboratory, for instance, whereas a ubiquitous and highly transmissible pathogen may be acquired following MCC in the clinical setting. Some cases of occupational infection, however, were described only in the laboratory. This may be due to the pathogen's prevalence being extremely low in the clinical setting but may principally reflect the higher risk of transmission associated with concentrated cultures used in the laboratory setting.
Some of these pathogens may be concentrated in body fluids other than blood: HIV is found in cerebrospinal or spermatic fluid; the hepatitis viruses may be present in ascites fluid. The clinicians responsible for risk evaluation must therefore think in terms of exposure to potentially infectious body fluids and not solely in terms of blood. Furthermore, they must bear in mind that the risk of BBF exposure does not only concern HCW but all personnel at risk of exposure to BBF or BBF-soiled devices. A high proportion of documented cases occurred in laboratory personnel, and a few others occurred in emergency assistance, police, or health care waste elimination personnel.60, 61, 62, 63 Finally, veterinary personnel or animal technicians in laboratories are exposed to infection risks with zoonotic pathogens following exposure to BBF.
Several factors are needed for successful infection to occur. First, a pathogen must have the capacity to replicate within a human host.64 The risk for HCW to be exposed to a given pathogen increases with its prevalence among treated patients and with the chronic character of the infection. In case exposure occurs, the risk of transmission increases with the importance of the infectious inoculum. This varies according to either the volume of blood inoculated (deep needlestick, large-bore needle, large surface of nonintact skin exposed) or to the number of infective agents (high viral load, bacteriemia, or parasitemia) contained in it.
These microbiologic notions are illustrated by existing data on transmission rates, which vary according to source patient parasitemia. The seroconversion rate following accidental needlestick in nonimmunized HCW is estimated at approximately 10% and as high as 30% in case of Ag-Hbe carriage in the source patient.65 A metaanalysis of available data estimated the global risk of transmission of HCV following needlestick injury at 1.5% to 2.0%.66 Studies conducted following exposure to BBF in Japan, however, found varying rates of seroconversion according to polymerase chain reaction (PCR) results in the source patient: 0% in case of negative HCV-PCR; 10% in case of positive PCR.67 Finally, the systematic serological follow-up of exposed HCW provided data on the rate of seroconversion for HIV following occupational needlestick injury, which was estimated at 0.29% (95% CI: 0.13%-0.70%).68 A case-control study was conducted by the CDC on the risk factors for seroconversion in HCW following needlestick exposure to HIV-infected blood.69 This study showed that this rate could be increased if risk factors were present: deep (bloodletting) injury; device visibly contaminated with blood; needle used for an intravascular procedure (therefore containing blood); patient in terminal phase of infection by HIV (therefore with a high viral load). It also showed the protective role of postexposure prophylaxis with AZT.
Although rates of exposure to blood in HCW are diminishing in industrialized countries, their risk of encountering little-known pathogens may increase with international travel and the emergence or reemergence of diseases.70, 71, 72 Data on transmission rates and possible prophylaxis for pathogens other than HBV, HCV, or HIV are needed to guide postexposure management experts.
Conclusion
The risk for HCW to be exposed to rare “exotic” pathogens increases with intercontinental travel and migrations. Emerging disease risks are also associated with progress in medical techniques, changes in nutritional or other social habits, or ecologic changes. Although the principal risks to which PEP experts are confronted are linked with HIV, HCV, and HBV, these experts must also be able to suspect other, less common transmissible pathogens. Clinicians should systematically follow up personnel who have sustained exposure to “inhabitual” pathogens. We hope that this article will be a useful tool for postexposure management experts. Extended national recommendations must nevertheless be developed to guide experts in the management of HCW following exposure to BBF.
Saint-Maurice and Paris, France
References
- 1.de Wazieres B., Gil H., Vuitton D.A., Dupond J.L. Nosocomial transmission of dengue from a needlestick injury. Lancet. 1998;351:498. doi: 10.1016/S0140-6736(05)78686-4. [DOI] [PubMed] [Google Scholar]
- 2.Seeff L.B. Hepatitis C from a needlestick injury. Ann Intern Med. 1991;115:411. doi: 10.7326/0003-4819-115-5-411_1. [DOI] [PubMed] [Google Scholar]
- 3.Cannon N., Walker S., Dismukes W. Malaria acquired by accidental needle puncture. JAMA. 1972;222:1425. [PubMed] [Google Scholar]
- 4.Casey J., Maayan S. The bacteriemic patient as a source of infection. N Engl J Med. 1981;305:582–583. [PubMed] [Google Scholar]
- 5.Glaser J., Garden A. Inoculation of cryptococcosis without transmission of the acquired immunodeficiency syndrome. N Engl J Med. 1985:266. doi: 10.1056/NEJM198507253130414. [DOI] [PubMed] [Google Scholar]
- 6.Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol Rev. 1973;37:1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Guidelines for the prevention of transmission of human immunodeficiency virus and hepatitis b virus to health-care and public safety workers. MMWR Morb Mortal Wkly Rep. 1989;38:S63–S87. [Google Scholar]
- 8.Lettau L.A., Alfred H.J., Glew R.H., Fields H.A., Alter M.J., Meyer R. Nosocomial transmission of delta hepatitis. Ann Intern Med. 1986;104:631–635. doi: 10.7326/0003-4819-104-5-631. [DOI] [PubMed] [Google Scholar]
- 9.Lot F., Migueres B., Yazdanpanah Y., Tarantola A., Abiteboul D., Domart M. Séroconversions professionnelles par le VIH et le VHC chez le personnel de santé en France, le point au 30 juin 2001. Bull Epidemiol Hebd. 2002;12:49–51. [Google Scholar]
- 10.Shibuya A., Takeuchi A., Sakurai K., Saigenji K. Hepatitis G virus infection from needle-stick injuries in hospital employees. J Hosp Infect. 1998;40:287–290. doi: 10.1016/s0195-6701(98)90305-x. [DOI] [PubMed] [Google Scholar]
- 11.Anonymous Needlestick transmission of HTLV-III from a patient infected in Africa. Lancet. 1984;2:1376–1377. [PubMed] [Google Scholar]
- 12.Health Protection Agency Centre for Infections & Collaborators. Occupational transmission of HIV—Summary of Published Reports. March 2005 Edition (data to December 2002). Available at http://www.hpa.org.uk/infections/topics_az/bbv/pdf/intl_HIV_tables_2005.pdf.
- 13.OMS Abrégé des communications présentées au séminaire interrégional OMS sur les fièvres hémorragiques transmises par moustiques dans les régions de l'Asie du Sud-Est et du Pacifique Occidental, 1964. Bull World Health Organ. 1966;35:1–95. [PMC free article] [PubMed] [Google Scholar]
- 14.Burney M.I., Ghafoor A., Saleen M., Webb P.A., Casals J. Nosocomial outbreak of viral hemorrhagic fever caused by Crimean hemorrhagic fever– Congo virus in Pakistan, January 1976. Am J Trop Med Hyg. 1980;29:941–947. doi: 10.4269/ajtmh.1980.29.941. [DOI] [PubMed] [Google Scholar]
- 15.Frame J.D., Baldwin J.M., Jr., Gocke D.J., Troup J.M. Lassa fever: a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19:670–676. doi: 10.4269/ajtmh.1970.19.670. [DOI] [PubMed] [Google Scholar]
- 16.Wells R., Sosa Estani S., Yadon Z., Enria D., Padula P., Pini N. An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Emerg Infect Dis. 1997;3:171–174. doi: 10.3201/eid0302.970210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitek C., Breiman R., Ksiazek T., Rollin P., McLaughlin J., Umland E. Evidence against person-to-person transmission of hantavirus to health care workers. Clin Infect Dis. 1996;22:824–826. doi: 10.1093/clinids/22.5.824. [DOI] [PubMed] [Google Scholar]
- 18.Coimbra T., Nassar E., Burattini M., Madia de Souza T., Ferreira I., Rocco I. New arenavirus isolated in Brazil. Lancet. 1994;343:391–392. doi: 10.1016/s0140-6736(94)91226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emond R., Evans B., Bowen E., Lloyd G. A case of Ebola virus infection. BMJ. 1977;2:541–544. doi: 10.1136/bmj.2.6086.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zweighaft R.M., Fraser D.W., Hattwick M.A., Winkler W.G., Jordan W.C., Alter M. Lassa fever: response to an imported case. N Engl J Med. 1977;297:803–807. doi: 10.1056/NEJM197710132971504. [DOI] [PubMed] [Google Scholar]
- 21.Gunther S., Emmerich P., Laue T., Kuhle O., Asper M., Jung A. Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerg Infect Dis. 2000;6:466–476. doi: 10.3201/eid0605.000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Laboratory-acquired West Nile Virus infections–United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1133–1135. [PubMed] [Google Scholar]
- 23.Douglas M., Walters J., Currie B. Occupational infection with herpes simplex virus type 1 after a needlestick injury. Med J Aust. 2002;176:240. doi: 10.5694/j.1326-5377.2002.tb04384.x. [DOI] [PubMed] [Google Scholar]
- 24.Perl T., Haugen T., Pfaller M., Hollis R., Lakman A., Whitley R. Transmission of herpes simplex virus type 1 infection in an intesive care unit. Ann Intern Med. 1992;117:584–586. doi: 10.7326/0003-4819-117-7-584. [DOI] [PubMed] [Google Scholar]
- 25.Su W., Muller S. Herpes zoster: case report of possible accidental inoculation. Arch Dermatol. 1976;112:1755–1756. doi: 10.1001/archderm.112.12.1755. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Fatal cercopithecine hepesvirus 1 (B virus) infection following a mucocutaneous exposure and interim recommendations for worker protection. MMWR Morb Mortal Wkly Rep. 1998;47:1073–1076. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Update: filovirus infection associated with contact with nonhuman primates or their tissues. MMWR Morb Mortal Wkly Rep. 1990;39:404–405. [PubMed] [Google Scholar]
- 28.Artenstein A.W., Hicks C.B., Goodwin B.S., Jr., Hilliard J.K. Human infection with B virus following a needlestick injury. Rev Infect Dis. 1991;13:288–291. doi: 10.1093/clinids/13.2.288. [DOI] [PubMed] [Google Scholar]
- 29.Kost T., Condreay J., Mickelson C. Biosafety and viral gene transfer vectors. In: Fleming D., Hunt D., editors. Biological safety. Principles and practices. ASM Press; Washington DC: 2000. [Google Scholar]
- 30.Openshaw P., Alwan W., Cherrie A., Record F. Accidental infection of laboratory worker with recombinant vaccinia virus. Lancet. 1991;338:459. doi: 10.1016/0140-6736(91)91093-a. [DOI] [PubMed] [Google Scholar]
- 31.Evans M., Lesnaw J. Infection control in gene therapy. Infect Control Hosp Epidemiol. 1999;20:568–576. doi: 10.1086/501674. [DOI] [PubMed] [Google Scholar]
- 32.Céline L.-F. Gallimard; Paris: 1999. Semmelweis (1923) [Google Scholar]
- 33.Turgenev I. Oxford Paperbacks; Oxford: 1998. Fathers and Sons (1862) [Google Scholar]
- 34.Collins C.H., Kennedy D.A. Microbiological hazards of occupational needlestick and “sharps” injuries: a review. J Appl Bacteriol. 1987;62:385–402. [PubMed] [Google Scholar]
- 35.Tarantola A., Rachline A.C., Konto C., Houzé S., Lariven S., Fichelle A. Occupational malaria following needlestick injury. Emerg Infect Dis. 2004;10:1878–1880. doi: 10.3201/eid1010.040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarantola A., Rachline A., Konto C., Houzé S., Sabah-Mondan C., Vrillon H. Occupational Plasmodium falciparum malaria following accidental blood exposure: a case, published reports and considerations for post-exposure prophylaxis. Scand J Infect Dis. 2005;37:131–140. [PubMed] [Google Scholar]
- 37.Alweis R.L., DiRosario K., Conidi G., Kain K.C., Olans R., Tully J.L. Serial nosocomial transmission of Plasmodium falciparum malaria from patient to nurse to patient. Infect Control Hosp Epidemiol. 2004;25:55–59. doi: 10.1086/502293. [DOI] [PubMed] [Google Scholar]
- 38.CDSC Needlestick malaria with tragic consequences. Commun Dis Rep CDR Wkly. 1997;7:247. [PubMed] [Google Scholar]
- 39.Herwaldt B.L. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev. 2001;14:659–688. doi: 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cossart Y. TTV a common virus, but pathogenic? Lancet. 1998;352:164. doi: 10.1016/S0140-6736(05)77802-8. [DOI] [PubMed] [Google Scholar]
- 41.Alter HJ. TT virus. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 5th ed. New York: Churchill Livingstone; 2000.
- 42.Update: investigation of rabies infections in organ donor and transplant recipients–Alabama, Arkansas, Oklahoma, and Texas, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:615–616. [PubMed] [Google Scholar]
- 43.Dickmeiss E., Gerstoft J. Blood infectivity in transmissible spongiform encephalopathies. APMIS. 2002;110:99–103. doi: 10.1034/j.1600-0463.2002.100112.x. [DOI] [PubMed] [Google Scholar]
- 44.Hunter N., Houston F. Can prion diseases be transmitted between individuals via blood transfusion: evidence from sheep experiments. Dev Biol (Basel) 2002;108:93–98. [PubMed] [Google Scholar]
- 45.Dobson R. Scientists show that vCJD can be transmitted through blood. BMJ. 2000;321:721. [PMC free article] [PubMed] [Google Scholar]
- 46.Deslys J., Lasmézas C., Dormont D. Selection of specific strains in iatrogenic Creutzfeldt-Jakob disease. Lancet. 1994;343:848–849. doi: 10.1016/s0140-6736(94)92046-x. [DOI] [PubMed] [Google Scholar]
- 47.Gajdusek D.C., Gibbs C.J., David P., Asher D., Brown P., Diwan A. Precautions in medical care of, and in handling materials from, patients with transmissible virus dementia (Creutzfeldt-Jakob Disease) N Engl J Med. 1977;297:1253–1258. doi: 10.1056/NEJM197712082972304. [DOI] [PubMed] [Google Scholar]
- 48.Ricketts M., Cashman N., Stratton E., ElSaadany S. Is Creutzfelkdt-Jakob disease transmissible in blood? Emerg Infect Dis. 1997;3:155–163. doi: 10.3201/eid0302.970208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dormont D. La transmission des agents transmissibles non conventionnels ou prions. Méd Mal Infect. 1996;26:455–464. [Google Scholar]
- 50.Schoene V., Masters C.L., Gibbs C.J., Jr., Gajdusek D.C., Tyler H.R., Moore F.D. Transmissible spongiform encephalopathy (Creutzfeldt-Jakob Disease). Atypical clinical and pathological findings. Arch Neurol. 1981;38:473–477. doi: 10.1001/archneur.1981.00510080035002. [DOI] [PubMed] [Google Scholar]
- 51.Gorman D., Beson F., Vogel D., Vinters H. Creutzfeldt-Jakob disease in a pathologist. Neurology. 1992;42:463. doi: 10.1212/wnl.42.2.463. [DOI] [PubMed] [Google Scholar]
- 52.Berger J., David N. Creutzfeldt-Jakob disease in a physician: a review of the disorder in health care workers. Neurology. 1993;43:205–206. doi: 10.1212/wnl.43.1_part_1.205. [DOI] [PubMed] [Google Scholar]
- 53.Miller D. Creutzfeldt-Jakob disease in a histopathology technician. N Engl J Med. 1988;318:853–854. doi: 10.1056/NEJM198803313181312. [DOI] [PubMed] [Google Scholar]
- 54.Weber T., Tumani H., Holdorff B., Collinge J., Palmer M., Kretzschmar H. Transmission of Creutzfeldt-Jakob disease by handling of dura mater. Lancet. 1993;341:123–124. doi: 10.1016/0140-6736(93)92608-v. [DOI] [PubMed] [Google Scholar]
- 55.HPA Possible case of transfusion-associated variant CJD: news. Commun Dis Rep CDR Wkly. 2003;13 [Google Scholar]
- 56.van Duijn C., Delasnerie-Lauprêtre N., Masulio C., Zerr I., de Silva R., Wientjens D. Case-control study of risk factors of Creutzfeldt-Jakob disease in Europe during 1993-1995. Lancet. 1998;351:1081–1085. doi: 10.1016/s0140-6736(97)09468-3. [DOI] [PubMed] [Google Scholar]
- 57.Ridley R., Baker H. Occupational risk of Creutzfeldt-Jakob disease. Lancet. 1993;341:641–642. doi: 10.1016/0140-6736(93)90413-b. [DOI] [PubMed] [Google Scholar]
- 58.Puro V., Cicalini S., De Carli G., Soldani F., Ippolito G. Towards a standard HIV post exposure prophylaxis for healthcare workers in Europe. Euro Surveill. 2004;9 doi: 10.2807/esm.09.06.00470-en. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.Gugel H., Sanders M. Needle-stick transmission of human colonic carcinoma. N Engl J Med. 1986;315:1487. doi: 10.1056/NEJM198612043152314. [DOI] [PubMed] [Google Scholar]
- 60.Jones P.D. HIV transmission by stabbing despite zidovudine prophylaxis. Lancet. 1991;338:884. doi: 10.1016/0140-6736(91)91535-3. [DOI] [PubMed] [Google Scholar]
- 61.Lot F., Abiteboul D. Infections professionnelles par le VIH en France. Le point au 31 Décembre 1993. Bull Epidemiol Hebdom. 1994;25:111–113. [Google Scholar]
- 62.Merchant R.C., Becker B.M., Mayer K.H., Fuerch J., Schreck B. Emergency department blood or body fluid exposure evaluations and HIV postexposure prophylaxis usage. Acad Emerg Med. 2003;10:1345–1353. doi: 10.1111/j.1553-2712.2003.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 63.Rosen H.R. Acquisition of hepatitis C by a conjunctival splash. Am J Infect Control. 1997;25:242–247. doi: 10.1016/s0196-6553(97)90011-0. [DOI] [PubMed] [Google Scholar]
- 64.Bruce-Chwatt L.J. Blood transfusion and tropical disease. Trop Dis Bull. 1972;69:825–862. [PubMed] [Google Scholar]
- 65.Seef L.B., Wright E.C., Zimmerman H.J., Alter H.J., Dietz A.A., Felsher B.F. Type B hepatitis after needle-stick exposures: prevention with hepatitis B immune globulin: final report of the Veterans Administration Cooperative Study. Ann Intern Med. 1978;88:285–293. doi: 10.7326/0003-4819-88-3-285. [DOI] [PubMed] [Google Scholar]
- 66.Puro V, Petrosillo N, Ippolito G, Jagger J. Mise à Jour sur les Etudes d'Incidence des Infections Professionnelles dues au VHC. 1er Colloque International sur les Infections Transmissibles par le Sang, Risques Professionnels et Prévention (abstract). June 8-9, 1995. Paris. Poster A8.
- 67.Sodeyama T., Kiyosawa K., Urushihara A., Matsumoto A., Tanaka E., Furuta S. Detection of hepatitis C virus markers and hepatitis C virus genomic-RNA after needlestick accidents. Arch Intern Med. 1993;153:1565–1572. [PubMed] [Google Scholar]
- 68.Henderson D.K., Fahey B.J., Willy M., Schmitt J.M., Carey K., Koziol D.E. Risk for occupational transmission of human immunodeficiency virus type 1 (HIV-1) associated with clinical exposures: a prospective evaluation. Ann Intern Med. 1990;113:740–746. doi: 10.7326/0003-4819-113-10-740. [DOI] [PubMed] [Google Scholar]
- 69.Cardo D.M., Culver D.H., Ciesielski C.A., Srivastava P.U., Marcus R., Abiteboul D. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 70.Dorolle P. Old plagues in the jet age: international aspects of present and future control of communicable disease. Br Med J. 1968;4:789–792. doi: 10.1136/bmj.4.5634.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drucker E., Alcabes P.G., Marx P.A. The injection century: massive unsterile injections and the emergence of human pathogens. Lancet. 2001;358:1989–1992. doi: 10.1016/S0140-6736(01)06967-7. [DOI] [PubMed] [Google Scholar]
- 72.Ostroff S.M., Kozarsky P. Emerging infectious diseases and travel medicine. Infect Dis Clin North Am. 1998;12:231–241. doi: 10.1016/s0891-5520(05)70420-7. [DOI] [PubMed] [Google Scholar]
- 73.Peters C.J. Churchill Livingstone; 2000. Lymphocytic Choriomeningitis virus, Lassa virus, and the South American hemorrhagic fevers. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 5th Edition (CD rom) [Google Scholar]
- 74.Peters C.J., Kuehne R., Mercado R., Le Bow R., Spertzel R., Webb P. Hemorrhagic fever in Cochabamba, Bolivia, 1971. Am J Epidemiol. 1974;99:425–433. doi: 10.1093/oxfordjournals.aje.a121631. [DOI] [PubMed] [Google Scholar]
- 75.Kilgore P., Peters C., Mills J., Rollin P., Armstrong L., Khan A. Prospects for the control of Bolivian hemorrhagic fever. Emerg Infect Dis. 1995;1:97–100. doi: 10.3201/eid0103.950308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peters C.J., Le Duc J.W. An introduction to Ebola: the virus and the disease. J Infect Dis. 1999;179(Suppl 1):9–16. doi: 10.1086/514322. [DOI] [PubMed] [Google Scholar]
- 77.Selvey L., Wells R., McCormack J., Ansford A., Murray K., Rogers R. Infection of humans and horses by a newly described morbilivirus. Med J Aust. 1995;162:642–645. doi: 10.5694/j.1326-5377.1995.tb126050.x. [DOI] [PubMed] [Google Scholar]
- 78.Dienstag J.L., Ryan D.M. Occupational exposure to hepatitis B virus in hospital personnel: infection or immunization? Am J Epidemiol. 1982;115:26–39. doi: 10.1093/oxfordjournals.aje.a113277. [DOI] [PubMed] [Google Scholar]
- 79.Gioannini P., Sinicco A., Cariti G. HIV infection acquired by a nurse. Eur J Epidemiol. 1988;4:119–120. doi: 10.1007/BF00152703. [DOI] [PubMed] [Google Scholar]
- 80.Gupta K., Pal Y. Kyasanur forest disease. J Indian Med Assoc. 1975;64:236–237. [PubMed] [Google Scholar]
- 81.Smith D., Johnson B., Isaacson M., Swanbepoel R., Johnson K., Killey M. Marburg-virus disease in Kenya. Lancet. 1982;1:816–820. doi: 10.1016/s0140-6736(82)91871-2. [DOI] [PubMed] [Google Scholar]
- 82.Gear J. Clinical aspects of African viral hemorrhagic fever. Rev Infect Dis. 1989;2:S777–S782. doi: 10.1093/clinids/11.supplement_4.s777. [DOI] [PubMed] [Google Scholar]
- 83.Sandstrom P., Phan K., Switzer W., Fredeking T., Chapman L., Heneine W. Simian foamy virus infection among zoo keepers. Lancet. 2000;355:551–552. doi: 10.1016/S0140-6736(99)05292-7. [DOI] [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention Seroconversion to simian immunodeficiency virus in two laboratory workers. MMWR Morb Mortal Wkly Rep. 1992;41:678–681. [PubMed] [Google Scholar]
- 85.Reif J., Webb P., Monath T.P., Emerson J., Poland J., Kemp G. Epizootic vesicular stomatitis in Colorado, 1982: infection in occupational risk groups. Am J Trop Med Hyg. 1987;36:177–182. doi: 10.4269/ajtmh.1987.36.177. [DOI] [PubMed] [Google Scholar]
- 86.de Manzione N., Salas R.A., Paredes H., Godoy O., Rojas L., Araoz F. Venezuelan hemorragic fever: clinical and epidemiological studies of 165 case. Clin Infect Dis. 1998;26:308–313. doi: 10.1086/516299. [DOI] [PubMed] [Google Scholar]
- 87.Cook G.C. Fatal yellow fever contracted at the Hospital for Tropical Diseases, London, UK, in 1930. Trans R Soc Trop Med Hyg. 1994;88:712–713. doi: 10.1016/0035-9203(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 88.Joffe B., Diamond M. Brucellosis due to self inoculation. Ann Intern Med. 1966;65:564–565. doi: 10.7326/0003-4819-65-3-564. [DOI] [PubMed] [Google Scholar]
- 89.Nicoletti P., Ring J., Boysen B., Buczek J. Illness in a veterinary student following accidental inoculation of Brucella abortus strain 19. J Am Coll Health. 1986;34:236–237. doi: 10.1080/07448481.1986.9938944. [DOI] [PubMed] [Google Scholar]
- 90.Srinivasan A., Kraus C.N., DeShazer D., Becker P.M., Dick J.D., Spacek L. Glanders in a military research microbiologist. N Engl J Med. 2001;345:256–258. doi: 10.1056/NEJM200107263450404. [DOI] [PubMed] [Google Scholar]
- 91.Baldwin A., McCallum F., Doull J. A case of pharyngeal diphteria probably due to auto-infection from a diphteric lesion of the thumb. JAMA. 1923;80:1375. [Google Scholar]
- 92.Cone L., Curry N., Wuesthoff M., O'Connell S., Feller J. Septic synovitis and arthritis due to Corynebacterium striatum following an accidental scalpel injury. Clin Infect Dis. 1998;27:1533–1544. doi: 10.1086/517737. [DOI] [PubMed] [Google Scholar]
- 93.Sarasin G., Tucker D., Arean V. Accidental laboratory infection caused by Leptospira icterohaemorrhagiae. Am J Clin Pathol. 1963;40:146–150. doi: 10.1093/ajcp/40.2.146. [DOI] [PubMed] [Google Scholar]
- 94.Porritt R., Olsen R. Two simultaneous cases of leprosy developing in tattoos. Am J Pathol. 1947;23:805–811. [PMC free article] [PubMed] [Google Scholar]
- 95.Chappler R.R., Hoke A.W., Borchardt K.A. Primary inoculation with Mycobacterium marinum. Arch Dermatol. 1977;113:380. [PubMed] [Google Scholar]
- 96.Fnini S., Ouarab M., Rafai M., Cohen D., Largab A., Trafeh M. An uncommon occupational accident: tuberculous tenosynovitis of the extensor tendons of the hand. Chir Main. 1999;18:309–312. [PubMed] [Google Scholar]
- 97.Hill A. Accidental infection of man with Mycoplasma caviae. Br Med J. 1971;2:711–712. doi: 10.1136/bmj.2.5763.711-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sears H. A cutaneous infection with Neisseria gonorrhoeae with development of lymphangitis resulting from a laboratory accident. Am J Syph Gonorr Vener Dis. 1947;31:60–64. [PubMed] [Google Scholar]
- 99.Buckland F., MacCallum F., Dudgeon A., Niven J., Edward D., Rowlands I. Scrub-typhus vaccine large-scale production. Lancet. 1947;2:734–737. [PubMed] [Google Scholar]
- 100.Olson L. Accidental penetration of hands with virulent and avirulent Pasteurella multocida of turkey origin. Avian Dis. 1980;24:1064–1066. [PubMed] [Google Scholar]
- 101.Sexton D.J., Gallis H.A., McRae J.R., Cate T.R. Possible needle-associated Rocky Mountain spotted fever. N Engl J Med. 1975;292:645. doi: 10.1056/nejm197503202921217. [DOI] [PubMed] [Google Scholar]
- 102.Centers for Disease Control and Prevention Laboratory-acquired endemic typhus–Maryland. MMWR Morb Mortal Wkly Rep. 1978;27:215–216. [Google Scholar]
- 103.Jacobson J., Burke J., Conti M. Injuries of hospital employees from needles and sharp objects. Infect Control. 1983;4:100–102. doi: 10.1017/s0195941700057830. [DOI] [PubMed] [Google Scholar]
- 104.Hawkey P.M., Pedler S.J., Southall P.J. Streptococcus pyogenes: a forgotten occupational hazard in the mortuary. BMJ. 1980;281:1058. doi: 10.1136/bmj.281.6247.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valenzuela T., Hooton T., Kaplan E., Schlievert P. Transmission of “toxic strep” syndrome from an infected child to a firefighter during CPR. Ann Emerg Med. 1991;20:90–92. doi: 10.1016/s0196-0644(05)81129-1. [DOI] [PubMed] [Google Scholar]
- 106.Chacko W. Accidental human infection in the laboratory with the Nichols rabbit-adapted virulent strain of Treponema pallidum. Bull World Health Organ. 1966;35:809–810. [PMC free article] [PubMed] [Google Scholar]
- 107.Willcox R. Treponema pallidum: a bibliographical reveiw of the morphology, culture and survival of T. pallidum and associated organisms. Bull World Health Organ. 1966;35:110–118. [PMC free article] [PubMed] [Google Scholar]
- 108.Knobloch J., Demar M. Accidental Leishmania mexicana infection in an immunosuppressed laboratory technician. Trop Med Int Health. 1997;2:1152–1155. doi: 10.1046/j.1365-3156.1997.d01-216.x. [DOI] [PubMed] [Google Scholar]
- 109.Herwaldt B.L., Juranek D.D. Laboratory-acquired malaria, leishmaniasis, trypanosomiasis, and toxoplasmosis. Am J Trop Med Hyg. 1993;48:313–323. doi: 10.4269/ajtmh.1993.48.313. [DOI] [PubMed] [Google Scholar]
- 110.Börsch G., Odendahl J., Sabin G., Ricken D. Malaria transmission from patient to nurse. Lancet. 1982;2:1212. doi: 10.1016/s0140-6736(82)91219-3. [DOI] [PubMed] [Google Scholar]
- 111.Freedman A.M. Unusual forms of malaria transmission: a report of 2 cases. S Afr Med J. 1987;71:183–184. [PubMed] [Google Scholar]
- 112.Neu H.C. Toxoplasmosis transmitted at autopsy. JAMA. 1967;202:844–845. [PubMed] [Google Scholar]
- 113.Field P.R., Moyle G.G., Parnell P.M. The accidental infection of a laboratory worker with Toxoplasma gondii. Med J Aust. 1972;2:196–198. doi: 10.5694/j.1326-5377.1972.tb47232.x. [DOI] [PubMed] [Google Scholar]
- 114.Larsh H.W., Schwarz J. Accidental inoculation blastomycosis. Cutis. 1977;19:334–337. [PubMed] [Google Scholar]
- 115.Ishizaki H., Ikeda M., Kurata Y. Lymphocutaneous sporotrichosis caused by accidental inoculation. J Dermatol. 1979;6:321–323. doi: 10.1111/j.1346-8138.1979.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 116.Dobroszycki J., Herwaldt B., Boctor F., Miller J., Linden J., Eberhard M. A cluster of transfusion-associated babesiosis cases traced to a single asymptomatic donor. JAMA. 1999;281:930. doi: 10.1001/jama.281.10.927. [DOI] [PubMed] [Google Scholar]
- 117.Hira P., Husein S. Some transfusion-induced parasitic infections in Zambia. J Hyg Epidemiol Microbiol Immunol. 2002;4:436–444. [PubMed] [Google Scholar]
- 118.Centers for Disease Control and Prevention Transmission of Colorado Tick Fever virus by blood transfusion. MMWR Morb Mortal Wkly Rep. 1975;24:422–427. [Google Scholar]
- 119.Barbara J., Contreras M. Infectious complications of blood transfusion: viruses. BMJ. 1990;300:450–453. doi: 10.1136/bmj.300.6722.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sato H., Okochi K. Transmission of human T-cell leukemia virus (HTLV-1) by blood transfusion: demonstration of proviral DNA in recipients' blood lymphocytes. Int J Cancer. 1986;37:395. doi: 10.1002/ijc.2910370311. [DOI] [PubMed] [Google Scholar]
- 121.Goodnough L., Brecher M., Kanter M., AuBuchon J. Transfusion medicine. N Engl J Med. 1999;340:438–446. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]


