Abstract
Background
Room ventilation is a key determinant of airborne disease transmission. Despite this, ventilation guidelines in hospitals are not founded on robust scientific evidence related to the prevention of airborne transmission.
Methods
We sought to assess the effect of ventilation rates on influenza, tuberculosis, and rhinovirus infection risk within 3 distinct rooms in a major urban hospital: a lung function laboratory, an emergency department negative-pressure isolation room, and an outpatient consultation room. Air-exchange rate measurements were performed in each room using CO2 as a tracer. The model developed by Gammaitoni and Nucci was used to estimate infection risk.
Results
Current outdoor air-exchange rates in the lung function laboratory and emergency department isolation room limited infection risks to 0.1%-3.6%. Influenza risk for individuals entering an outpatient consultation room after an infectious individual departed ranged from 3.6% to 20.7%, depending on the duration for which each person occupied the room.
Conclusion
Given the absence of definitive ventilation guidelines for hospitals, air-exchange measurements combined with modeling afford a useful means of assessing, on a case-by-case basis, the suitability of room ventilation for preventing airborne disease transmission.
Key Words: Infection control, air exchange, influenza, tuberculosis, rhinovirus
Room ventilation acts to dilute and remove infectious airborne droplet nuclei (aerosols), and several epidemiologic investigations have underscored its significant role in determining airborne transmission of tuberculosis (TB), influenza, measles, rhinovirus and severe acute respiratory syndrome in various indoor settings.1, 2, 3, 4, 5, 6, 7, 8 An extensive multi-disciplinary review conducted in the wake of the 2003 severe acute respiratory syndrome epidemic9 concluded that the relationship between ventilation and indoor airborne transmission of disease was supported by strong and sufficient evidence. Despite this, the potential for transmission in hospitals has received little attention, and there are insufficient data on which to base minimum ventilation rate guidelines.9 Thus, existing ventilation guidelines, of which there are several (summarized by Beggs et al10), are not founded on robust scientific evidence related to prevention of airborne transmission.
Although some respiratory infections may be communicated by fomites or over short distances (less than a few meters) by large droplets (>20 μm) that subsequently result in direct contact with the respiratory tract, airborne transmission is likely to contribute to person-to-person spread over relatively long distances, due the ability of small droplet nuclei (<5 μm) to remain suspended in air for extended periods.11 In addition to clear evidence indicating the airborne route is a mode of spread for TB and measles, there is mounting evidence of its role in influenza and rhinovirus transmission.7, 12, 13, 14, 15, 16
Given that the significance of knowledge gaps outlined above was amplified by the 2009 H1N1 pandemic, we sought to determine the effect of ventilation on the risk of airborne infection posed by 3 common pathogens in a major teaching hospital. We aimed to provide general information for use in the evaluation of hospital design and infection control strategies and also to inform in-house patient management guidelines.
Methods
Setting
The Prince Charles Hospital (TPCH) is a major tertiary referral and university hospital located in southeastern Queensland, Australia. TPCH has 588 beds, including a large Pulmonology Unit with 54 inpatient beds. The hospital had ∼77,000 outpatient consultations and performed ∼17,000 lung function tests in 2009-2010, and a large Emergency Department (ED) delivered 43,000 occasions of service. Clinical services at TPCH were built in recent years, and the Outpatient Department and Lung Function Laboratory, both commissioned in 1999, underwent redevelopment in 2007. The ED was completed and commissioned in 2007. In 2009, a total of 286 confirmed cases of H1N1 influenza were diagnosed and managed at TPCH. An average of 7 cases of Mycobacterium tuberculosis are managed at TPCH’s Pulmonology Unit each year.
Our study targeted rooms within TPCH that encompassed a range of uses and were potential airborne transmission locations: (1) the Respiratory Investigation (Lung Function) Laboratory (169 m3 volume), a negative-pressure isolation room within the ED (24 m3 volume), and 2 separate but proximate outpatient consulting rooms (room A, 32 m3; room B, 36 m3). All rooms were mechanically ventilated. The Lung Function Laboratory and outpatient consulting rooms were served by air-handling units (AHUs), with the 2 outpatient consulting rooms sharing a common AHU. The ED isolation room was ventilated entirely by outdoor air drawn in by an exhaust fan.
Air-exchange rate measurements
Air-exchange rate measurements in the Lung Function Laboratory and ED isolation room were performed with the room doors in their typical position (fully open and fully closed, respectively), and measurements in the 2 outpatient consulting rooms were performed under both closed-door and open-door conditions, representing a typical functioning outpatient clinic session. All measurements were performed when the rooms were unoccupied. Background concentrations of CO2 were monitored with a Sable Systems CA-10 CO2 analyzer (Las Vegas, NV) for at least 20 minutes. High-purity (99.9%) CO2 was then released and vigorously mixed with room air by 2 fans until concentrations stabilized. The approximate homogeneity of concentration was confirmed by measurements at a minimum of 3 points within each room before the cessation of CO2 release. A single sampling point was then sited at a central location. The decay of CO2 was recorded every second until background concentrations were reached. Three repeat measurements were conducted in each room, and for each door position in the outpatient consulting rooms. A total of 18 air-exchange measurements were obtained.
The gradient of the line of best fit through the natural logarithm of the background-corrected decay was recorded as the number of air changes per hour (ACH). The standard error of the line-fitting procedure was calculated.
For all rooms except the ED isolation room, the proportion of outdoor air in the total air volume supplied by their respective AHUs (ie, the combination of outdoor and recirculated air) was determined by mass balance of CO2 concentrations measured in return, supply, and outdoor air.17 The precision of calculated values was estimated using the method of Persily.17
Infection risk modeling
We used the model developed by Gammaitoni and Nucci18 (G-N) to estimate airborne transmission risk. The G-N model is a variation of the traditional steady-state Wells-Riley (W-R) model.1 Both models assume that an infectious person constantly generates a number of infectious quanta over time, with a quantum defined as the dose of airborne droplet nuclei required to cause infection in , or 63%, of susceptible persons. Unlike the W-R model, however, the G-N model is capable of incorporating non–steady-state quanta levels. Detailed discussions of each model’s merits and underlying assumptions have been provided by Beggs et al19 and Sze To and Chao.20
The W-R model is
| (1) |
where I is the number of infectious source cases, q is the number of infectious quanta produced per source case (quanta/hour), p is the average respiratory ventilation rate of susceptible persons (m3/hour), t is the duration of exposure (hours), and Q is the volume of infection-free (ie, outdoor) air supplied to the room (m3/hour).
The G-N model (when the initial quanta concentration is nonzero)19 is as follows:
| (2) |
where V is the volume of the room (m3), N is the air-change rate (ie, Q/V), and n0 is the total number of quanta in the room at t = 0.
We modeled 3 diseases spread by the airborne route that spanned a range of infectiousness and frequency of presentation at the study site: influenza, TB, and rhinovirus. Quanta generation rates for these 3 diseases were 67, 12.7, and 5 quanta/hour, respectively, with these values chosen to represent relatively typical cases.5, 21 Although we did not explicitly model H1N1, the influenza quanta generation rate that we used is within its suspected range.22 We assumed that all susceptible individuals had a standard adult respiratory rate of 0.6 m3/hour.5, 19 The modeling approach and additional equations used are described in the Appendix.
Based on typical patient occupancy times and patterns, we modeled 2 general scenarios for each airborne pathogen: the risk of infection for susceptible individuals occupying the Lung Function Laboratory with an infectious patient (exposure times ranging from 15 to 45 minutes), and the risk of infection for susceptible individuals occupying the ED isolation room for between 30 minutes and 8 hours immediately after the departure of an infectious individual who spent 30 minutes or more in the room. We also modeled a third, more complex, situation: the risk of infection for a susceptible individual occupying an outpatient consulting room for up to 120 minutes after previous occupation by an infectious individual for each of 15, 60, and 120 minutes, which spans the range of consultation times for brief to complex multidisciplinary consultations. To mimic typical practice, a 5-minute period during which the door was open was incorporated into each scenario (ie, between the departure of the infectious person and arrival of the susceptible individual). To best assess the capability of the outpatient consulting room's ventilation system at preventing airborne transmission, we investigated the most infectious pathogen (influenza) in that room.
Ethical approval
This study was approved by the Human Research Ethics Committees of TPCH (HREC/09/QPCH/163) and Queensland University of Technology (0900001290). Individual patient consent was not required for this study, although signs explaining the purpose of the measurements were displayed to staff, patients, and visitors.
Results
Table 1 summarizes the results of air-exchange and outdoor air proportion measurements. The proportion of outdoor air supplied to the Lung Function Laboratory by its AHU was approximately twice that supplied to the outpatient consulting rooms. This discrepancy was traced to a modification of the outdoor air intake dampers performed by technicians 2 years before our investigation. All outdoor air proportions were fixed and did not vary with season, reflecting the small seasonal temperature variation at the study location.
Table 1.
Summary of air-exchange and outdoor air proportion measurements
| Location | Volume, m3 | N∗ | Total ACH, mean ± SD | MSE† | Outdoor air proportion | Precision, %‡ | Mean outdoor ACH§ | Outdoor air, m3/h |
|---|---|---|---|---|---|---|---|---|
| Lung Function Laboratory | 168.5 | 3 | 8.5 ± 0.8 | 0.03 | 0.57 | 25.3 | 4.9 | 817.4 |
| ED isolation room | 23.5 | 3 | 23.8 ± 1.5 | 0.16 | 1.0 | – | 23.8 | 559.7 |
| Consulting room A (closed) | 31.5 | 3 | 7.0 ± 0.1 | 0.01 | 0.28 | 14.2 | 2.0 | 62 |
| Consulting room A (open) | – | 3 | 13.2 ± 1.1 | 0.06 | – | – | 3.7 | 117.3 |
| Consulting room B (closed) | 36.0 | 3 | 6.1 ± 0.1 | 0.01 | 0.28 | 14.2 | 1.7 | 62.1 |
| Consulting room B (open) | – | 3 | 9.1 ± 1.1 | 0.02 | – | – | 2.6 | 92.6 |
Number of ventilation measurements.
Mean standard error of line fit to CO2 decays.
Precision of outdoor air proportion measurement.17
The number of outdoor ACH was equal to the measured ACH rate multiplied by the proportion of outdoor air supplied.
A significant finding was the effect of door position on air exchange rates in outpatient consulting rooms A and B, which was most marked in the former and resulted in a near-doubling of air exchange compared with the closed-door situation. We ascribe this to the presence of a large air return air vent in the corridor immediately adjacent to consulting room A that promoted air movement out of the room. This effect was enhanced under open-door conditions.
Lung Function Laboratory
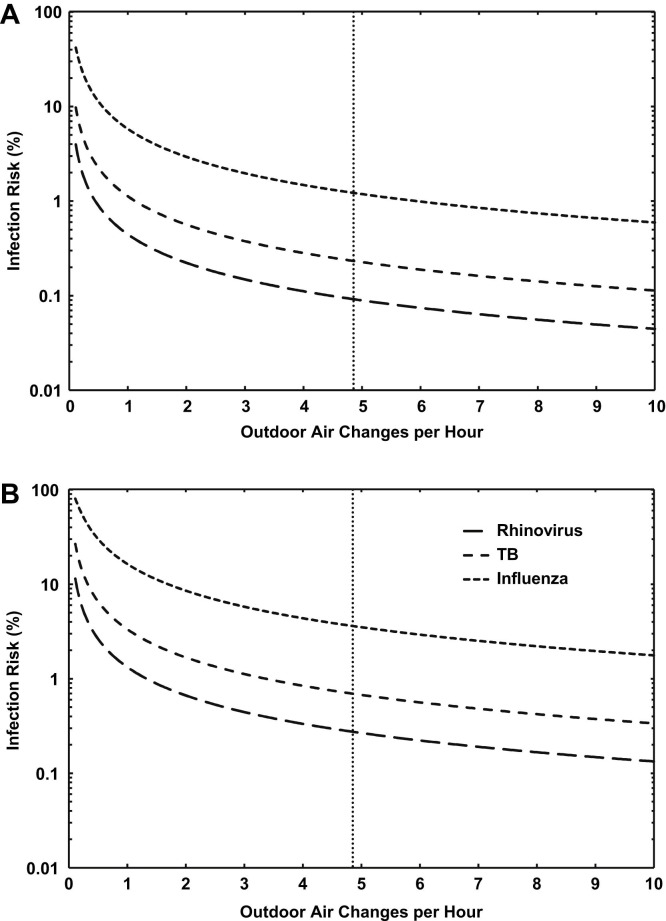
Figure 1 shows the effect of outdoor air-exchange rate on the infection risk of susceptible individuals occupying the Lung Function Laboratory for 15 minutes and for 45 minutes. For all scenarios, risk decreased rapidly with increasing air exchange. The outdoor air-exchange rate in the Lung Function Laboratory (4.9 ACH; shown as the vertical dotted line in the figure) was relatively high and resulted in risks ranging from 0.1% after a 15-minute exposure to rhinovirus to 3.6% after a 45-minute exposure to influenza.
Fig 1.
(A) Modeled influenza, TB, and rhinovirus infection risk as a function of outdoor air exchange rate for individuals occupying the Lung Function Laboratory for 15 minutes in the presence of an infectious person. The current outdoor air-exchange rate is indicated by the vertical dotted line. (B) Equivalent risks for 45 minutes of exposure.
ED isolation room
Because of the high air-exchange rate in the ED isolation room (23.8 ACH), steady-state quanta concentrations were achieved after approximately 15 minutes. Consequently, these values were low: 0.12 quanta/m3 for influenza, 0.02 quanta/m3 for TB, and 0.009 quanta/m3 for rhinovirus. The estimated time required to achieve a 99.9% reduction in quanta concentrations after the departure of an infectious individual was 18 minutes. The risk posed to an individual entering the room immediately after the departure of an infectious influenza case (ie, worst-case scenario) and remaining there for 30 minutes or 8 hours was 0.3%. The additional 7.5 hours of occupancy time in the latter case did not have a significant effect on risk, as there were no new sources of influenza quanta in the room.
Outpatient consulting rooms
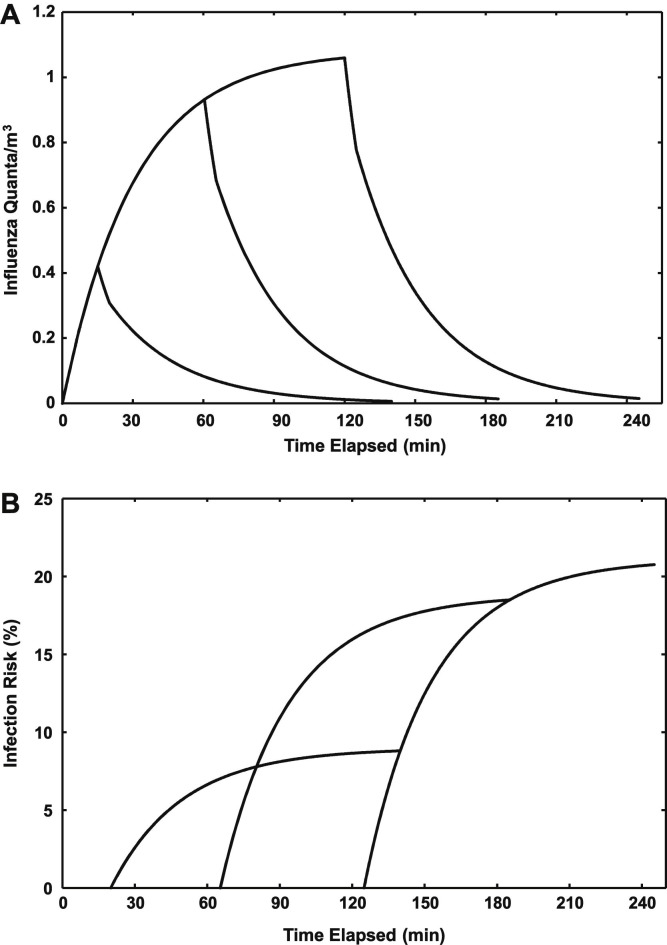
Figure 2 A shows the modeled influenza quanta concentrations in outpatient consulting room A during a consultation with an infectious individual for up to 120 minutes. The figure also shows the decays in quanta concentrations following departure of the individual after 15, 60, and 120 minutes, including an initial 5-minute period with the room door open. Figure 2B shows the estimated risk of infection for a susceptible individual entering the room after each of these periods.
Fig 2.
(A) Modeled influenza quanta concentrations in outpatient consulting room A for consultations with an infectious individual for up to 120 minutes. The decays in quanta concentrations following the departure of the infectious person after 15-, 60-, and 120-minute consultations, including an initial 5-minute period with the door open, are shown. (B) Corresponding infection risks for a susceptible person entering the room after the 5-minute open-door period and remaining in the room for up to 120 minutes.
During a 15-minute consultation with an infectious individual, there was insufficient time for the quanta concentration to reach its steady-state value (1.08 quanta/m3). An initially sharp decrease in quanta when the door was opened and the infectious individual departed was curtailed as the air exchange rate decreased once the susceptible individual entered and the door was closed. As shown in Figure 2B, the subsequent risk of infection to the susceptible individual ranged from 3.6% for the 15-minute consultation to 8.8% for the 120-minute consultation.
A similar pattern, albeit of greater magnitude, was seen for the 60- and 120-minute consultations with an infectious individual. In these scenarios, quanta concentrations approached their steady-state values at the conclusion of the consultation. When the room had been previously been occupied for 60 minutes by an infectious person, the susceptible individual’s estimated infection risk ranged from 8.1% for a 15-minute consultation to 18.5% for a 120-minute consultation. The equivalent range assuming previous occupation by an infectious individual for 120 minutes was 8.8%-20.7%.
Discussion
The scarcity of scientific evidence available to underpin ventilation guidelines in hospitals makes modeling studies an attractive approach to developing customized airborne infection control policies. In the present study, we focused on producing conservative risk estimates that reflect the real-world activities of individuals (staff, patients, and visitors) at the study location. It is important to keep in mind that our risk estimates are expressed as percentages, and although this is a convenient and intuitive metric, it might not translate to a significant absolute number of infections in a room with low occupancy.19, 23
The points at which the existing air exchange rate in the Lung Function Laboratory intersects the curves shown in Figure 1 indicate that increasing air exchange further would provide a negligible reduction to an already very low infection risk. The attendant increase in energy consumption required would be difficult to justify when the size of the room and its typical occupancy of up to 10 persons are considered.24 A similar situation exists in the ED isolation room, where occupancy was low and outdoor air exchange was very high; even in the worst-case scenario, a very low risk of 0.3% was estimated. The ventilation rate in the ED isolation room afforded substantial protection from the 3 pathogens modeled and was approximately twice that recommended by the Centers for Disease Control and Prevention for airborne infection isolation rooms.25 Although the real-world bases of prescribed ventilation guidelines are limited, in these 2 scenarios, modeling demonstrated that measured air exchange limited the infection risk to relatively low levels. For both clinical rooms, in the case of a highly contagious airborne infection and/or highly susceptible group, modeling could be useful when conducted on a case-by-case basis to assess the benefit of increasing air exchange on infection risk.
Infections arising from airborne transmission during time spent in a physician’s waiting room or consulting room after the departure of an infectious individual have been documented previously.3, 4 However, those occurrences resulted from a combination of a pediatric source case and a highly infectious airborne pathogen (measles), which were compounded by low outdoor air ventilation rates. Airborne transmission is widely acknowledged as the mechanism of spread of tuberculosis, although its role in influenza and rhinovirus transmission is less well established.16 Nonetheless, there is evidence to support increased airborne transmission of the latter 2 diseases under low outdoor air exchange conditions.7, 15 Our results suggest that the risk of influenza infection for susceptible individuals entering outpatient consulting room A, although relatively low, are not negligible despite the fact that the total and outdoor air change rates meet guidelines recommended for patient examination rooms and general wards.10 This further emphasizes the need to develop a rigorous scientific basis for prescribing minimum ventilation rates within a diverse range of hospital environments.9 It is also noteworthy that natural ventilation can reduce airborne infection risk and energy consumption compared with mechanical ventilation,26, 27 although this requires a climate amenable to this practice and appropriate planning of the hospital environment.
While ventilation rates increased in outpatient consulting rooms A and B when doors were opened compared to when doors were closed, the increase was approximately 50% greater in consulting room A. Although not modeled, the risks to a susceptible individual entering room B thus would be greater than those shown in Figure 2B. Such room-specific idiosyncrasies underscore the potential pitfalls of generalizing results, even between 2 proximate and similar rooms. Notwithstanding this, the infection risk in both rooms could be further reduced by allowing their doors to remain open for longer periods after occupation by a potentially infectious individual. However, reducing the risk in one room occupied by a handful of individuals at the expense of increased risk in more densely occupied adjacent areas (eg, waiting room) would represent a false economy. The infectiousness of the pathogen, the air volume into which it would be mixed, and the number of susceptible persons located nearby would need to be carefully considered and balanced against existing risks before opening doors could be recommended as a general control strategy. Further research addressing this issue is needed.
Certain locations within hospitals are likely to be airborne infection hot spots, especially those in which large numbers of untriaged individuals assemble. Beggs et al23 estimated the risks of airborne transmission of TB, influenza, and measles in a hypothetical hospital waiting area containing a single infectious individual and found respective mean risks of infection for susceptible persons of 0.3%, 2.6%, and 13.5% for a 30-minute wait and 0.8%, 6.6%, and 30.9% for a 60-minute wait. Despite differences in methodology, the present study and that of Beggs et al23 highlight the approximate relative risks faced by individuals during the time spent waiting for and during medical consultations. The risk of an individual acquiring influenza appears more likely during the actual consultation, if the room was previously occupied by an infectious individual, than during the period spent in a waiting room based on the limited scenarios modeled in the 2 studies.
Infection risk modeling using the W-R or G-N model has several limitations that reflect the varying degrees to which its assumptions represent real-world conditions; these have been discussed in detail elsewhere.19, 20, 23, 24 A key limitation is the reliance on quanta generation rates that have been calculated by a handful of previous epidemiologic investigations, although recent work suggests that this can be ameliorated somewhat by adopting a stochastic approach.23 We sought to minimize this limitation by using values approximately representative of median cases reported in the literature.5, 21 Nonetheless, it is prudent to view the output of infection risk models from a relative perspective.23, 24, 26 Moreover, we did not assess risks posed to health care workers,6, 28 which are undoubtedly greater than those presented here, given these individuals’ longer exposure times.
In locations with suitably accurate ventilation control systems, it may be possible to infer outdoor air exchange for some rooms from known total air flow rates and outdoor air intake proportions, thus enabling calculation of real-time infection risk estimates when combined with occupancy at a given time. However, such an approach would need to be capable of representing air-exchange rates at the room rather than AHU scale, and would be appropriate only for locations in which air exchange from nonmechanical means (eg, infiltration) is small relative to that delivered mechanically. For greatest accuracy, a measurement-oriented approach should be used, even if only to validate the utility of the foregoing method.
This study has built on previous work by estimating the airborne infection risk posed to individuals both simultaneous with and subsequent to the presence of an infectious person. Infection risk was found to vary considerably in the different locations assessed. A simple model provided useful information regarding relative infection risks and the role of room ventilation as a determinant. The risk of influenza infection in susceptible individuals entering an outpatient consultation room after the departure of an infectious person was related to the occupancy time of both parties and the outdoor air-exchange rate. Allowing the door to remain open for longer periods between consultations in the room that we investigated could reduce transmission risk by significantly increasing the air-exchange rate. However, such a basic infection control strategy cannot be recommended without an appropriately detailed assessment of its effects on infection risks in proximate areas.
We have highlighted the utility of a customized approach that accounts for typical occupancy patterns of individuals at our study site. Ventilation measurements and modeling can be used to produce location-specific risk estimates that err on the side of caution and inform airborne infection control and patient management practices. Such an approach may find increasing applications in the wake of the 2009 H1N1 pandemic, and in locations dealing with particularly susceptible individuals.
Acknowledgments
We thank the Hospital Executive and staff in the Emergency Department, Lung Function Laboratory and Outpatients Department at the study site, all of whom generously accommodated the research team and their equipment. We are grateful to the Engineering Department staff at TPCH for their expert advice regarding the ventilation systems with the hospital.
Footnotes
Financial support was provided by National Health and Medical Research Council Project Grant 455919, a Queensland University of Technology Institute of Health and Biomedical Innovation Human Health and Well Being Collaborative Research Development Grant, and a grant from the Center for Advanced Studies at Warsaw University of Technology (to P.G.).
Conflict of interest: None to report.
Appendix: Equations
In addition to (1), (2) in the text body, we also used the following equations (see main text for nomenclature): Total number of quanta within a room under steady-state conditions, n :
| (3) |
The quanta concentration (q/m3) in the room at time t (nt ) whilst occupied by an infectious person:
| (4) |
The quanta concentration (q/m3) in the room at time t2 following the departure of an infectious person at time t1 4:
| (5) |
Modeling approach
To calculate risk estimates that were conservative (ie, not likely to be underestimates), we used the steady-state variation of the G-N model by setting n0 to the steady-state value [eq (3)] for situations where the initial quanta concentration was lower than its steady-state value.19 Under these conditions, G-N and W-R model outputs are the same. When modeling risk to an individual entering a room after the departure of infectious individual, steady-state, initial and decaying quanta concentrations were calculated using (3), (4), (5), and the G-N model shown as eq (2) was used.
References
- 1.Riley E.C., Murphy G., Riley R.L. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978;107:421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 2.Moser M.R., Bender T.R., Margolis H.S., Noble G.R., Kendal A.P., Ritter D.G. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 3.Bloch A.B., Orenstein W.A., Ewing W.M., Spain W.H., Mallison G.F., Herrmann K.L. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics. 1985;75:676–683. [PubMed] [Google Scholar]
- 4.Remington P.L., Hall W.N., Davis I.H., Herald A., Gunn R.A. Airborne transmission of measles in a physician’s office. JAMA. 1985;253:1574–1577. [PubMed] [Google Scholar]
- 5.Nardell E.A., Keegan J., Cheney S.A., Etkind S.C. Airborne infection: theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144:302–306. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 6.Menzies D., Fanning A., Yuan L., Fitzgerald M. Hospital ventilation and risk for tuberculosis in Canadian health care workers. Ann Intern Med. 2000;133:779–789. doi: 10.7326/0003-4819-133-10-200011210-00010. [DOI] [PubMed] [Google Scholar]
- 7.Myatt T.A., Johnston S.L., Zuo Z., Wand M., Kebadze T., Rudnick S. Detection of airborne rhinovirus and its relation to outdoor air supply in office environments. Am J Respir Crit Care Med. 2004;169:1187–1190. doi: 10.1164/rccm.200306-760OC. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Huang X., Yu I.T.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2004;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Leung G.M., Tang J.W., Yang X., Chao C.Y.H., Lin J.Z. Role of ventilation in airborne transmission of infectious agents in the built environment: a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 10.Beggs C.B., Kerr K.G., Noakes C.J., Hathaway E.A., Sleigh P.A. The ventilation of multiple-bed hospital wards: review and analysis. Am J Infect Control. 2008;36:250–259. doi: 10.1016/j.ajic.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Stilianakis N.I., Drossinos Y. Dynamics of infectious disease transmission by inhalable respiratory droplets. J R Soc Interface. 2010;7:1355–1366. doi: 10.1098/rsif.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick E.C., Jennings L.C., Mink K.A., Wartgow C.D., Inhorn S.L. Aerosol transmission of rhinovirus colds. J Infect Dis. 1987;156:442–448. doi: 10.1093/infdis/156.3.442. [DOI] [PubMed] [Google Scholar]
- 13.Roy C.J., Milton D.K. Airborne transmission of communicable infection: the elusive pathway. N Engl J Med. 2004;350:1710–1712. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 14.Tellier R. Aerosol transmission of influenza A: a review of new studies. J R Soc Interface. 2009;6:S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persily A.K. Evaluating building IAQ and ventilation with indoor carbon dioxide. ASHRAE Trans. 1997;103:193–204. [Google Scholar]
- 18.Gammaitoni L., Nucci M.C. Using a mathematical model to evaluate the efficacy of TB control measures. Emerg Infect Dis. 1997;3:335–342. doi: 10.3201/eid0303.970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beggs C.B., Noakes C.J., Sleigh P.A., Fletcher L.A., Siddiqi K. The transmission of tuberculosis in confined spaces: an analytical review of alternative epidemiological models. Int J Tuberc Lung Dis. 2003;7:1015–1026. [PubMed] [Google Scholar]
- 20.Sze To G.N., Chao C.Y.H. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20:2–16. doi: 10.1111/j.1600-0668.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myatt T.A., Minegishi T., Allen J.G., MacIntosh D.L. Control of asthma triggers in indoor air with air cleaners: a modeling analysis. Environ Health. 2008;7:43. doi: 10.1186/1476-069X-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner B.G., Coburn B.J., Blower S. Calculating the potential for within-flight transmission of influenza A (H1N1) BMC Med. 2009;7:81. doi: 10.1186/1741-7015-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beggs C.B., Shepherd S.J., Kerr K.G. Potential for airborne transmission of infection in the waiting areas of healthcare premises: stochastic analysis using a Monte Carlo model. BMC Infect Dis. 2010;10:247. doi: 10.1186/1471-2334-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardell E.A. The role of ventilation in preventing nosocomial transmission of tuberculosis. Int J Tuberc Lung Dis. 1998;2:S110–S117. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Morb Mortal Wkly Rep. 2005;54:1–142. [Google Scholar]
- 26.Escombe A.R., Oeser C.C., Gilman R.H., Navincopa M., Ticona E., Pan W. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:309–317. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian H., Li Y., Seto W.H., Ching P., Ching W.H., Sun H.Q. Natural ventilation for reducing airborne infection in hospitals. Build Environ. 2010;45:559–565. doi: 10.1016/j.buildenv.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fennelly K.P., Nardell E.A. The relative efficacy of respirators and room ventilation in preventing occupational tuberculosis. Infect Cont Hosp Epidemiol. 1998;19:754–759. doi: 10.1086/647719. [DOI] [PubMed] [Google Scholar]