Highlights
-
•
Novel color additive for chlorine disinfectants aids visibility and coverage.
-
•
Chlorine with and without color additive achieved the same antimicrobial efficacy.
-
•
The color additive improved the efficacy of 0.5% sodium hypochlorite against coronavirus.
-
•
Chlorine with and without color additive were negligible skin irritants.
-
•
The color additive did not adversely affect efficacy or skin safety of chlorine.
Key Words: Chlorine disinfectant, Color additive, Efficacy test, Skin irritation, Antimicrobial efficacy, Skin safety
Abstract
Objective
A novel color additive colorizes chlorine disinfectants blue to improve visibility and enhance spray surface coverage, and it fades to colorless to indicate elapsed contact time. We investigated its interactions with 3 chlorine disinfectants to determine if the additive would adversely affect the disinfectants' antimicrobial efficacy or skin safety.
Methods
We tested 0.5% sodium hypochlorite, 0.2% calcium hypochlorite, and 0.5% sodium dichloroisocyanurate (NaDCC) alone versus with color additive. An independent laboratory tested efficacy against Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholerae, and human coronavirus 229E. An independent laboratory also tested direct skin irritation.
Results
Chlorine disinfectants with and without color additive achieved equal levels of efficacy against the tested pathogens. Against S. aureus, 0.5% sodium hypochlorite with and without color additive met Environmental Protection Agency criteria for disinfection success. Against human coronavirus 229E, 0.5% sodium hypochlorite alone failed disinfection success criteria, whereas 0.5% sodium hypochlorite with color additive achieved full viral inactivation (≥4.50 log10 reduction). Against V. cholerae, 0.2% calcium hypochlorite alone and with color additive achieved 5.99 log10 and >6.03 log10 reductions, respectively. Against S. aureus and P. aeruginosa, 0.5% NaDCC with and without color additive achieved >4.9 log10 and >3.54 log10 reductions, respectively. All 3 chlorine disinfectants with color additive tested as negligible skin irritants.
Conclusions
This color additive can be combined with chlorine disinfectants without adversely affecting antimicrobial efficacy or skin safety.
Environmental surface cleaning is a key tenet of infection prevention programs, and its proper execution has been shown to reduce healthcare-associated infections (HAIs).1 However, current disinfection practices often fail because of human error, lack of protocol compliance, and the difficulty of monitoring cleaning techniques.2 Furthermore, the optimal use of disinfectants in accordance with manufacturer guidelines is typically infeasible in real-world settings, as spray disinfectants have been shown to cover less than 33% of a surface or evaporate in less than 2 minutes, without reaching required wet-contact times.3, 4, 5
These shortfalls severely limit the effectiveness of environmental surface cleaning as an infection prevention strategy. Less than 50% of high-touch surfaces in healthcare facilities are properly cleaned.6 One study found that despite housekeepers being instructed to use 10% bleach to clean rooms with Clostridium difficile-infected patients, 78% of these rooms still tested positive for C. difficile after terminal cleaning.7 There is a consensus that improving compliance with disinfection protocols and techniques is critical to reducing HAIs.2, 7, 8, 9
A novel color additive, Highlight® (Kinnos Inc, Brooklyn, New York), colorizes chlorine disinfectants blue to allow for improved visibility and thoroughness of application. Upon spraying on a surface, chlorine disinfectants with the color additive fade from blue to colorless to indicate when the proper contact time has been met. They also exhibit lowered surface tension that improves spray surface coverage to >99.9%.4, 10 This novel additive addresses the major shortfalls of environmental cleaning by ensuring compliance with wet-contact time, improving coverage of sprayed surfaces, and providing real-time ability to monitor cleaning techniques.
This color additive is considered an adjuvant under U.S. Environmental Protection Agency (EPA) guidelines and does not require registration as a pesticide.11 However, given the novel nature of this product, it is important to demonstrate that its addition to chlorine disinfectants does not compromise antimicrobial efficacy or pose any safety risks. We evaluated the effect of color additive on the antimicrobial efficacy and skin safety of 3 major chlorine disinfectants: 0.5% sodium hypochlorite, 0.2% calcium hypochlorite and 0.5% sodium dichloroisocyanurate (NaDCC). This study presents the efficacy test results of an independent Good Laboratory Practice (GLP)-compliant facility against Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholerae, and human coronavirus 229E. This study also presents results from another independent GLP-compliant laboratory that tested skin irritation from chlorine disinfectants with and without color additive. Our results confirm that when added to chlorine disinfectants, the color additive does not reduce antimicrobial efficacy or cause skin irritation.
METHODS
An independent GLP-compliant facility, Microchem Laboratory (Round Rock, Texas), performed efficacy tests in accordance with test methods established by the Association of Official Analytical Chemists (AOAC) and the American Society for Testing and Materials (ASTM), as outlined in Table 1 . Another independent GLP-compliant facility, Toxikon Corporation (Bedford, Massachusetts), performed skin irritation studies in accordance with test methods established by the International Organization for Standardization (ISO). The same batches of commercially produced chlorine disinfectants were tested with or without the addition of the Highlight® color additive to directly compare efficacy and skin safety results. Detailed test methods used in these studies are detailed in Appendix 1.
Table 1.
Overview of efficacy test methodology and organisms for 3 chlorine disinfectants with and without color additive
| Test Substances | Test Organisms | Contact Time | Test Method | Study ID Number |
|---|---|---|---|---|
| 0.5% sodium hypochlorite vs. 0.5% sodium hypochlorite with color additive | Staphylococcus aureus (ATCC 6538) | 10 min ± 5 sec | AOAC 961.02 | GLP1540+GLP1550 |
| Human coronavirus 229E (ATCC VR-740) | 10 min ± 5 sec | ASTM E1053 | GLP1536+GLP1538 | |
| Pseudomonas aeruginosa (ATCC 15442) | 5 min ± 5 sec | ASTM E1153 | NG8698* | |
| 0.2% calcium hypochlorite vs. 0.2% calcium hypochlorite with color additive | Vibrio cholerae (ATCC 39050) | 3 min ± 5 sec | ASTM E2315 | NG8285 |
| Vibrio cholerae (ATCC 39050) | 3 min ± 5 sec | ASTM E2315 | NG9676* | |
| Pseudomonas aeruginosa (ATCC 15442) | 5 min ± 5 sec | ASTM E1153 | NG8698* | |
| 0.5% NaDCC control vs. 0.5% NaDCC with color additive | Staphylococcus aureus (ATCC 6538) | 5 min ± 5 sec | ASTM E1153 | NG8156 |
| Pseudomonas aeruginosa (ATCC 15442) | 5 min ± 5 sec | ASTM E1153 | NG8156 | |
| Pseudomonas aeruginosa (ATCC 15442) | 5 min ± 5 sec | ASTM E1153 | NG9375* |
NaDCC = sodium dichloroisocyanurate.
Studies in which the test substances were prepared and left at room temperature for four hours prior to efficacy testing.
Efficacy test against S. aureus using AOAC method 961.02
This study was conducted using a previously described protocol, AOAC 961.02.12, 13, 14 Three lots of 0.5% sodium hypochlorite were tested against S. aureus (ATCC 6538) at the contact time of 10 minutes, based on the label claims of a common household bleach.15 First, 0.5% sodium hypochlorite was prepared by diluting regular bleach (Clorox, Oakland, California) in sterile AOAC hard water. This study was repeated using 3 lots of the color additive mixed into the same lots of 0.5% sodium hypochlorite solution immediately before testing. Next, 60 carriers inoculated with dried S. aureus were sprayed with the test substance and left undisturbed for a contact time of 10 minutes ± 5 seconds. After neutralization, the carriers were transferred into tubes and assessed for growth.
Efficacy test against human coronavirus 229E using ASTM method E1053
This study was conducted using a previously described protocol, ASTM E1053.16, 17, 18 Two lots of 0.5% sodium hypochlorite were tested against human coronavirus strain 229E (ATCC VR-740) at the contact time of 10 minutes, based on the label claims of a common household bleach.15 This test was repeated using the same lots of 0.5% sodium hypochlorite mixed with 2 lots of the color additive. Carriers of dried human coronavirus 229E film were sprayed with the test substance and left undisturbed for 10 minutes ± 5 seconds, then neutralized and filtered. Serial 10-fold dilutions of the resultant filtrate were then prepared and applied in quadruplicate to multi-well plates containing normal lung fibroblast cell monolayers. After incubation, each well was examined for cytopathic effects indicating infectious virus. The Spearman-Karber method was used to quantify the amount of infectious virus recovered.
Efficacy test against V. cholerae using ASTM method E2315
A suspension efficacy test was performed using a previously described protocol, ASTM E2315.19, 20, 21 Calcium hypochlorite was tested against V. cholerae (ATCC 39050) with a 0.2% concentration based on World Health Organization and U.S. Centers for Disease Control and Prevention cholera guidelines and a contact time of 3 minutes, based on the standard practices of a nongovernmental organization that field-tested the color additive during the 2017 Haiti outbreak.22, 23, 24 The suspension test method is commonly used for efficacy testing against pathogens like V. cholerae that cannot survive the drying phase of standard hard surface tests.25
For study NG8285, 0.2% calcium hypochlorite was prepared by dissolving 68% Calcium Hypochlorite Granular (Lonza, Basel, Switzerland) in AOAC hard water immediately before efficacy testing. This test was repeated using the same lot of 0.2% calcium hypochlorite combined with color additive. For study NG9676, both test substances were prepared and allowed to rest at room temperature for 4 hours before testing to determine if the color additive affected antimicrobial efficacy of 0.2% calcium hypochlorite over time. Ten mL of test substance was inoculated with 0.5 mL of V. cholerae culture for 3 minutes, neutralized, then plated and enumerated for colony-forming units (CFU) to determine log reduction after exposure.
Efficacy tests against S. aureus and P. aeruginosa using ASTM method E1153
This study was conducted using a previously established protocol, ASTM E1153.26, 27, 28, 29 In study NG8156, 0.5% NaDCC was tested against both S. aureus and P. aeruginosa (ATCC 15442) with a contact time of 5 minutes, based on the label claims of an existing 0.5% chlorine NaDCC disinfectant.30 This test was repeated using the same lot of 0.5% NaDCC with the addition of color additive. 0.5% NaDCC was prepared by dissolving OASIS NaDCC Granules (Hydrachem, West Sussex, United Kingdom) in sterile AOAC hard water.
In study NG8698, 4 test substances were prepared (0.5% sodium hypochlorite and 0.2% calcium hypochlorite, both with and without color additive) and allowed to rest at room temperature for 4 hours. Then, efficacy testing against P. aeruginosa was conducted with a contact time of 5 minutes to determine if prolonged exposure to the color additive interfered with the ability of these chlorine solutions to meet the ASTM E1153 passing criteria of >3 log10 reduction. Similarly, in study NG9375, 0.5% NaDCC solutions with and without color additive were prepared, allowed to rest at room temperature for 4 hours, then tested against P. aeruginosa with a contact time of 5 minutes. Test carriers inoculated with dried pathogen were sprayed with the test substance, neutralized, then plated and enumerated to determine log reduction after exposure.
Direct primary skin irritation test using ISO 10993-10
We evaluated the potential for 6 test substances (0.5% sodium hypochlorite, 0.5% calcium hypochlorite, and 0.5% NaDCC either alone or in combination with their formula of the color additive) to produce primary dermal irritation after 4 hours of direct exposure. An independent GLP-compliant facility, Toxikon Corporation, performed these studies in accordance with U.S. Food and Drug Administration (FDA)-recognized test methods established by the ISO.31, 32
Three New Zealand white rabbits for each test substance were acclimatized for a minimum of 5 days before treatment. Then, 0.5 mL of test substance was applied directly to 2 test sites per animal, which were then bandaged. Two control sites per animal were left untreated and bandaged in the same manner as the test sites. After 4 hours, the dressings were removed and sites observed for signs of erythema and edema after 1, 24, 48, and 72 hours. Signs of erythema and edema were both scored from 0 to 4. The final Primary Irritation Index (PII) of each test substance was determined by summing erythema and edema scores and averaging across time points (3), number of test sites (2), and number of animals (3).
RESULTS
Effect of color additive on 0.5% sodium hypochlorite efficacy against S. aureus using AOAC 961.02
Table 2 shows the results of studies GLP1540 and GLP1550, in which 0.5% sodium hypochlorite was tested alone and in combination with color additive against S. aureus at a contact time of 10 minutes. To pass EPA performance criteria for successful disinfection, 3 lots of the test substance must be tested, with at least 59 of the 60 test carriers for each lot confirmed negative for any surviving S. aureus. Three lots of 0.5% sodium hypochlorite successfully disinfected 60, 60, and 59 of the 60 carriers. Three lots of 0.5% sodium hypochlorite with color additive successfully disinfected 60, 60, and 59 of the 60 carriers. Both met EPA efficacy performance guidelines.
Table 2.
Study GLP1540 & GLP1550: efficacy of 0.5% sodium hypochlorite with and without color additive against S. aureus after 10 minutes ± 5 seconds contact time
| Test substance | Lot number | Test organism | Number of carriers |
Carriers disinfected | ||
|---|---|---|---|---|---|---|
| Treated | Showing growth | Confirmed as test organism | ||||
| 0.5% sodium hypochlorite | E616050 | S. aureus (ATCC 6538) | 60 | 0 | 0 | 60/60 |
| E616060 | 60 | 1 | 0 | 60/60 | ||
| E615306 | 60 | 1 | 1 | 59/60 | ||
| 0.5% sodium hypochlorite with color additive | 3-16-HLTS1 | S. aureus (ATCC 6538) | 60 | 0 | 0 | 60/60 |
| 3-16-HLTS2 | 60 | 0 | 0 | 60/60 | ||
| 3-16-HLTS3 | 60 | 1 | 1 | 59/60 | ||
Effect of color additive on 0.5% sodium hypochlorite efficacy against human coronavirus 229E using ASTM E1053
Table 3 and Table 4 show the results of studies GLP1538 and GLP1536, in which 0.5% sodium hypochlorite was tested alone and in combination with color additive against human coronavirus 229E at a contact time of 10 minutes. To pass EPA performance criteria for virucidal efficacy, test substances must demonstrate complete inactivation of the virus at all dilutions.
Table 3.
Study GLP1538: efficacy of 0.5% sodium hypochlorite after 10 minutes ± 5 seconds contact time to human coronavirus strain 229E dried on inanimate surface
| 0.5% sodium hypochlorite: Lot E616050 | 0.5% sodium hypochlorite: Lot E616060 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dilution | Dried Virus Plate Recovery Control | Human Coronavirus 229E Test Film | Human Coronavirus 229E Test Film | |||||||||
| 10−1 | + | + | + | + | + | O | O | O | O | O | O | O |
| 10−2 | + | + | + | + | O | O | O | O | O | O | O | O |
| 10−3 | + | + | + | O | O | O | O | O | O | O | O | O |
| 10−4 | + | O | O | + | O | O | O | O | O | O | O | O |
| 10−5 | O | O | O | O | O | O | O | O | O | O | O | O |
| 10−6 | O | O | O | O | O | O | O | O | O | O | O | O |
| TCID50/1.0 mL | 3.75 log10 | 0.75 log10 | ≤0.50 log10 | |||||||||
| TCID50/carrier | 4.35 log10 | 1.35 log10 | ≤1.10 log10 | |||||||||
| Reduction of virus due to inactivation by test substance: | 3.00 log10 | ≥3.25 log10 | ||||||||||
Recovery of 229E virus (+) or absence of 229E virus and cytotoxicity (O) in wells containing MRC-5 host cells. 229E virus (200 µL) was previously dried on an inanimate surface, treated with a control media or test substance, neutralized and filtered, and then serially diluted in quadruplicate.
Table 4.
Study GLP1536: efficacy of 0.5% sodium hypochlorite with color additive after 10 minutes ± 5 seconds contact time to human coronavirus strain 229E dried on inanimate surface
| Color additive: Lot HLT 3-16-HLTS1 | Color additive: Lot HLT 3-16-HLTS1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5% sodium hypochlorite: Lot E616050 | 0.5% sodium hypochlorite: Lot E616060 | |||||||||||
| Dilution | Dried Virus Plate Recovery Control | Human Coronavirus 229E Test Film | Human Coronavirus 229E Test Film | |||||||||
| 10−1 | + | + | + | + | O | O | O | O | O | O | O | O |
| 10−2 | + | + | + | + | O | O | O | O | O | O | O | O |
| 10−3 | + | + | + | O | O | O | O | O | O | O | O | O |
| 10−4 | + | O | + | + | O | O | O | O | O | O | O | O |
| 10−5 | O | O | O | + | O | O | O | O | O | O | O | O |
| 10−6 | O | O | + | + | O | O | O | O | O | O | O | O |
| TCID50/1.0 mL | 5.00 log10 | ≤0.50 log10 | ≤0.50 log10 | |||||||||
| TCID50/carrier | 5.60 log10 | ≤1.10 log10 | ≤1.10 log10 | |||||||||
| Reduction of virus due to inactivation by test substance: | ≥4.50 log10 | ≥4.50 log10 | ||||||||||
Recovery of 229E virus (+), or absence of both 229E virus and cytotoxicity (O) in wells containing MRC-5 host cells. 229E virus (200 µL) was previously dried on an inanimate surface, treated with a control media or test substance, neutralized and filtered, and then serially diluted in quadruplicate.
The 2 lots of 0.5% sodium hypochlorite alone demonstrated 3.00 log10 and ≥3.25 log10 reduction of virus, and infectious viral particles were recovered in 1 of the 10−1 dilution wells. The 2 lots of 0.5% sodium hypochlorite with color additive both demonstrated ≥4.50 log10 reduction of virus. Under these test conditions, the combination of 0.5% sodium hypochlorite with color additive completely inactivated human coronavirus 229E and met EPA performance criteria for virucidal efficacy, whereas 0.5% sodium hypochlorite alone failed the disinfection success criteria due to recovery of infectious viral particles from 1 lot.
Effect of color additive on 0.2% calcium hypochlorite efficacy against V. cholerae using ASTM E2315
Figure 1 shows the results of studies NG8285 and NG9679, in which 0.2% calcium hypochlorite was tested alone and in combination with color additive against V. cholerae at a contact time of 3 minutes. 0.2% calcium hypochlorite alone and with color additive were prepared immediately before efficacy testing and demonstrated 5.99 log10 and >6.03 log10 reduction of V. cholerae, respectively. For study NG9679, both test substances were prepared and allowed to rest at room temperature 4 hours before efficacy testing, and both demonstrated 6.58 log10 reduction.
Fig 1.

Studies NG8285 and NG9676: log10 reduction of V. cholerae when treated with 0.2% calcium hypochlorite with and without color additive for 3 minutes ± 5 seconds. Test solutions were tested for efficacy either immediately or 4 hours after preparation.
Effect of color additive on 0.5% NaDCC efficacy against P. aeruginosa and S. aureus using ASTM E1153
Figure 2 shows the results of study NG8156, in which 0.5% NaDCC was tested alone and with color additive against P. aeruginosa and S. aureus at a contact time of 5 minutes. Against S. aureus, 0.5% NaDCC alone and with color additive both reduced the bacteria below detectable limits, with a reduction of >4.9 log10 (>99.9988%). Against P. aeruginosa, both test substances demonstrated >3.54 log10 (>99.97%) reduction. Overall, the efficacy results of 0.5% NaDCC with and without color additive both met ASTM passing criteria of >3 log10 reduction.
Fig 2.
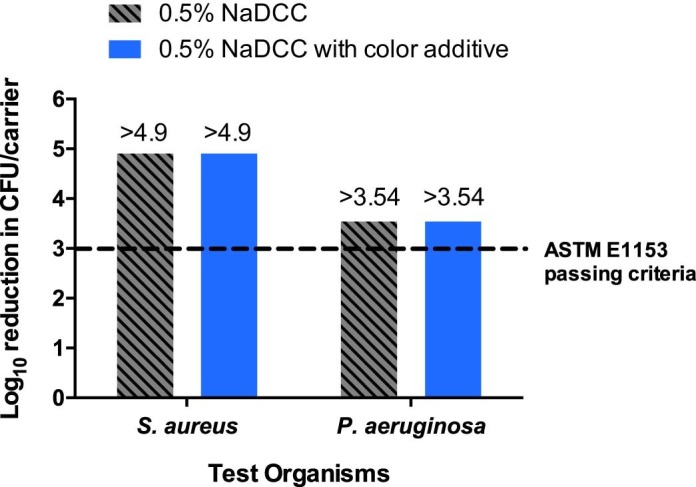
Study NG8156: efficacy of 0.5% NaDCC with and without color additive after 5 minutes ± 5 seconds contact time against S. aureus and P. aeruginosa. The ASTM E1153 passing criterion of 3 log10 reduction is represented by the dashed line.
Effect of color additive on chlorine disinfectant efficacy against P. aeruginosa after 4-hour resting time using ASTM E1153
Figure 3 shows the results of studies NG8698 and NG9375, in which 0.5% sodium hypochlorite, 0.2% calcium hypochlorite, and 0.5% NaDCC were prepared alone and in combination with their respective formulas of color additive, allowed to rest for 4 hours, then tested against P. aeruginosa using a contact time of 5 minutes. 0.5% sodium hypochlorite alone and with color additive achieved >4.1 log10 (>99.992%) and 3.8 log10 (99.98%) reduction, respectively. 0.2% calcium hypochlorite alone and with color additive both demonstrated 3.8 log10 (99.98%) reduction. 0.5% NaDCC alone and with color additive both achieved >4.34 log10 (>99.995%) reduction. Overall, the addition of color additive to all 3 chlorine solutions did not affect their ability to achieve the ASTM-required >3 log10 reduction, even after prolonged resting time.
Fig 3.

Studies NG8698 and NG9375: efficacy of chlorine disinfectants with and without color additive after 5 minutes ± 5 seconds contact time against P. aeruginosa, when prepared and allowed to rest 4 hours in advance of efficacy testing. The ASTM E1153 passing criterion of 3 log10 reduction is represented by the dashed line.
Skin irritation profile of chlorine disinfectants with and without color additive
Table 5 shows the results of direct primary skin irritation tests performed on rabbits using the 3 chlorine disinfectants alone or with color additive. The PII is a quantitative evaluation of the ability of a test substance to provoke inflammatory response in the skin and is determined based on triplicate testing of animals on 2 test sites through 3 time points. Any PII score above 0 indicates that erythema or edema occurred at least once during the observation period. Based on FDA evaluation criteria, test substances with a PII score of less than 0.5 are considered negligible irritants; a PII score of 0.5-<2.0 indicates a slight irritant; and a PII score of 2.0-<5.0 indicates a moderate irritant. Substances with a PII score of >5.0 are considered severe irritants.31
Table 5.
Skin irritation testing: erythema and edema irritation scores of individual rabbits after 4-hour substance exposure
| Test substance | Animal no. | Primary Irritation Index (PII) score (erythema/edema)* |
Final PII‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 hour† |
24 hours |
48 hours |
72 hours |
|||||||
| Test site 1 | Test site 2 | Test site 1 | Test site 2 | Test site 1 | Test site 2 | Test site 1 | Test site 2 | |||
| 0.5% sodium hypochlorite | 60135 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| 60136 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.0 | |
| 60137 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | ||
| 0.5% sodium hypochlorite + color additive | 60073 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| 60074 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.0 | |
| 60075 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | ||
| 0.5% calcium hypochlorite | 60615 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| 60616 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.1 | |
| 60617 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2/0 | 0/0 | ||
| 0.5% calcium hypochlorite + color additive | 60612 | 1/0 | 1/0 | 1/0 | 1/0 | 1/0 | 1/0 | 0/0 | 0/0 | |
| 60613 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.2 | |
| 60614 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | ||
| 0.5% NaDCC | 60702 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| 60704 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.0 | |
| 60706 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | ||
| 0.5% NaDCC + color additive | 60696 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| 60698 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.0 | |
| 60700 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | ||
NaDCC = sodium dichloroisocyanurate.
All readings were taken after the dressing removal.
1-hour scores are not included in determining PII.
PII = sum of (erythema + edema scores) / (2 test sites x 3 observation points) for each animal / 3 animals.
No signs of erythema or edema were found on any of the untreated bandaged control sites for every animal. 0.5% sodium hypochlorite alone or with color additive and 0.5% NaDCC alone or with color additive produced no signs of erythema or edema on any animal, thus yielding a PII score of 0. For 0.5% calcium hypochlorite alone, a small area of moderate erythema (score of 2) was found on Test Site 1 of Animal 60617 at the 72-hour time point, yielding a final PII score of 0.1 when averaged across all animals and test sites. For 0.5% calcium hypochlorite in combination with color additive, 1 rabbit (Animal 60612) was observed with slight erythema (score of 1) on both test sites at the 1-, 24-, and 48-hour time points, yielding a final PII score of 0.2. All test substances yielded PII scores of below 0.5 and were considered negligible irritants.
DISCUSSION
Efficacy testing performed by an independent GLP-compliant laboratory confirmed across a variety of test methods and pathogens that the addition of the color additive did not adversely affect the antimicrobial efficacy of 3 major chlorine disinfectants. In these studies, chlorine disinfectants with color additive achieved the same level of disinfection as the chlorine disinfectants alone and passed different efficacy criteria established by EPA and ASTM guidelines.
Both 0.5% sodium hypochlorite with and without color additive met EPA criteria for disinfection of S. aureus at a 10-minute contact time. 0.5% sodium hypochlorite combined with color additive also fully inactivated human coronavirus 229E after a 10-minute contact time. Interestingly, 0.5% sodium hypochlorite alone failed due to recovery of infectious viral particles in 1 of the lots. This result may have been due to factors such as inconsistencies in spray application and coverage between lots. Given the design of this study, in which a plate of dried virus film was sprayed with the disinfectant, it is possible that the surface coverage-improving properties of the color additive contributed to improved efficacy compared to 0.5% sodium hypochlorite alone, which tends to bead up and form droplets when sprayed.33 A recent study demonstrated that typical bleach disinfectants cover <33% of a sprayed surface and lose coverage over the duration of the contact time, whereas the addition of color additive improved bleach surface coverage to >99.9% and maintained this level of coverage for 15 minutes.4 When used in real-world settings, this color additive may maximize the efficacy of bleach disinfectants due to superior spray coverage.
Both 0.2% calcium hypochlorite with and without color additive demonstrated equal efficacy against V. cholerae, achieving >6.03 log10 and 5.99 log10 reduction. Also, 0.5% NaDCC with and without color additive were equally efficacious against S. aureus and P. aeruginosa after a contact time of 5 minutes, passing the ASTM criteria for disinfection success of >3.00 log10 reduction.
We were interested to see if chlorine solutions remained efficacious when combined with the color additive for a prolonged period of time, given that its blue coloration is stable for 4–6 hours in bulk solution. We therefore conducted studies in which chlorine disinfectants were prepared with or without the color additive and allowed to rest at room temperature for 4 hours before efficacy testing. After the resting period, all 3 chlorine disinfectants, with and without color additive, met the ASTM criteria of >3.00 log10 reduction for disinfection success against P. aeruginosa after a contact time of 5 minutes. Although disposal of the chlorine solutions is recommended once the blue color has faded, additional testing is warranted on the effect of the color additive on long-term shelf-life.
Evaluating the skin safety of the color additive in combination with chlorine disinfectants was a major priority, given the risk of occupational exposure to disinfectants and skin diseases in healthcare personnel.34, 35, 36 An independent GLP-compliant facility conducted direct primary skin irritation studies on all 3 chlorine disinfectants with and without color additive and found all to be negligible irritants. Thus, the addition of color additive to chlorine disinfectants does not increase their potential to cause skin irritation.
While the adoption of multifaceted infection prevention programs has helped combat HAIs, significant work remains to be done to ensure the proper execution of standard prevention practices.37 Environmental surface cleaning is a critical component of these programs, but there is a consensus that current cleaning and disinfection practices are suboptimal.1, 2, 3 Lack of adherence to protocol and poor techniques can be mitigated with careful monitoring and educational interventions. One study detailing the successful reduction of C. difficile contamination from 78% to 11% concluded that environmental cleaning interventions should provide a way to both monitor cleaning techniques and provide feedback to housekeeping staff.7 However, these monitoring programs are difficult to enforce and sustain given that housekeeper turnover reaches up to 50% in some facilities.38, 39
The color-fading properties of the color additive allow it to function as a visible, real-time feedback mechanism for thoroughness of cleaning and adherence to wet-contact times. One recent study found that healthcare personnel were more successful in correctly identifying surfaces that had been sprayed with bleach if the color additive had been added.40 Another study demonstrated that the color additive improved the surface coverage properties of bleach sprays from <33% to >99.9%.4 We recently found that workers at Ebola treatment units in Liberia and Guinea reported increased coverage and confidence of disinfection when combining the color additive with their currently used chlorine solutions.10 Although this color additive is suggested for use only on hard, nonporous surfaces, as is similarly recommended for chlorine disinfectants, it has potential applications in other settings like veterinarian clinics, food sanitation, and forensic restoration.
CONCLUSIONS
This study provided a comprehensive evaluation of the antimicrobial efficacy and skin safety profile of chlorine solutions in combination with this color additive. In conjunction with data from a recent study showing that the color additive did not adversely affect the efficacy of commercial bleach against C. difficile spores, our study demonstrated that this color additive is highly compatible with chlorine disinfectants.40 This novel color additive could play a significant role in improving cleaning compliance and environmental surface disinfection, and further studies on its ability to enhance cleaning practices in healthcare settings are warranted.
Footnotes
Funding/support: This work was supported by the United States Agency for International Development Fighting Ebola Grand Challenge (Grant no. AID-OAA-F-15-00026). The funding source played no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or in the decision to submit the paper for publication.
Conflicts of interest: KT, JK, and KJ are founders and shareholders of Kinnos Inc. and have patents pending on the Highlight® technology. AMK declares no conflicts of interest relevant to this article. All authors declare no relationship or conflicts of interest with the manufacturers of chlorine disinfectants used in this study.
Appendix 1. Detailed efficacy test and skin irritation test methods
AOAC method 961.02
AOAC Method 961.02, or the AOAC Germicidal Spray Test, is a standard test protocol specified by the EPA for antimicrobial pesticide registration. This protocol, followed for studies GLP1540 and GLP1550, was conducted in agreement with EPA guideline OCSPP 810.2200 and GLP requirements, as defined in 40 CFR § 160.12
A daily culture of S. aureus was prepared from frozen stock culture, and test cultures were initiated in 20x150-mm tubes containing 10 mL AOAC Synthetic Broth. After a 48-hour incubation at 36°C ± 1°C, all test culture tubes were pooled together with the addition of 5% ± 0.1% (v/v) fetal bovine serum (FBS) as organic bioburden. Carriers were prepared by placing autoclaved 18x36-mm glass slides into sterile petri dishes matted with sterile 9-cm filter rounds. Each carrier was inoculated by evenly spreading 10 µL of pooled test culture onto the glass slide and drying for 30–40 minutes at 36°C ± 1°C. Two sets of 3 inoculated carriers were selected for enumeration of the amount of S. aureus present on the carriers, with 1 set harvested before and after the test.
Sixty inoculated carriers were treated with 3 sprays of the test substance (0.5% sodium hypochlorite with or without color additive) on mist setting at a distance of 6–8 inches and an angle of 45° and left undisturbed for a contact time of 10 minutes ± 5 seconds. The treated carriers were then individually transferred into 25x150-mm test tubes containing Letheen Broth supplemented with 0.1% sodium thiosulfate and 0.5% Tween 80, to both neutralize the test substance and allow any surviving S. aureus to grow. After a 48-hour incubation at 36°C ± 1°C, the 60 test tubes were assessed for growth. Any tubes showing growth were confirmed to not be a result of contamination by plating on growth media and testing for S. aureus. Three lots of each test substance were tested, with 60 carriers for each lot test.
All of the EPA-specified criteria for experimental success were met in these studies. The set of carriers from each lot that were enumerated for S. aureus before and after the test all demonstrated the required mean log10 density of between 5.0 and 6.5. Additional details around the control criteria that were met for this study to proceed (media sterility control, viability growth control, and neutralization control) may be found in the cited study protocols.13, 14
ASTM Method E1053
ASTM E1053, or Standard Test Method for Efficacy of Virucidal Agents Intended for Inanimate Environmental Surfaces, is the primary testing method accepted by the EPA for virucidal claims. This protocol, followed for studies GLP1536 and GLP1538, was conducted in agreement with EPA guideline OCSPP 810.2200 and GLP requirements, as defined in 40 CFR § 158.16
Stock aliquots of human coronavirus 229E were thawed on the day of use, and viral inoculum was prepared by combining the viral suspension to a 5% protein organic soil load composed of phosphate buffered saline (PBS), bovine serum albumin, bovine mucin, and yeast extract. Carriers were prepared by autoclaving 100x15-mm glass petri dishes. Then, 200 µL of viral inoculum was spread evenly inside the bottom surfaces of the carriers using a sterile cell scraper, then left to dry for 20 minutes under ambient conditions. Three carriers were prepared: 1 as a plate recovery control to determine the baseline dried virus titer and 1 for each of the 2 lots of test substance to determine levels of infectious virus after exposure to the test substance.
One dried virus test film carrier for each lot of test substance was treated with 3 sprays of the test substance (0.5% sodium hypochlorite with or without color additive) on mist setting at a distance of 6–8 inches and an angle of 45° and left undisturbed for 10 minutes ± 5 seconds. The treated carriers were then neutralized with 2 mL of 0.1% lecithin in 10% FBS Eagle's Minimum Essential Medium. The virus films were then mechanically detached from the carriers using sterile cell scrapers and filtered through pre-equilibrated Sephacryl (S-1000 SF) gel filtration columns for secondary neutralization. Serial 10-fold dilutions of the filtrate were then prepared from 10−1 to 10−6, and each dilution was applied in quadruplicate to multi-well cell culture plates containing monolayers of normal lung fibroblast (MRC-5) cells (ATCC CCL-171).
Assay plates were incubated at 37°C ± 2°C, 5% ± 1% CO2, for 7 days, and then each well was examined under a microscope for cytopathic effects, indicating presence of infectious virus. The Spearman-Karber method was used to quantify the amount of infectious virus recovered at the end of the assays.
The virus stock titer, cell culture, cytotoxicity, and test substance neutralization controls were all assayed concurrently with the plate recovery control and virus-substance exposure tests and met all criteria necessary to validate this study, additional details of which may be found in the cited study protocols.17, 18
ASTM Method E2315
ASTM E2315, or Assessment of Antimicrobial Activity Using a Time-Kill Procedure, is used to quantify the log reduction of a test microorganism after exposure in an antimicrobial suspension.19 The suspension test method is commonly used for efficacy testing against pathogens like V. cholerae that cannot survive the drying phase of standard hard surface tests.25
A culture of V. cholerae was prepared in tryptic soy broth medium as the inoculum. Ten mL of the test substance and 10 mL of the control substance (PBS) were dispensed into separate sterile tubes. Both the test and control substances were then inoculated with 500 µL of V. cholerae in growth medium and mixed. One mL of the inoculated control substance was immediately harvested to determine the starting concentration of the microorganism. After the contact time of 3 minutes ± 5 seconds, 1 mL of the inoculated test substance was harvested and chemically neutralized with 9 mL of Dey/Engley broth supplemented with 0.5% sodium thiosulfate, 0.5% lecithin, and 0.5% Tween 80. The neutralized test solution was plated on tryptic soy agar for 48 hours at 36°C ± 1°C, then enumerated to determine the number of CFUs. The surviving microbial population from the test substance exposure was compared to the population recovered from the control to determine the log reduction.
The appropriate controls (positive/growth, negative/purity, neutralization, and media sterility) were performed and confirmed to validate these studies, additional details of which may be found in the cited study protocols.20, 21
ASTM Method E1153
ASTM E1153, or Efficacy of Sanitizers Recommended for Inanimate Non-Food Contact Surfaces, is a test method typically performed to substantiate sanitizer claims for registered disinfectants. This protocol was followed for studies NG8156, NG8698, and NG9375.27, 28, 29
Cultures of S. aureus and P. aeruginosa were initiated from stock in tubes containing 10 mL of tryptic soy broth and AOAC synthetic broth, respectively. After a 24-hour incubation at 37°C ± 2°C, a 4-mm transfer loop was used to transfer the culture into 10 mL of fresh broth. Three consecutive daily transfers were made, with the final transfer of S. aureus incubated for 24 hours and the final transfer of P. aeruginosa incubated for 48 hours. Carriers were prepared by placing autoclaved 18x36-mm glass slides into sterile petri dishes. Each carrier was then inoculated with 10 µL of the culture, then dried for 30 minutes ± 2 minutes at 36°C ± 1°C. Test carriers were then treated with approximately 5 mL of the test substance by spraying 6 times from a distance of 6–8 inches at a 45° angle. Control carriers were treated with 5 mL of PBS. After a contact time of 5 minutes ± 5 seconds in ambient conditions, all carriers were chemically neutralized with 20 mL of Dey/Engley broth supplemented with 0.5% sodium thiosulfate and 0.5% Tween 80. Using standard pour-plate techniques, the neutralized substances were plated on nutrient agar and incubated for 48 hours at 36°C ± 1°C. The surviving microbial populations (CFU) on the test plates were compared to the controls to determine log reduction.
The appropriate controls (positive/growth, negative/purity, neutralization, and media sterility) were performed and confirmed to validate these studies, additional details of which may be found in the cited study protocols.26, 27, 28, 29
Direct primary skin irritation test
Toxikon Corporation performed direct primary skin irritation studies in accordance with FDA-recognized test methods established by the ISO. The studies were based on standard protocols ISO 10993-10, ISO 10993-12, and ISO/IEC 17025 and conducted in agreement with GLP requirements, as defined in 21 CFR § 58.31, 32
Three 2–4 kg New Zealand white rabbits were purchased from Covance Laboratories (Denver, Pennsylvania) for each test substance, for a total of 18 rabbits. The rabbits were maintained at 20°C ± 3°C under 30%–70% humidity, fed ad libitum, and acclimatized for a minimum of 5 days before treatment. The trunk of each rabbit was clipped free of hair within 24 hours before application of the test substance. Four application sites were designated, with 2 sites for the test substance and 2 sites for untreated control. 0.5 mL of the test substance was then applied directly to the 2 test sites, which were then covered with non-occlusive dressing and wrapped with a semi-occlusive bandage. The control sites were left untreated and wrapped in the same manner as the test sites. After a 4-hour contact period, the dressings were removed, the application sites were marked, and any residual test substance was rinsed off with sterile water.
The rabbits were observed for signs of erythema and edema at 1, 24, 48, and 72 hours after the dressings were removed. Signs of erythema and edema were both scored from 0 to 4 according to the ISO 10993-10 scoring system for skin reaction.31 The observation values for each animal were obtained by adding the erythema and edema scores at the 24-, 48-, and 72-hour time points and dividing the sum by 6 (2 test sites x 3 observation time points). The same was done for the control sites of each animal. The control scores were then subtracted from the test site scores. To obtain the final PII score of a test substance, the calculated values of the 3 animals were totaled and divided by 3.
References
- 1.Donskey C.J. Does improving surface cleaning and disinfection reduce health care-associated infections? Am J Infect Control. 2013;41:S12–S19. doi: 10.1016/j.ajic.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016;5:10. doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J.H., Sullivan N., Leas B.F., Pegues D.A., Kaczmarek J.L., Umscheid C.A. Cleaning hospital room surfaces to prevent health care-associated infections. A technical brief. Ann Intern Med. 2015;163:598–607. doi: 10.7326/M15-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyan K., Jin K., Kang J., Kyle A.M. Novel color additive for chlorine disinfectants corrects deficiencies in spray surface coverage and wet-contact time, and checks for correct chlorine concentration. Am J Infect Control. 2018;46:624–627. doi: 10.1016/j.ajic.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Rutala W.A., Weber D.J. Selection of the ideal disinfectant. Infect Control Hosp Epidemiol. 2014;35:855–865. doi: 10.1086/676877. [DOI] [PubMed] [Google Scholar]
- 6.Carling P.C., Bartley J.M. Evaluating hygienic cleaning in healthcare settings: what you do not know can harm your patients. Am J Infect Control. 2010;38:S41–S50. doi: 10.1016/j.ajic.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Eckstein B.C., Adams D.A., Eckstein E.C., Rao A., Sethi A.K., Yadavalli G.K. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis. 2007;7:61. doi: 10.1186/1471-2334-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramphal L., Suzuki S., McCracken I.M., Addai A. Improving hospital staff compliance with environmental cleaning behavior. Proc (Bayl Univ Med Cent) 2014;27:88–91. doi: 10.1080/08998280.2014.11929065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson P.A., Watson L.R., Torress-Cook A. Efficacy of a hospital-wide environmental cleaning protocol on hospital-acquired methicillin-resistant Staphylococcus aureus rates. J Infect Prev. 2016;17:171–176. doi: 10.1177/1757177416645342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J., Tyan K.S., Jin K., Kyle A.M. Field-testing of a novel color indicator added to chlorine solutions used for decontamination of surfaces in Ebola Treatment Units. J Hosp Infect. 2018;99:188–191. doi: 10.1016/j.jhin.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 11.United States Environmental Protection Agency Inert Ingredient Frequently Asked Questions. 2014. https://www.epa.gov/sites/production/files/2014-05/documents/faqs.pdf Published; Available from:
- 12.AOAC International . Official methods of analysis of AOAC International. 2016. AOAC Official Method 961.02 Germicidal spray products as disinfectants. Chapter 6. [Google Scholar]
- 13.Grant K. 2016. GLP1550: GLP AOAC Germicidal Spray Products Test. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 14.Grant K. 2016. GLP1540: GLP AOAC Germicidal Spray Products Test. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 15.US EPA Pesticide Product Label, CLOROX BLEACH. United States Environmental Protection Agency website. 2011. https://www3.epa.gov/pesticides/chem_search/ppls/005813-00001-20110913.pdf Published; Available from:
- 16.Standard Test Method to Assess Virucidal Activity of Chemicals Intended for Disinfection of Inanimate, Nonporous Environmental Surfaces. 2011. E1053-11www.astm.org ASTM. ASTM International, West Conshohocken, PA; Available from: Accessed March 8, 2018. [Google Scholar]
- 17.Guin E. 2017. GLP1536: GLP ASTM E1053 Modified for Spray Products. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 18.Guin E. 2017. GLP1538: GLP ASTM E1053 Modified for Spray Products. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 19.Standard Guide for Assessment of Antimicrobial Activity Using a Time-Kill Procedure. 2008. E2315-03www.astm.org ASTM. ASTM International, West Conshohocken, PA; Available from: Accessed March 8, 2018. [Google Scholar]
- 20.Saad C. 2017. NG8285: Antibacterial Efficacy of Kinnos's Test Substances Using a Suspension Time-Kill Procedure. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 21.Weeks C. 2017. NG9676: Antibacterial Efficacy of Kinnos' Test Substances Using a Suspension Time-Kill Procedure. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 22.International Medical Corps, Haiti . 2017. Feedback on the Use of the “Highlights” at two Cholera Treatment Units in Haiti. [Google Scholar]
- 23.United States Centers for Disease Control and Prevention Infection Control for Cholera in Health Care Settings. 2017. https://www.cdc.gov/cholera/infection-control-hcp.html Updated; Available from:
- 24.World Health Organization . World Health Organization; Geneva: 2008. Essential environmental health standards in health care. [Google Scholar]
- 25.Jones M.V., Wood M.A., Herd T.M. Comparative sensitivity of Vibrio cholerae 01 E1 Tor and Escherichia coli to disinfectants. Lett Appl Microbiol. 1992;14:51–53. [Google Scholar]
- 26.Standard Test Method for Efficacy of Sanitizers Recommended for Inanimate, Hard, Nonporous Non-Food Contact Surfaces. 2014. E1153-14www.astm.org ASTM. ASTM International, West Conshohocken, PA; Available from: Accessed March 8, 2018. [Google Scholar]
- 27.Saad C. 2017. NG8156: Antibacterial Activity and Sanitizing Efficacy of Kinnos Inc.'s Test Substances against S. aureus & P. aeruginosa. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 28.Lutz I. 2017. NG8698: Antibacterial Activity and Sanitizing Efficacy of Kinnos Inc.'s Test Substances against P. aeruginosa. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 29.Grant K. 2017. NG9375: Antibacterial Activity and Sanitizing Efficacy of Kinnos, Inc.'s Test Substances. Microchem Laboratory, Round Rock, TX. [Google Scholar]
- 30.US EPA Pesticide Product Label, XHC-S. United States Environmental Protection Agency website. 2016. https://www3.epa.gov/pesticides/chem_search/ppls/001677-00255-20160629.pdf Published; Available from:
- 31.ISO 10993-10:2010 Biological evaluation of medical devices – Part 10: Tests for irritation and skin sensitization. International Organization for Standardization website. 2010. https://www.iso.org/standard/40884.html Published; Available from:
- 32.Lister S. 2016. Final GLP Reports. Toxikon Corporation, Bedford, MA. [Google Scholar]
- 33.Omidbakhsh N. Theoretical and experimental aspects of microbicidal activities of hard surface disinfectants: are their label claims based on testing under field conditions? J AOAC Int. 2010;93:1944–1951. [PubMed] [Google Scholar]
- 34.Stingni L., Lapomarda V., Lisi P. Occupational hand dermatitis in hospital environments. Contact Dermatitis. 1995;33:172–176. doi: 10.1111/j.1600-0536.1995.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 35.Singgih S.I.R., Lantinga H., Nater J.P., Woest T.E., Kruyt-Gaspersz J.A. Occupational hand dermatoses in hospital cleaning personnel. Contact Dermatitis. 1986;14:14–19. doi: 10.1111/j.1600-0536.1986.tb01146.x. [DOI] [PubMed] [Google Scholar]
- 36.Quinn M.M., Henneberger P.K. Cleaning and disinfecting environmental surfaces in health care: toward an integrated framework for infection and occupational illness prevention. Am J Infect Control. 2015;43:424–434. doi: 10.1016/j.ajic.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 37.Dick A.W., Perencevich E.N., Pogorzelska-Maziarz M., Zwanziger J., Larson E.L., Stone P.W. A decade of investment in infection prevention: a cost-effectiveness analysis. Am J Infect Control. 2015;43:4–9. doi: 10.1016/j.ajic.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoutman D.E., Ford B.D., Sopha K. Environmental cleaning resources and activities in Canadian acute care hospitals. Am J Infect Control. 2014;42:490–494. doi: 10.1016/j.ajic.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Appelbaum E., Berg P., Frost A., Preuss G., Appelbaum E. The effects of work restructuring on low-wage, low-skilled workers in U.S. hospitals. In: Bernhadt A., Murnane R., editors. Low-wage America: how employers are reshaping opportunity in the workplace. Russel Sage Foundation; New York (NY): 2003. pp. 77–117. [Google Scholar]
- 40.Mustapha A., Cadnum J.L., Alhmidi H., Donskey C.J. Evaluation of novel chemical additive that colorizes chlorine-based disinfectants to improve visualization of surface coverage. Am J Infect Control. 2018;46:119–121. doi: 10.1016/j.ajic.2017.09.019. [DOI] [PubMed] [Google Scholar]


