Abstract
An RNA-based, non-cytopathic replicon vector system, based on the flavivirus Kunjin, has shown considerable promise as a new vaccine delivery system. Here we describe the testing in mice of four different SIVmac239 gag vaccines delivered by Kunjin replicon virus-like-particles. The four vaccines encoded the wild type gag gene, an RNA-optimised gag gene, a codon-optimised gag gene and a modified gag-pol gene construct. The vaccines behaved quite differently for induction of effector memory and central memory responses, for mediation of protection, and with respect to insert stability, with the SIV gag-pol vaccine providing the optimal performance. These results illustrate that for an RNA-based vector the RNA sequence of the antigen can have profound and unforeseen consequences on vaccine behaviour.
Keywords: SIV, Vaccine, Replicon
1. Introduction
A number of RNA-based vector systems have been used in the construction of HIV-1 and SIV vaccines. These include vectors based on negative strand RNA viruses [1], [2], [3], positive strand RNA viruses such as Sendai virus [4], coronavirus [5] and lentiviruses [6], and positive strand RNA replicon-based vectors derived from alphaviruses [7], [8], [9], picornaviruses [10], and flaviviruses [11]. Replicon-based vectors exploit the ability of viral non-structural proteins to amplify greatly (in the cytoplasm of infected/transfected cells) the replicon RNA, which encodes both the heterologous protein and the viral non-structural proteins. This results in high-level expression of antigens and provides potent adjuvant activity via double-stranded RNA replication intermediates [12]. We have previously described a replicon vector system based on the Kunjin virus [12], a largely non-pathogenic flavivirus found in northern Australia [13]. Kunjin replicon RNA can be packaged into virus-like particles (VLPs) using a packaging cell line expressing Kunjin structural proteins in trans [14]. The resulting VLP vaccines are capable of inducing potent, long-lived and protective CD8 T cell responses in several murine systems [12], [15]. Kunjin replicon VLP vaccines encoding HIV-1 gag have also been shown to induce CD8 T cell immunity comparable to that seen after immunisation with recombinant vaccinia [11]. In contrast to most alphavirus replicon-infected cells, Kunjin replicon-infected cells (i) do not show overt cytopathic effects, so both double strand RNA-induced “danger signals” and antigen production are maintained for extended periods and (ii) do not produce replication-competent viruses via recombination during VLP manufacture, with recombination in flavivirus systems appearing to be extremely low [12].
The evaluation of SIV vaccines in non-human primates has proved to be an invaluable and enduring model system for testing new concepts in HIV vaccine development [16], [17], [18], [19]. Most SIV and HIV subunit vaccines encode gag, as Gag proteins are believed to be an important target of protective cellular immunity [20], [21]. Herein we describe the construction and immunogenicity of four SIV gag (pol) Kunjin replicon VLP vaccines. The first encodes SIV gag using the wild-type nucleic acid sequence (WT). The second encodes SIV gag using an RNA-optimised nucleic acid sequence (DX). HIV and SIV gag contain RNA inhibitory sequence (INS) elements, which have been shown to act at several levels to inhibit gag expression; primarily via nuclear retention of RNA in the absence of the Rev protein. RNA-optimisation strategies, which remove these INS elements by changing the codon usage, result in significant increases in protein expression from DNA-based expression vectors [22], and such RNA-optimised SIV gag vaccines have been used in a number of monkey trials [23], [24]. Inhibition of nuclear export of gag RNA by INS sequences is unlikely to be an issue for the exclusively cytoplasmic Kunjin replicon RNA, however, INS elements have also been reported to reduce RNA stability and translation [25], [26]. The third SIV gag Kunjin replicon VLP vaccine encodes a human codon-optimised SIV gag gene (OPT). Codon optimisation has been used extensively to improve recombinant antigen expression in a number of systems and the process involves altering the codon usage to the one most commonly utilized in a given species [27], [28]. The fourth vaccine encodes wild-type matrix and capsid from gag-linked in-frame to reverse transcriptase from pol (Gag-pol). This approach has been described previously for HIV Gag and removes from the gag gene sequences that encode proteins with potentially immunosuppressive properties, whilst retaining sequences frequently recognised by T cells [29]. Although all four vaccines expressed Gag protein at comparable levels, they behaved quite differently in terms of immunogenicity, protection and insert stability.
2. Materials and methods
2.1. Plasmids and construction of Kunjin SIV gag (pol) vaccines
The RNA-based Kunjin replicon vector C20UbHDVrep (SP6KUNrep1) [30] was used to construct WT, DX and OPT vaccines, and SP6KUNrep6 [15] was used to construct the Gag-pol vaccine. These two RNA-based Kunjin replicon vectors are the same, except SP6KUNrep6 has two copies of the foot and mouse virus 2A autoprotease, one upstream of MluI (replacing the ubiquitin gene) and another one downstream of the SnaBI cloning sites [12]. The Kunjin SIV gag WT construct encodes SIV mac239 gag subcloned from p239SpSp5′ [31] (catalogue #829, NIH AIDS Research & Reference Reagent Program, Germantown, MD, USA) using AscI sites. The Kunjin SIV gag DX construct was generated by subcloning RNA-optimised SIV mac239 gag DX [23], [24] from pFB-SIVgag DX CTE Neo [32] using AscI/SnaBI sites. Kunjin SIVgag OPT was constructed using AscI/SnaBI sites and p01-426 (catalogue #9422, NIH AIDS Research & Reference Reagent Program), which encodes human codon-optimised SIVmac239 gag. The Kunjin SIVmac239 Gag-pol vaccine was constructed by separately PCR-amplifying matrix (MA)/nucleocapsid (NC) and reverse transcriptase (RT) genes from p239SpSp5′ plasmid (NIH AIDS Research & Reference Reagent Program) and joining at SacII. The fragment was then cloned into the Mlu I and SnaB I sites of SP6KUNrep6 vector. Construction of the HIV-1 gag vaccine has been described previously and was expressed from the SP6KUNrep1 vector [11]. All gag constructs used in this study-encoded alanine in place of the initial glycine in Gag.
2.2. Production of virus-like particles (VLPs) and determination of their titre
Kunjin replicon RNAs were transcribed in vitro using SP6 RNA polymerase and electroporated into tetKUNCprME BHK packaging cells as described previously [14]. Briefly, RNA (20–30 μg) was electroporated into 3 × 106 packaging cells in suspension and the cells were seeded into a 100-mm dish and incubated at 37 °C for 3–5 days and culture fluid collected at days 3–5. The titre of infectious VLPs in infectious units (IUs) was determined by infection of Vero cells with 10-fold serial dilutions of the culture fluid. At 30–40 h post-infection the cells were fixed with methanol/acetone (1:1, v/v) and analysed by immunofluorescence antibody staining using the 4G4 monoclonal anti-NS1 antibody [33], [34] and the 55-2F12 anti-SIV Gag monoclonal antibody (catalogue # 1610, NIH AIDS Research & Reference Reagent Program) and the number of positive cells counted using Zeiss Axiophot 2 fluorescent microscope (Carl Zeiss Microimaging GmbH, Berlin, Germany).
2.3. Detection of SIV gag and Kunjin NS5 protein by western blot
Vero or BHK cells were infected with VLPs at MOI = 1 or electroporated with 5–10 μg of Kunjin SIV gag RNA constructs and cultured for 2 days in a 60-mm dish. The cells were lysed with RIPA buffer and cell debris was removed by centrifugation. The protein concentration for each sample was determined using the BioRad Protein assay (Biorad, Regents Park, NSW, Australia). Fifteen microgram of total cell protein was separated on a 12.5% gel by SDS-PAGE and transferred onto Hybond-P membrane (Amersham-Pharmacia Biotech, UK) using the Mini Trans Blot transfer tanks (Biorad, Regents Park, NSW, Australia) at 4 °C.
The HIS-tagged recombinant SIV mac239 and HIV-1 (clade B SF2/BH10) gag proteins were expressed from the pET28a+ plasmid (Merck Pty. Ltd., VIC, Australia) under control of T7-promoter in Escherichia coli C43(DE3) [35] and purified using a Ni-agarose column. Gag proteins were detected using the 55-2F12 or the AG3.0 monoclonal antibody (catalogue # 4121. NIH AIDS Research & Reference Reagent Program). Kunjin NS5 protein was detected by an anti-Kunjin NS5 monoclonal antibody (5H1.1) (Hall et al., in press). The secondary antibody was sheep anti-mouse-HRP (Chemicon, Melbourne, Australia). The proteins were visualised by the Western Lightning Chemiluminescence Reagent (PerkinElmer, Boston, MA, USA).
2.4. Deletion mutant analyses
Vero cells were transfected with Kunjin SIV gag VLPs at MOI = 1 and were cultured for 72 h. Total RNA was collected using Trizol reagent (Invitrogen) and SuperScript III reverse transcriptase (Invitrogen) was used to produce cDNA. PCR primers that bind to C20 and E22 of the Kunjin replicon vector backbone were then used to amplify fragments from the cDNA using DyNAzyme EXT (Finnzyme, Diagnostics, Espoo, Finland); forward primer 5′ ggctgtcaatatgctaaaacg 3′, reverse primer 5′ tctttctctccgtgaacgtgcat 3′.
2.5. Vaccination of mice
Female BALB/c (H-2d) mice (6–8 weeks) were supplied by the Animal Resources Centre (Perth, Western Australia). Mice were vaccinated with the Kunjin replicon SIV gag VLP vaccines WT, OPT, DX, or Gag-pol, the Kunjin HIV-1 gag VLP vaccine [11] or control VLPs diluted in RPMI 1640 and 106 IU injected by the intraperitoneal (i.p.) route in a final volume of 200 μl.
2.6. ELISPOT assays
Interferon γ (IFNγ) ELISPOT assays were conducted using 125 or 123 overlapping 15-mer peptides spanning the entire SIVmac239 or HIV-1 subtype B gag protein, respectively (catalogue #6204 and 8117, respectively, NIH AIDS Research & Reference Reagent Program). The peptides were divided into six pools and the total final concentration of the peptide mix in each pool was 10 μg/ml. For Kunjin-specific responses directed to NS3, GYISTRVEL (H-2Kd) was used (AusPep, Parkville Victoria, Australia). Ex vivo ELISPOT assays were conducted as described previously [36] except that MultiScreen-IP hydrophobic PVDF membrane microtitre plates (Millipore Australia Ltd., North Ryde, Australia) and 25 IU/ml of IL-2 (kindly provided by Cetus Corp., Emeryville, CA, USA) was used. The highest cell concentration was 2.5 × 105 cells/well in triplicate, followed by four doubling dilutions. Spots were counted using a KS ELISPOT reader (Carl Zeiss Vision GmbH, Hallbergmoos, Germany). Mean background spots from wells without peptide were subtracted from the mean number of spots obtained from wells with peptide.
Cultured ELISPOT assays were performed using splenocytes cultured in vitro for 6 days in 24 well plates (5 × 106 cells/well in 1 ml medium) with a single pool of the entire overlapping gag peptides (10 μg/ml of the peptide pool) or GYISTRVEL (10 μg/ml). (Culture of splenocytes in the presence of 100 μg/ml of the SIV peptide pool resulted in excessive responses being detected in naïve mice—data not shown). On day 4 a further 1 ml of medium was added to each well. On day 6 the cells were used in the ELISPOT assay (as described above) starting at 1.25 × 105 cells per well followed by four doubling dilutions.
Groups (n = 3 or 4) were compared (using the total peptide response for each mouse) with the non-parametric Median test and the Monte Carlo significance given (SPPS for Windows, Version 15.0, 2007, SPSS Inc., Chicago, IL, USA).
2.7. Challenge assay using A20 cells expressing SIV gag
A plasmid encoding an EGFP-SIV gag DX fusion protein was generated by subcloning human RNA-optimised SIV gag DX from the plasmid pCMVSIVgagDX [24] (provided by Dr B. Felber) into XhoI/EcoRI sites of the pEGFP C3 plasmid (catalogue #6082-1. BD Biosciences, Franklin Lakes, NJ, USA) to generate pEGFP SIVgagDX. This plasmid was used to transfect the BALB/c-derived A20 murine lymphoblastic leukemia cells (ATCC, TIB-208) using the GeneJammer Transfection Reagent (StrataGene, La Jolla, CA, USA). Cells were cultured for 24 h and sorted using MoFlo High-Performance Cell Sorter (Dako, Glostrup, Denmark) for high EGFP expression. The sorted cells were cultured in medium [37] containing G418 (800 μg/ml) for 1–2 weeks then sorted again for high EGFP expression. This procedure was repeated eight times at which point expression had stabilised with >95% of cells expressing EGFP as assessed by FACS after a week of culture under G418 selection. Expression of EGFP-SIV Gag DX fusion protein was also confirmed by western blot using anti-SIV Gag (55-2F12) and anti-EGFP antibodies (Molecular Probes, Leiden, Netherlands). (We were unable to generate a Gag-expressing line using J774 macrophage cells).
Groups of BALB/c mice were vaccinated twice separated by 2–3 weeks with 106 IU of the four different VLPs encoding SIV mac239 gag, control VLPs, or RPMI 1640 by the i.p. route. Approximately 9 months after the last immunisation mice were challenged with the A20-EGFP-SIVgag cells (2.5 × 106 cells/mouse) on the back by the subcutaneous route. Hair around the injection site was removed with an electric shaver prior to the challenge to facilitate injection and measurement of tumours. The tumour sizes were measured three times a week and mice were sacrificed when the tumour area reached 100 mm2. Differences between Kaplan–Meier survival curves were calculated using the log-rank test (SPSS for Windows).
3. Results
3.1. Construction of Kunjin replicon vaccines expressing SIV gag
Four different Kunjin replicon VLP vaccines encoding SIV mac239 gag were constructed; SIVgag WT (WT), SIV gag DX (DX), SIV gag OPT (OPT), and SIV gag-pol (Gag-pol) (Fig. 1 ). WT encodes full-length wild-type SIV gag, DX and OPT encode RNA- and codon-optimised versions of the SIV gag gene, respectively, and Gag-pol encodes wild type matrix and capsid from gag connected in-frame with reverse transcriptase from pol. An HIV-1 gag construct encoding wild-type HIV gag was used as a positive control (see below) and has the same vector backbone as that shown in Fig. 1 [11].
Fig. 1.
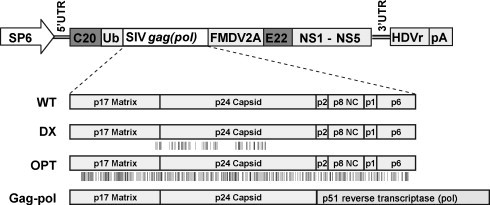
Schematic representation of RNA Kunjin replicon constructs encoding the SIV gag and gag-pol antigens. The Kunjin replicon RNA contained the SP6 RNA promoter, the 5′ and 3′ untranslated regions (UTR), the first 20 amino acids of the Kunjin core protein (C20), ubiquitin (Ub) to allow N-terminal cleavage by ubiquitin hydrolase, the SIV gag or gag-pol genes, the 2A autoprotease sequence of the foot and mouse disease virus (FMDV2A) to allow C-terminal cleavage, the last 22 amino acids of the Kunjin envelope protein (E22), the non-structural proteins (NS1-NS5) that are responsible for RNA replication, the anti-genomic sequence of the hepatitis delta virus ribozyme (HDVr) and the polyadenylation signal from simian virus 40 (pA) [12]. The wild type vaccine construct (WT) contains the full-length native SIV mac239 gag gene. The DX and OPT constructs contain RNA- and codon-optimised versions of this gene, respectively, with the positions of the codon changes schematically represented by vertical lines below the gene box. DX sequence was provided by Dr. Felber and 6% of nucleotides have been changed. The OPT sequence is available from the AIDS Research and Reference Reagent Program web site (catalogue #9422) and 25% of nucleotides have been changed. The Gag-pol construct contains wild-type matrix and capsid from gag and in-frame reverse transcriptase from pol. The gag-pol construct contained a second FMDV2A site in place of the Ub sequence at the N-terminus of gag.
In vitro transcribed RNA derived from each Kunjin replicon DNA construct was used to manufacture VLPs in packaging cells [14]. Each VLP vaccine was used to infect Vero cells in vitro and western blotting experiments (using the anti-SIV gag 55-2F12 antibody) illustrated that each vaccine expressed a protein of the expected molecular weight (Fig. 2A). Similar data was produced when Kunjin RNA (rather than VLPs) was used to transfect BHK cells (Fig. 2B), indicating there was no major differences in expression between monkey and rodent cells. In these experiments no major improvements in protein expression levels were seen for DX or OPT vaccines despite RNA- and codon optimisation. (No in vitro SIV Gag particle formation was observed by electron microscopy for these vaccine—data not shown.)
Fig. 2.
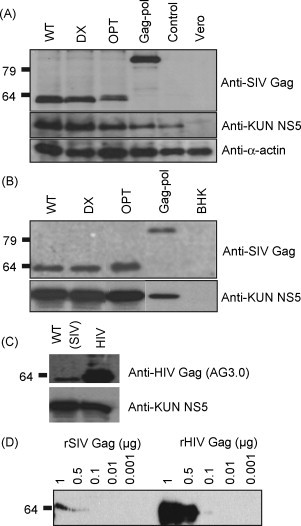
Analysis of SIV gag expression from the four different Kunjin SIVgag constructs. (A) Analysis of SIV Gag protein expression after infection of Vero cells with Kunjin VLPs. Cells were lysed after 48 h incubation and total cell lysates analysed by western blot using anti-Gag antibody (55-2F12). (B) As for A except that BHK cells were transfected with RNA for each of the vaccine constructs. (C) Comparison of SIV Gag and HIV Gag protein expression after infection of Vero cells as above with WT and HIV-1 VLPs using the AG3.0 antibody. (D) Comparison of the reactivity of AG3.0 in western analysis with serial dilutions of purified recombinant SIV Gag and recombinant HIV Gag proteins.
When the lysates from SIV gag and HIV-1 gag VLP-infected cells were initially compared side by side in western analyses using the AG3.0 antibody, we were surprised to find that the expression of SIV Gag appeared to be substantially lower (Fig. 2C). It emerged, however, that this antibody recognises the SIV mac239 Gag protein much less effectively than the HIV-1 Gag protein (Fig. 2D). SIV Gag expression was thus probably not in reality substantially lower than HIV Gag expression in these Kunjin replicon systems.
A more extensive analysis of a panel of antibodies used for detecting SIV Gag proteins also revealed some hitherto unpublished differences in reactivity with Gag proteins from different SIV strains using different techniques. We have shown the data in Table 1 and have added published information for completeness. This information will hopefully allow others to identify more easily the appropriate antibody for future SIV Gag studies.
Table 1.
Reactivity of anti-SIVgag antibodies in different settings
| Ab (NIH cat) | AG3.0 (4121) |
55-2F12 (1610) |
KK59 (2320) |
KK64 (2321) |
183-H12-5C (3537) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Technique | W | E | I | W | E | I | W | E | I | W | E | I | W | E | I |
| SIVmac239 | +c | +c | +d (poor) | +c,d | +c | +d | + [62] | +c | + [62] | +c | + [63] | +c | |||
| SIVmne | +a | −a | + [64] | ||||||||||||
| SIVmac251 | +b | −b | −b | + [65] | |||||||||||
| HIV-1 | +b | + [66] | + [67] | + [68] | + [69] | ||||||||||
W, Western blotting; E, ELISA; I, IFA. The source of antigens: arecombinant vaccinia infected cells, brecombinant antigen (catalogue #1845, NIH AIDS Research & Reference Reagent Program), crecombinant antigen (see Section 2) and dKunjin SIVgag VLP-infected cells.
3.2. Gag-specific T cell induction by Kunjin replicon SIV gag vaccines
To compare the immunogenicity of the Kunjin SIV gag vaccine constructs, BALB/c mice were immunised twice with 106 IU of each of the four VLP vaccines. IU represents the titre of VLPs expressing Gag (see Table 2 ), so each mouse received the same number of Kunjin VLPs encoding gag. Two negative control groups were included to account for non-specific responses, one group received no vaccination (control naïve) and the second was vaccinated with VLPs not encoding gag (control VLP). Immunisations with Kunjin VLPs encoding HIV-1 gag was also included. Ten weeks after the last immunisation splenocytes were assayed by ex vivo and cultured IFNγ ELISPOT assays using six pools of overlapping peptides spanning the entire SIV Gag protein or in the case of HIV gag immunised mice, the HIV Gag protein. The use of peptide pools covering Gag to measure T cell responses in mice has been described previously [38], [39].
Table 2.
Percentage of NS1-expressing cells also expressing SIV Gag following infection with SIV gag VLP vaccines
| SIVgag construct | % gag positive |
|---|---|
| WT | 69.3 ± 0.08 |
| DX | 22.5 ± 0.49 |
| OPT | 40.2 ± 0.43 |
| gag-pol | 87.0 ± 0.01 |
Each VLP vaccine was titred on Vero cells and cultured for 48 h. IFA was then performed using anti-NS1 and anti-SIV Gag (55-2F12) monoclonal antibodies and the IU titre calculated for each antibody. The ratio of the titres is represented as a percentage (Gag titre/NS1 titre × 100).
Ex vivo ELISPOT assays measure immediate effector function and thus tend to measure effector memory T cell activity, whereas cultured ELISPOT assays measure the ability of cells to replicate and form effector cells and thus represents a measure of central memory cell activity [40], [41], [42]. Ex vivo ELISPOT assays illustrated that responses induced by the WT vaccine were not significantly higher than the control VLP (p = 0.092) (Fig. 3A). DX induced responses that approached significance over control VLPs (p = 0.054), and OPT, Gag-pol and the HIV gag vaccine induced responses that were significantly higher than the control VLP (p = 0.047, 0.029 and 0.039, respectively) (Fig. 3A). The OPT and Gag-pol vaccine-induced responses were comparable to those induced by the Kunjin HIV-1 gag VLP vaccine tested under the same conditions (Fig. 3A). Using the cultured ELISPOT assay, WT and OPT failed to produce significant responses over the controls (Fig. 3B). The Gag-pol construct induced significantly higher responses than WT (p = 0.015), whereas DX (due to the very high mouse to mouse variation in this group, ranging from 2688 to 17,000 spots) did not reach significance over WT using the Median test (although it did reach asymptotic significance using the Mann–Whitney test, p = 0.034). DX and Gag-pol vaccines also induced responses comparable to those induced by HIV-1 gag VLP vaccination (Fig. 3B). Pool 3 dominated the HIV-1 Gag responses (Fig. 3B) as this pool contains the immunodominant AMQMLKETI epitope [11].
Fig. 3.
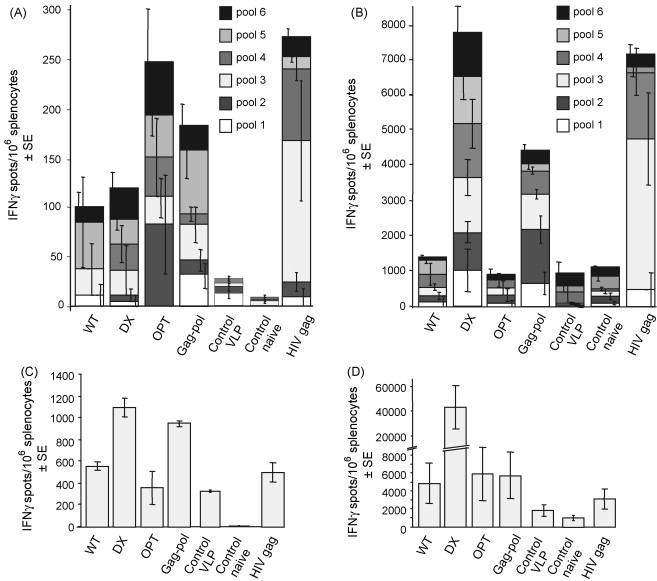
T cells responses induced after immunisation with Kunjin-SIVmac239 gag VLP vaccines. (A) Measurement of Gag-specific responses by ex vivo ELISPOT assays. BALB/c mice (n = 3 or 4 per group) were immunised twice with 106 IU of each of the indicated Kunjin VLP vaccines. There were two control groups, one received 106 IU of a control VLP encoding an irrelevant antigen (control VLP) and the other received no vaccination (control naïve). Ten weeks after the second immunisation, the mice were sacrificed and the splenocytes were assayed for Gag-specific T cell responses by ex vivo IFNγ ELISPOT using six pools of overlapping peptides spanning either the SIV Gag protein for SIV gag vaccinated animals, or the HIV-1 Gag protein for HIV-1 gag vaccinated animals. Error bars illustrate the variation in responses to each pool. (B) Measurement of Gag-specific responses by cultured IFNγ ELISPOT assays. The splenocytes from the animals described in (A) were also cultured for 6 days with the pooled SIV or HIV Gag peptides, prior to the ELISPOT assay. (C) Measurement of NS3-specific responses by ex vivo IFNγ ELISPOT assays. The splenocytes from the animals described in (A) were also used in an ex vivo ELISPOT assays using the NS3 CD8 T cell peptide epitope, GYISTRVEL. (D) Measurement of NS3-specific responses by cultured IFNγ ELISPOT assays. The splenocytes from the animals described in (A) were cultured for 6 days with GYISTRVEL peptide prior to an ELISPOT assay using the same peptide.
3.3. Vector-specific CD8 T cell induction by Kunjin replicon VLP vaccines
Spaulding et al. identified a region in Dengue virus NS3, which represents a dominant CD8 T cell epitope [43]. We have confirmed that the homologous region (299GYISTRVEL207) in Kunjin virus NS3, which contains a substitution (M to L) in the last amino acid, also represents a dominant CD8 T cell epitope for Kunjin replicon vaccines (data not shown). To analyse the levels of anti-vector CD8 T cell responses for the different vaccines shown in Fig. 3A and B, ELISPOT assays using the GYISTRVEL peptide epitope (at 10 μg/ml) were undertaken. Ex vivo IFNγ ELISPOT analysis showed that responses ranged from ≈400 to 1000 spots/106 splenocytes (Fig. 3C). Cultured IFNγ ELISPOT analysis showed a range of ≈2000–5000 spots/106 splenocytes for all the vectors except DX, which gave a large mean response in excess of 40,000 spots/106 splenocytes (Fig. 3D). The very large response for DX might be due to the large number of VLPs not expressing Gag present in the DX preparation (Table 2). These results illustrate that flavivirus replicon-based vectors, like other vector systems [44], [45], also induce CD8 T cell responses specific for vector proteins.
3.4. Insert stability
We and others [46] have noted that flavivirus VLPs can lose expression of certain inserted genes. To investigate to what extent the different SIV gag VLP vaccines suffered from this problem, Vero cells were infected with VLPs and dual-labelled with anti-Kunjin NS1 and anti-Gag antibodies. For WT and Gag-pol the majority of VLP-infected cells expressed both NS1 and Gag, however, for DX and OPT only about 22% and 40% of NS1 positive cells, respectively, also expressed Gag (Table 2). For the HIV gag VLPs this value was >90% (data not shown). The DX and OPT constructs contained RNA- and codon-optimised genes, perhaps suggesting that altering the coding sequence somehow reduces the stability of these inserted genes in the Kunjin replicon constructs. The in vitro transcribed RNA used to transfect the packaging cells did not contain any detectable truncated replicon RNA species (Fig. 4A) indicating that these insert deletion events likely occurred during VLP manufacture. Gene deletion events from flavivirus vectors have not been extensively studied. To gain some insight into the process, RNA from WT and DX VLP-infected cells was analysed by RT-PCR using primers flanking the SIV gag gene insert. The full-length gene is visible at ≈2000 bp, with shorter PCR products evident in both WT and DX infected cells. Gene deletions in WT and DX appear to be different, with DX showing a major band at ≈180 bp (Fig. 4B, lane 3). A band of the same size was also observed in samples from OPT-infected cells (data not shown). This ≈180 bp fragment is actually smaller than that obtained from the empty vector (Fig. 4B, lane 4), suggesting that at least one end of the deletion must lie within the ubiquitin cloning site or the FMDV 2A protease region (see Fig. 1). Sequencing of the ≈180 bp fragment from DX illustrated that the deletion occurred 15 nucleotides past the 5′ end of the 225 nucleotide Ub sequence and 29 nucleotides past the 5′ end of the 48 nucleotide FMDV2A sequence (Fig. 4B). There are no obvious homologies between the sequences either side of the insert deletion site that would suggest strand switching or homologous recombination events.
Fig. 4.
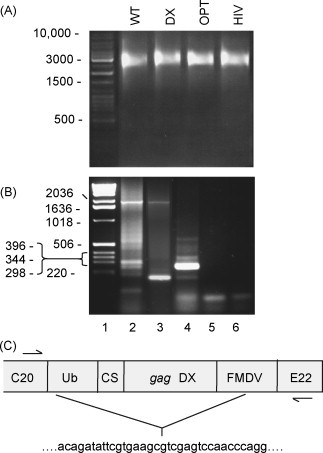
Analysis of insert deletions in the Kunjin SIV gag vaccines. (A) Non-denaturing agarose gel and ethidium bromide staining of in vitro transcribed RNA for the indicated constructs prior to transfection of packaging cells and VLP manufacture. Note the double-stranded DNA markers do not accurately illustrate the size of the transcribed single-stranded RNA species, which are about 10.5 kb. (B) Vero cells were infected with WT (lane 2), DX (lane 3), empty VLPs (lane 4) or nothing (lane 5) and RT-PCR performed on extracted RNA using primers flanking the multiple cloning site region into which the gag genes were inserted for the former two constructs. Lane 1 shows the markers and lane 6 no template control. (C) Sequencing of the ≈180 bp fragment from DX transfected cells. The insert deletion is schematically represented with the sequence either side of the insert deletion site given beneath.
3.5. Protection assays
Recombinant vaccinia viruses encoding HIV-1 antigens have been used extensively as surrogate viral challenge models in mice to assess protective immune responses induced by different HIV-1 vaccines [11], [47]. We determined whether two recombinant vaccinia viruses, one encoding SIV MNE gag/pol [48] (kind gift from Dr. Shiu-Lok Hu, University of Washington, Seattle, WA) and the other SIV mac251 gag (EVA261, Centre for AIDS Reagents, National Institute for Biological Standards and Controls, UK) could be used in such assays. Unfortunately, although they grew well in vitro, both vaccinia viruses replicated very poorly in mice following i.p., intranasal or intravenous inoculations of 106 or 107 pfu of virus (data not shown), making them unsuitable for such assays. A vaccinia virus encoding HIV-1 gag was run in parallel and replicated efficiently as described previously [11]. A vaccinia encoding SIVmac239 gag has been reported to replicate in mice [1], however, this vaccinia virus has not been made publicly available, and the reason for its better replication in mice is unclear.
To develop an alternative challenge assay we generated an A20 lymphoblastic leukemia line stably expressing SIV gag DX fused to EGFP (A20-EGFP-SIVgag cells). Western blotting illustrated that these cells expressed the Gag-EGFP fusion protein, which has an expected molecular weight of ≈82 kDa (Fig. 5A). These cells were used to challenge animals ≈9 months after vaccination with the different SIV VLP vaccines. The OPT, Gag-pol and DX vaccines all provided significantly better protection than both the controls and the WT vaccine (log-rank tests all p ≤ 0.006) (Fig. 5B). OPT vaccination also provided significantly better protection than DX (p = 0.031), with no other comparisons showing significant differences (Fig. 5B). Mean tumour growth curves from the same experiment are shown in Fig. 5C. The percentage of mice surviving after challenge with A20-EGFP-SIVgag cells (0, 33, 67 and 100% for WT, DX, Gag-pol, and OPT, respectively, Fig. 5B) correlated well with the mean ex vivo ELISPOT data shown in Fig. 3A (100, 119, 183 and 248 spots/106 splenocytes, respectively), R 2 = 0.95. A similar comparison with the cultured ELISPOT data (Fig. 3B) failed to show any correlation, R 2 = 0.04, suggesting that effector memory rather than central memory cell activity had a major role in mediating protection in this model.
Fig. 5.
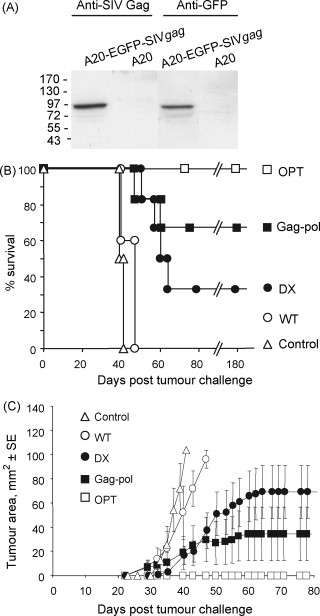
Challenge of Kunjin SIV gag VLP vaccinated mice with A20 cells expressing SIV mac239 Gag. (A) Western blot of A20-EGFP-SIVgag and parental A20 cells with anti-SIV Gag antibody (55-2F12) and anti-GFP antibody. 15 μg of total protein was loaded in each well. (B) Kaplan Meier plot of survival. Groups of mice (n = 4–6) were immunised twice with the indicated VLP vaccines and were then challenged ≈9 months later with A20-EGFP-SIVgag cells. Animals were euthanased when tumours reached 100 mm2. (C) Mean growth curves for the tumours from the same experiment. When an animal was euthansed a value of 100 mm2 was included for that animal in the mean for all subsequent time points.
4. Discussion
Vaccines against a series of pathogens including HIV/SIV are being developed using a number of RNA-based viral vector systems. Here we describe the behaviour of four different Kunjin replicon VLP vaccines encoding SIV gag and show that only the Gag-pol vaccine (i) induced good levels of both effector memory and central memory T cell responses 10 weeks post-vaccination, comparable to those induced by the previously described HIV-1 gag Kunjin replicon VLP vaccine [11], (ii) showed good levels of protection against challenge with A20 cells expressing SIV Gag ≈9 months post-vaccination, and (iii) displayed high levels of insert stability. WT and DX vaccines induced poor effector memory responses and provided poor protective activity, and OPT and DX showed high levels of insert deletion.
The potential for genetic instability for many recombinant viral vectors is well described and a range of largely vector-specific strategies have been adopted to limit the problem [46], [49], [50], [51], [52], [53]. In the Kunjin replicon system deletion of inserted genes appears to occur in packaging cells during VLP manufacture. For constructs with low insert stability the proportion of VLPs without inserts increases if the time between RNA transfection of the packaging line and VLP harvest is extended, and appropriate reductions of this time period can result in VLP preparations with substantially fewer empty VLPs (unpublished observation). These observations parallel those obtained for West Nile replicons where VLPs with insert deletions were also rapidly selected during serial passage of VLPs in a packaging cell line [46]. In contrast, we have generated by antibiotic selection several cell lines stably transfected with recombinant Kunjin replicon vectors and these could be extensively passaged without loss of reporter gene inserts [12], suggesting that insert deletion may be rare once a cell is transfected. One might speculate that insert deletion is associated with the cellular stress that leads to the cytopathic effects, which are observed in the packaging cells during VLP manufacture due to the high level of structural protein expression. Conceivably, in such stressed cells replicon RNA may gain access to the nuclear RNA splicing machinery. RNA splicing is well known to be influenced by complex RNA structure interactions, with distant RNA sequences able to regulate splicing events [54]. Why DX and OPT vaccines would be more susceptible to insert deletion remains unclear, but may be associated with the loss of native RNA structures.
The DX gene was engineered to allow Rev-independent expression from DNA-based vector systems [23], [24], however, this did not provide significant improvements in antigen expression. The negative influence of SIV gag INS sequences on RNA stability and translation [25], [26] therefore do not appear to be significant in this system. Successful expression of wild-type SIV Gag by other vector systems where mRNA is generated in the cytoplasm (e.g. alphavirus replicons and vaccinia) also supports the view that the major influence of INS elements is on RNA export [9], [55]. The human codon-optimised Kunjin OPT construct did not result in significantly higher Gag expression in Vero (monkey) or BHK (hamster) cells, suggesting that tRNAs are not particularly limiting in these replicon-based expression systems when tested in vitro.
Why OPT and Gag-pol might provide slightly improved effector memory responses and WT and OPT induce such poor central memory responses when delivered by Kunjin replicon VLPs remains unclear, but is likely to be a reflection of multiple factors including the number of empty VLPs in the vaccine inocula, the level and length of antigen expression in vivo, and the level of “danger signals” induced by the replicating replicon RNA. The better memory responses induced by the Gag-pol vaccine may be due to the removal of the potentially immunosuppressive gag-encoded proteins [29]. An alphavirus replicon SIV vaccine with these regions deleted also showed improved longevity of T cell responses over full-length gag constructs [8]. The high (albeit variable) central memory responses induced by DX may arise from increased levels of IFNα/β produced [56] as a result of the large number of empty VLPs that compromised ≈80% of the VLP inocula for this vaccine preparation (Table 2).
The A20-EGFP-SIV gag challenge model (Fig. 5) represents a new model for testing the effectiveness of T cell induction by SIV gag vaccines. This model is likely to represent a measure of SIV Gag-specific CD8 T cell activity as rejection of this tumour has previously been shown to be CD8 T cell dependent [57]. Protection from this model also correlated well with the magnitude of ex vivo ELISPOT (effector memory) responses rather than cultured ELISPOT (central memory) responses induced by each of the Kunjin SIV vaccines. This might be expected since tumours are generally poor at stimulating the differentiation of central memory cells into effectors, so prophylactic protection would rely on effector memory cells [58]. The relative importance of effector memory and/or central memory cells for prophylactic protection against HIV-1/SIV has not been extensively studied. Some evidence suggests central memory cells are more important than effector memory cells [59], whereas others have argued that effector activity early in infection (when effector memory T cells would be most active) is important for protection [60] and for slowing progression to AIDS [61].
In summary we describe here a Kunjin replicon SIV Gag-pol VLP vaccine, which showed high insert stability, good induction of effector and central memory responses, and good protection against a model challenge. Other SIV gag vaccines failed in one or more of these criteria, illustrating that antigen construction and the insert's nucleotide sequences can have unforeseen consequences for the performance of these RNA replicon vectors.
Acknowledgements
We would like to thank Drs. Barbara K. Felber and George N. Pavlakis (Human Retrovirus Pathogenesis Section, National Cancer Institute-Frederick, Frederick, MD, USA) for providing pCMV SIVgagDX plasmid. We would also like to thank Dr. Felber for critical review of the manuscript. The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: p239SpSp5′ from Dr Ronald Desrosiers; p01-426 from Dr Stephen Dewhurst; monoclonal antibody to HIV 1 p24 (AG3.0) from Dr. Jonathan Allan; and SIVmac p27 monoclonal antibody (55-2F12) from Dr. Niels Pedersen; HIV-1 Con B Gag peptides-Complete Set; SIVmac 239 Gag (15-mer) Peptides-Complete Set; SIVmac251 (BK28) Pr55 Gag.
References
- 1.Nakaya Y., Zheng H., Garcia-Sastre A. Enhanced cellular immune responses to SIV Gag by immunization with influenza and vaccinia virus recombinants. Vaccine. 2003;21:2097–2106. doi: 10.1016/s0264-410x(02)00781-8. [DOI] [PubMed] [Google Scholar]
- 2.Nakaya Y., Nakaya T., Park M.S., Cros J., Imanishi J., Palese P. Induction of cellular immune responses to simian immunodeficiency virus gag by two recombinant negative-strand RNA virus vectors. J Virol. 2004;78:9366–9375. doi: 10.1128/JVI.78.17.9366-9375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Messling V., Cattaneo R. Toward novel vaccines and therapies based on negative-strand RNA viruses. Curr Top Microbiol Immunol. 2004;283:281–312. doi: 10.1007/978-3-662-06099-5_8. [DOI] [PubMed] [Google Scholar]
- 4.Griesenbach U., Inoue M., Hasegawa M., Alton E.W. Sendai virus for gene therapy and vaccination. Curr Opin Mol Ther. 2005;7:346–352. [PubMed] [Google Scholar]
- 5.Eriksson K.K., Makia D., Maier R., Ludewig B., Thiel V. Towards a coronavirus-based HIV multigene vaccine. Clin Dev Immunol. 2006;13:353–360. doi: 10.1080/17402520600579168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negri D.R., Michelini Z., Baroncelli S., Spada M., Vendetti S., Buffa V. Successful immunization with a single injection of non-integrating lentiviral vector. Mol Ther. 2007;15:1716–1723. doi: 10.1038/sj.mt.6300241. [DOI] [PubMed] [Google Scholar]
- 7.Johnston R.E., Johnson P.R., Connell M.J., Montefiori D.C., West A., Collier M.L. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine. 2005;23:4969–4979. doi: 10.1016/j.vaccine.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Cecil C., West A., Collier M., Jurgens C., Madden V., Whitmore A. Structure and immunogenicity of alternative forms of the simian immunodeficiency virus gag protein expressed using Venezuelan equine encephalitis virus replicon particles. Virology. 2007;362(2):362–373. doi: 10.1016/j.virol.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong W., Tian C., Liu B., Yu X.F. Stable expression of primary human immunodeficiency virus type 1 structural gene products by use of a noncytopathic sindbis virus vector. J Virol. 2002;76:11434–11439. doi: 10.1128/JVI.76.22.11434-11439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson M.J., Porter D.C., Moldoveanu Z., Fletcher T.M., 3rd, McPherson S., Morrow C.D. Characterization of the expression and immunogenicity of poliovirus replicons that encode simian immunodeficiency virus SIVmac239 Gag or envelope SU proteins. AIDS Res Hum Retroviruses. 1997;13:53–62. doi: 10.1089/aid.1997.13.53. [DOI] [PubMed] [Google Scholar]
- 11.Harvey T.J., Anraku I., Linedale R., Harrich D., Mackenzie J., Suhrbier A. Kunjin virus replicon vectors for human immunodeficiency virus vaccine development. J Virol. 2003;77:7796–7803. doi: 10.1128/JVI.77.14.7796-7803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pijlman G.P., Suhrbier A., Khromykh A.A. Kunjin virus replicons: an RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Expert Opin Biol Ther. 2006;6:135–145. doi: 10.1517/14712598.6.2.135. [DOI] [PubMed] [Google Scholar]
- 13.Hall R.A., Broom A.K., Smith D.W., Mackenzie J.S. The ecology and epidemiology of Kunjin virus. Curr Top Microbiol Immunol. 2002;267:253–269. doi: 10.1007/978-3-642-59403-8_13. [DOI] [PubMed] [Google Scholar]
- 14.Harvey T.J., Liu W.J., Wang X.J., Linedale R., Jacobs M., Davidson A. Tetracycline-inducible packaging cell line for production of flavivirus replicon particles. J Virol. 2004;78:531–538. doi: 10.1128/JVI.78.1.531-538.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anraku I., Harvey T.J., Linedale R., Gardner J., Harrich D., Suhrbier A. Kunjin virus replicon vaccine vectors induce protective CD8+ T-cell immunity. J Virol. 2002;76:3791–3799. doi: 10.1128/JVI.76.8.3791-3799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Gegerfelt A.S., Rosati M., Alicea C., Valentin A., Roth P., Bear J. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency virus SIVmac251 after therapeutic DNA immunization. J Virol. 2007;81:1972–1979. doi: 10.1128/JVI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson N.A., Reed J., Napoe G.S., Piaskowski S., Szymanski A., Furlott J. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattapallil J.J., Douek D.C., Buckler-White A., Montefiori D., Letvin N.L., Nabel G.J. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J Exp Med. 2006;203:1533–1541. doi: 10.1084/jem.20060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh Y.S., Park K.S., Sauermann U., Franz M., Norley S., Wilfingseder D. Reduction of viral loads by multigenic DNA priming and adenovirus boosting in the SIVmac-macaque model. Vaccine. 2006;24:1811–1820. doi: 10.1016/j.vaccine.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Sacha J.B., Chung C., Rakasz E.G., Spencer S.P., Jonas A.K., Bean A.T. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS–virus integration and viral protein expression. J Immunol. 2007;178:2746–2754. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawada M., Tsukamoto T., Yamamoto H., Takeda A., Igarashi H., Watkins D.I. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J Virol. 2007;81:5202–5211. doi: 10.1128/JVI.02881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider R., Campbell M., Nasioulas G., Felber B.K., Pavlakis G.N. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hel Z., Tsai W.P., Thornton A., Nacsa J., Giuliani L., Tryniszewska E. Potentiation of simian immunodeficiency virus (SIV)-specific CD4(+) and CD8(+) T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J Immunol. 2001;167:7180–7191. doi: 10.4049/jimmunol.167.12.7180. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J., Lou Y., Pinczewski J., Malkevitch N., Aldrich K., Kalyanaraman V.S. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine. 2003;21:4022–4035. doi: 10.1016/s0264-410x(03)00266-4. [DOI] [PubMed] [Google Scholar]
- 25.Zolotukhin A.S., Michalowski D., Bear J., Smulevitch S.V., Traish A.M., Peng R. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol Cell Biol. 2003;23:6618–6630. doi: 10.1128/MCB.23.18.6618-6630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz S., Felber B.K., Pavlakis G.N. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992;66:150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W.J., Zhao K.N., Gao F.G., Leggatt G.R., Fernando G.J., Frazer I.H. Polynucleotide viral vaccines: codon optimisation and ubiquitin conjugation enhances prophylactic and therapeutic efficacy. Vaccine. 2001;20:862–869. doi: 10.1016/s0264-410x(01)00406-6. [DOI] [PubMed] [Google Scholar]
- 28.Davies M.N., Flower D.R. Harnessing bioinformatics to discover new vaccines. Drug Discov Today. 2007;12:389–395. doi: 10.1016/j.drudis.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Bolesta E., Gzyl J., Wierzbicki A., Kmieciak D., Kowalczyk A., Kaneko Y. Clustered epitopes within the Gag-Pol fusion protein DNA vaccine enhance immune responses and protection against challenge with recombinant vaccinia viruses expressing HIV-1 Gag and Pol antigens. Virology. 2005;332:467–479. doi: 10.1016/j.virol.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 30.Varnavski A.N., Young P.R., Khromykh A.A. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J Virol. 2000;74:4394–4403. doi: 10.1128/jvi.74.9.4394-4403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 32.Negri D.R., Buffa V., Leone P., Bona R., Borghi M., Carlini F. Use of retroviral vectors for the analysis of SIV/HIV-specific CD8 T cell responses. J Immunol Methods. 2004;291:153–163. doi: 10.1016/j.jim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Westaway E.G., Mackenzie J.M., Kenney M.T., Jones M.K., Khromykh A.A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macdonald J., Tonry J., Hall R.A., Williams B., Palacios G., Ashok M.S. NS1 protein secretion during the acute phase of West Nile virus infection. J Virol. 2005;79:13924–13933. doi: 10.1128/JVI.79.22.13924-13933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miroux B., Walker J.E. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 36.Le T.T., Drane D., Malliaros J., Cox J.C., Rothel L., Pearse M. Cytotoxic T cell polyepitope vaccines delivered by ISCOMs. Vaccine. 2001;19:4669–4675. doi: 10.1016/s0264-410x(01)00243-2. [DOI] [PubMed] [Google Scholar]
- 37.Elliott S.L., Pye S., Le T., Mateo L., Cox J., Macdonald L. Peptide based cytotoxic T-cell vaccines; delivery of multiple epitopes, help, memory and problems. Vaccine. 1999;17:2009–2019. doi: 10.1016/s0264-410x(98)00468-x. [DOI] [PubMed] [Google Scholar]
- 38.Someya K., Xin K.Q., Matsuo K., Okuda K., Yamamoto N., Honda M. A consecutive priming-boosting vaccination of mice with simian immunodeficiency virus (SIV) gag/pol DNA and recombinant vaccinia virus strain DIs elicits effective anti-SIV immunity. J Virol. 2004;78:9842–9853. doi: 10.1128/JVI.78.18.9842-9853.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barouch D.H., Pau M.G., Custers J.H., Koudstaal W., Kostense S., Havenga M.J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 40.Goonetilleke N., Moore S., Dally L., Winstone N., Cebere I., Mahmoud A. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006;80:4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reece W.H., Pinder M., Gothard P.K., Milligan P., Bojang K., Doherty T. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–410. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 42.Godkin A.J., Thomas H.C., Openshaw P.J. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J Immunol. 2002;169:2210–2214. doi: 10.4049/jimmunol.169.4.2210. [DOI] [PubMed] [Google Scholar]
- 43.Spaulding A.C., Kurane I., Ennis F.A., Rothman A.L. Analysis of murine CD8(+) T-cell clones specific for the Dengue virus NS3 protein: flavivirus cross-reactivity and influence of infecting serotype. J Virol. 1999;73:398–403. doi: 10.1128/jvi.73.1.398-403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tscharke D.C., Suhrbier A. From mice to humans—murine intelligence for human CD8+ T cell vaccine design. Expert Opin Biol Ther. 2005;5:263–271. doi: 10.1517/14712598.5.2.263. [DOI] [PubMed] [Google Scholar]
- 45.Molinier-Frenkel V., Gahery-Segard H., Mehtali M., Le Boulaire C., Ribault S., Boulanger P. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J Virol. 2000;74:7678–7682. doi: 10.1128/jvi.74.16.7678-7682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fayzulin R., Scholle F., Petrakova O., Frolov I., Mason P.W. Evaluation of replicative capacity and genetic stability of West Nile virus replicons using highly efficient packaging cell lines. Virology. 2006;351:196–209. doi: 10.1016/j.virol.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 47.Coupar B.E., Purcell D.F., Thomson S.A., Ramshaw I.A., Kent S.J., Boyle D.B. Fowlpox virus vaccines for HIV and SHIV clinical and pre-clinical trials. Vaccine. 2006;24:1378–1388. doi: 10.1016/j.vaccine.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 48.Kent S.J., Hu S.L., Corey L., Morton W.R., Greenberg P.D. Detection of simian immunodeficiency virus (SIV)-specific CD8+ T cells in macaques protected from SIV challenge by prior SIV subunit vaccination. J Virol. 1996;70:4941–4947. doi: 10.1128/jvi.70.8.4941-4947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kriajevska M.V., Zakharova L.G., Altstein A.D. Genetic instability of vaccinia virus containing artificially duplicated genome regions. Virus Res. 1994;31:123–137. doi: 10.1016/0168-1702(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 50.Junker U., Bohnlein E., Veres G. Genetic instability of a MoMLV-based antisense double-copy retroviral vector designed for HIV-1 gene therapy. Gene Ther. 1995;2:639–646. [PubMed] [Google Scholar]
- 51.Lee S.G., Kim D.Y., Hyun B.H., Bae Y.S. Novel design architecture for genetic stability of recombinant poliovirus: the manipulation of G/C contents and their distribution patterns increases the genetic stability of inserts in a poliovirus-based RPS-Vax vector system. J Virol. 2002;76:1649–1662. doi: 10.1128/JVI.76.4.1649-1662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dufresne A.T., Dobrikova E.Y., Schmidt S., Gromeier M. Genetically stable picornavirus expression vectors with recombinant internal ribosomal entry sites. J Virol. 2002;76:8966–8972. doi: 10.1128/JVI.76.17.8966-8972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raju R., Subramaniam S.V., Hajjou M. Genesis of Sindbis virus by in vivo recombination of nonreplicative RNA precursors. J Virol. 1995;69:7391–7401. doi: 10.1128/jvi.69.12.7391-7401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valadkhan S. The spliceosome: caught in a web of shifting interactions. Curr Opin Struct Biol. 2007;17:310–315. doi: 10.1016/j.sbi.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Swanson C.M., Puffer B.A., Ahmad K.M., Doms R.W., Malim M.H. Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J. 2004;23:2632–2640. doi: 10.1038/sj.emboj.7600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mescher M.F., Curtsinger J.M., Agarwal P., Casey K.A., Gerner M., Hammerbeck C.D. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 57.Liu A., Guardino A., Chinsangaram L., Goldstein M.J., Panicali D., Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of recombinant fowlpox virus encoding CD40 ligand. Cancer Res. 2007;67:7037–7044. doi: 10.1158/0008-5472.CAN-07-0224. [DOI] [PubMed] [Google Scholar]
- 58.Fuchs E.J., Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8:271–280. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 59.Vaccari M., Trindade C.J., Venzon D., Zanetti M., Franchini G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J Immunol. 2005;175:3502–3507. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- 60.Altes H.K., Price D.A., Jansen V.A. Effector cytotoxic T lymphocyte numbers induced by vaccination should exceed levels in chronic infection for protection from HIV. Vaccine. 2001;20:3–6. doi: 10.1016/s0264-410x(01)00318-8. [DOI] [PubMed] [Google Scholar]
- 61.Jansen C.A., van Baarle D., Miedema F. HIV-specific CD4+ T cells and viremia: who's in control? Trends Immunol. 2006;27:119–124. doi: 10.1016/j.it.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Brown K., Gao W., Alber S., Trichel A., Murphey-Corb M., Watkins S.C. Adenovirus-transduced dendritic cells injected into skin or lymph node prime potent simian immunodeficiency virus-specific T cell immunity in monkeys. J Immunol. 2003;171:6875–6882. doi: 10.4049/jimmunol.171.12.6875. [DOI] [PubMed] [Google Scholar]
- 63.Brandt S., Blissenbach M., Grewe B., Konietzny R., Grunwald T., Uberla K. Rev proteins of human and simian immunodeficiency virus enhance RNA encapsidation. PLoS Pathog. 2007;3:e54. doi: 10.1371/journal.ppat.0030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu Y., Koo K., Bradshaw J.D., Sutton W.F., Kuller L.R., Bucala R. Macaque blood-derived antigen-presenting cells elicit SIV-specific immune responses. J Med Primatol. 2000;29:182–192. doi: 10.1034/j.1600-0684.2000.290312.x. [DOI] [PubMed] [Google Scholar]
- 65.Brandt S., Grunwald T., Lucke S., Stang A., Uberla K. Functional replacement of the R region of simian immunodeficiency virus-based vectors by heterologous elements. J Gen Virol. 2006;87:2297–2307. doi: 10.1099/vir.0.81883-0. [DOI] [PubMed] [Google Scholar]
- 66.McDonald D., Vodicka M.A., Lucero G., Svitkina T.M., Borisy G.G., Emerman M. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin-Serrano J., Zang T., Bieniasz P.D. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 68.Pfeiffer T., Pisch T., Devitt G., Holtkotte D., Bosch V. Effects of signal peptide exchange on HIV-1 glycoprotein expression and viral infectivity in mammalian cells. FEBS Lett. 2006;580:3775–3778. doi: 10.1016/j.febslet.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 69.Moses A.V., Stenglein S.G., Strussenberg J.G., Wehrly K., Chesebro B., Nelson J.A. Sequences regulating tropism of human immunodeficiency virus type 1 for brain capillary endothelial cells map to a unique region on the viral genome. J Virol. 1996;70:3401–3406. doi: 10.1128/jvi.70.6.3401-3406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


