Abstract
Epitope-based DNA vaccines designed to induce T cell responses specific for Mycobacterium tuberculosis (M. tb) are being developed as a means of addressing vaccine potency. In this study, we predicted 4 T cell epitopes from ESAT-6, Ag85A/B and CFP-10 antigens and constructed an ECANS (epitopes casted in a natural structure) DNA vaccine by inserting the epitope DNA segments separately into the gene backbone of M. tb-derived HSP65 (heat shock protein 65) carrier. The immunogenicity and protective efficacy of pECANS DNA vaccine were assessed in BALB/c mice after intramuscular immunization with 4 doses of 50 μg ECANS DNA and followed by mycobaterial challenge 4 weeks after the last immunization. Compared to plasmid encoding HSP65, pECANS DNA immunization elicited remarkably higher levels of IFN-γ production by both CD4+ and CD8+ T cells, which were coupled with higher frequencies of antigen-specific T cells and higher CTL activity. Significantly enhanced levels of Th1 cytokines (IFN-γ and IL-12) and increased serum IgG2a/IgG1 ratio were also noted, indicating a predominant Th1 immune response achieved by pECANS DNA immunization. In the consequence, a better protection against Mycobacterium bovis BCG challenge was achieved which was evidenced by reduced bacterial loads in lungs and spleens and profound attenuation of lung inflammation and injury. Our results suggested that multi-T cell-epitope based ECANS gene vaccine induced T cell response to multiple T cell epitopes and led to enhanced protection against mycobacterial challenge. This strategy might be a useful platform to design multi-T cell epitope-based vaccine against M. tb infection.
Keywords: DNA vaccine, Epitope, ECANS, Mycobacterium tuberculosis
1. Introduction
Tuberculosis (TB) remains the single largest infectious disease and a leading cause of death throughout the world. It accounts for 4 deaths every minute and 2 million deaths annually and severely threats the health of humankind [1]. At present, the only available TB vaccine is the attenuated strain of Mycobacterium bovis Bacillus Calmette-Guérin (BCG). Although effective in preventing Mycobacterium tuberculosis (M. tb) infection in newborns and toddlers, BCG provides poor protection in adult pulmonary tuberculosis with varying protective efficacy [2]. The pre-exposure to environmental mycobacteria may prevent or mask BCG-induced protective immunity [3]. In addition, the protective efficacy of BCG is weakened by its continuing attenuation and loss of immunodominant antigens such as RD-1 antigens (CFP-10 and ESAT-6 proteins) [4]. Therefore, alternative preventive TB vaccines are urgently needed.
It is well-known that T cells play a primary role in the protective immune responses against M. tb. However, searching for suitable antigens to elicit potent T cell immune response has been thwarted by the complexity of the M. tb proteome. Vaccines based on T cell epitopes represent an ideal approach to generate effective cellular immunity in the prophylactic and therapeutic settings, because multiple epitopes can be incorporated into one vaccine to induce broadly immune responses, reacting with various immunogenic epitopes present or absent in BCG [5]. Epitopes that are highly conserved among bacterial types or dominant epitopes from numerous gene products could be preferentially selected. Furthermore, the immune response would be restricted to the specific epitopes and required type of protective immune response would be generated [6], [7], overcoming any potential safety concerns associated with the other vaccine regimens.
Development of epitope-based vaccines has been hampered for its relatively low antigenicity. Thus, different vaccine formats and delivery methodologies have been utilized including DNA vaccine, viral vector or protein carrier to solve the problem. Muti-CTL epitope DNA vaccines have been reported to induce broad CTL responses against HIV, HBV, SARS-CoV and so on [8], [9], [10]. Introduction of heterologous epitopes into protein carriers by genetic engineering or synthetic coupling method [11] can enhance the Th1 immune responses, induce more potent humoral and cell-mediated immune responses and promote epitope spreading [12]. Mycobacterial heat shock protein (HSP) 65, a chaperon protein, has received immense attention as a suitable and safe protein carrier because of its considerable instinct T helper epitopes, ability to chaperone antigenic peptides and facilitate the cross-presentation in APC [13]. It has been demonstrated that BCG-derived HSP65 is capable of delivering antigenic peptides into the MHC class I presentation pathway of APCs, and thus stimulating antigen-specific CD8+ T-cell responses [14]. It is also used for delivering B cell epitopes in the absence of adjuvants [15]. Besides, DNA vaccine encoding HSP65 protects mice from a virulent strain of M. tb challenge and exhibits cure effects, indicating HSP65 itself as an immunodominant TB antigen. Therefore, HSP65 is selected as a carrier for incorporation of multiple epitopes from the immunodominant antigens of M. tb.
In this study, we predicted 4 T cell epitopes from 4 well-defined M. tb protective antigens, 6-kDa early secretory antigen of T cells (ESAT-6), Ag85A, 10-kDa culture filtrate protein (CFP-10) and Ag85B via epitope prediction software online. The epitope-encoding genes were grafted into the various inter-domain region of the H37Rv-derived HSP65 scaffold gene and obtained a multi-epitope DNA vaccine designated as ECANS (epitopes casted in a natural structure). Intramuscular immunization of this DNA vaccine was performed to evaluate its efficacy in inducing specific and protective immune responses against mycobacterial tuberculosis.
2. Materials and methods
2.1. Prediction of T cell epitopes
Potential MHC I- or II-binding T cell epitopes were screened from ESAT-6, Ag85A, CFP-10 and Ag85B proteins using epitope prediction softwares online (http://www.syfpeithi.com/scripts/MHCSr.dll/home.htm, http://www.jenner.ac.uk/MHCPred/ and http://www.imtech.res.in/raghava/propred/). Similarity was scored using position-specific scoring matrixes derived from aligned peptides. Four epitopes, including ESAT-664–76 (ELNNALQNLARTI), Ag85A124–135 (GLSMAASSALTL), CFP-1055–69 (VVRFQEAANKQKQEL) and Ag85B141–153 (QQFIYAGSLSALL), were sorted.
2.2. Construction of pECANS DNA vaccine
M. tb-derived HSP65 DNA fragment (BX842573) was yielded from H37Rv genome by PCR using a forward primer (5′-GAAGAATTCATGGCCAAGACA ATTGCG-3′) and a reverse primer (5′-CATAAGCTTTCAGAAATCCATGCCACC-3′), and then inserted into pcDNA3.1 (Invitrogen) to generate pHSP65 plasmid. The 4 selected T cell epitopes were engineered into the HSP65 scaffold separately except that CFP-1055–69 and Ag85B141–153 were separated one from another by an AAY spacer (Fig. 1 A). The chimera ECANS gene was yielded by method of gene splicing through overlap extension of several synthetic nucleotide sequences and was then incorporated into pcDNA3.1 plasmid driven by a CMV promoter.
Fig. 1.
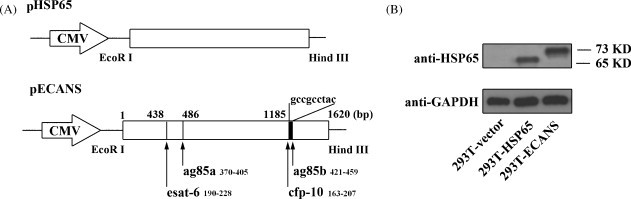
Construction and expression of pHSP65 and pECANS. (A) Schematic representation of pHSP65 and pECANS. (B) Western Blot analysis to characterize the expressions of the plasmids. 293T cells were transfected with the indicated plasmids and the cell lysis was subjected to Western Blot with anti-HSP65 or anti-GAPDH antibody. Individual experiments were conducted three times, with one representative shown for each group.
2.3. Preparation of antigen peptides and proteins
BCG and purified protein derivative (PPD) were purchased from Shanghai Institute of Biological Products. Recombinant HSP65 protein were expressed as an his-tagged protein in the pET-28a Escherichia coli expression system (Novagen) and purified as previously described [14]. Polypeptides, ESAT-664–76, Ag85A124–135, CFP-1055–69 and Ag85B141–153 were commercially synthesized by GL Biochem Ltd. (Shanghai) with the purity >95% and then dissolved in PBS and stored at −70 °C until use.
2.4. Animals and vaccination
Six to eight weeks female BALB/c mice (H-2d) were purchased from the experimental animal centre of Chinese Academy of Science (Shanghai, P.R. China) and remained in pathogen-free conditions. All animal experiments were performed according to the guidelines for the Care and Use of Laboratory Animals (Ministry of Health, P.R. China, 1998) and the guidelines of the Laboratory Animal Ethical Commission of Fudan University. Endotoxin-free plasmids were prepared using an EndoFree plasmid purification mega prep kit (Qiagen). Mice were immunized intramuscularly with 50 μg pECANS, pHSP65 or pcDNA3.1 for 4 times biweekly. Serum was collected 2 weeks after the final immunization by retro-orbital bleeding and stored at −70 °C for further analysis.
2.5. Western Blot assay
293T cells were transfected with pECANS or pHSP65 by lipofectamine (Invitrogen) and cultured for 48 h. The supernatants were electrophoresed on SDS-PAGE gels and transferred to PVDF membrane. The membrane was probed with anti-HSP65 mAb (Santa Cruz), followed by horseradish peroxidase (HRP) conjugated goat anti-mouse antibody (SouthernBiotech), the signals were developed using chemiluminescence (Amersham Biosciences).
2.6. ELISA measurement of TB-specific antibody
Plates were coated with PPD, HSP65 protein or the predicted peptides at a final concentration of 10 μg/ml at 4 °C overnight. After blocking with 1% BSA–PBS, serum (1:100 dilution) was added in duplicate. HRP-conjugated goat anti-mouse IgG, IgG1 or IgG2a (SouthernBiotech) was added, followed by OPD substrate addition. Absorbance at 490 nm was measured in a microplated reader (BioLab).
2.7. IFN-γ ELISPOT assays
ELISPOT was performed with ELISPOT assay kit (BD PharMingen). Briefly, plates were coated with the capture anti-IFN-γ mAb overnight at 4 °C and then blocked with complete media for 2 h at room temperature. Splenocytes from immunized mice were isolated, plated (5 × 105 cells/well) and cultured with each of the predicted peptides, the pool of the peptides, HSP65 protein, PPD or inactivated H37Rv (all at the final concentration of 10 μg/ml) at 37 °C for 48 h. After washing the plates with deionized water and PBST, the biotinylated anti-IFN-γ mAb was added for 2 h at room temperature. The plate was supplemented with streptavidin–alkaline phosphatase for 1 h, and the color was developed by AP-colorimetric substrate. An immunospot analyzer (Cellular Technology) was used to enumerate the spots.
2.8. ELISA assay of cytokines
Splenocytes from immunized mice were plated (5 × 106 cells/well) and cultured with mixed peptides or inactivated H37Rv (10 μg/ml) for 48 h, The concentrations of IFN-γ, IL-12, IL-4 and IL-10 in the culture supernatants were measured by ELISA kit (R&D) following the manufacturer's procedures.
2.9. Intracellular IFN-γ staining
Two weeks after the final immunization, splenocytes were isolated and stained with anti-CD4-FITC and anti-CD8-PerCP antibodies (BD PharMingen). After washing, the cells were treated with fixation/permeabilization solution (BD PharMingen) for 30 min at 4 °C, and then stained with anti-IFN-γ-PE antibody (BD PharMingen). The percentages of CD4+IFN-γ+ and CD8+IFN-γ+ T cells were determined by flow cytometry using a FACScalibur instrument (BD Biosciences).
2.10. In vivo CTL assays
In vivo CTL assays were performed as described earlier [16]. Briefly, 2 weeks after the final immunization, mice were adoptively transferred with 1 × 107 cells of a 1:1 mix of unpulsed-CFSElow:peptides-pulsed-CFSEhigh syngeneic target cells. The peptides used to pulse target cells were the pool of the 4 predicted peptides. 18 h later, adoptively transferred mice were killed; splenocytes were isolated and resuspended in PBS for acquisition on a FACScalibur instrument (BD Biosciences). To evaluate the percentage of specific lysis, the ratio of CFSEhigh/CFSElow in vaccinated mice was compared to the ratio in transferred naive control mice.
2.11. M. bovis BCG challenge and CFU determination
BCG (Denmark strain 1331) was grown in Middlebrook 7H9 broth supplemented with Middlebrook OADC enrichment (BD PharMingen), 0.002% glycerol, and 0.05% Tween 80 for 10–15 days and then aliquoted and stored in −70 °C. Before each use, the bacteria were washed with PBS containing 0.05% Tween 80 twice and passed through a needle 10 times to disperse clumps. Immunized mice were infected intranasally with 1 × 107 CFU of BCG at 4 weeks post-immunization. The level of bacterial burden was determined 6 weeks post-challenge in the lung and spleen by plating serial dilutions of tissue homogenates in triplicates onto Middlebrook 7H10 agar plates. Plates were incubated for 3 weeks at 37 °C, colonies were then counted, calculated, and presented as log10 CFU per organ.
2.12. Histopathology
Lung tissues were harvested for histological evaluation 6 weeks after BCG challenge, fixed in 10% buffered formalin, and embedded in paraffin. Sections of 4 μm were stained with hematoxylin and eosin (HE) and analyzed by a pathologist who was blinded for the treatment allocation. To score lung inflammation and damage, the entire lung section was analyzed with confluent inflammatory infiltration which was quantified and expressed as a percentage of the lung surface.
2.13. Statistical analysis
All data are given as mean ± SD. Statistical analysis of the data was performed with the two-tailed independent Student's t-test analysis using SPSS, version 12.0 (SPSS Inc). P < 0.05 was considered statistically significant.
3. Results
3.1. Prediction of T cell epitopes with online software and construction of pECANS DNA vaccine
To design a multivalent DNA vaccine against M. tb, ESAT-6, Ag85A, CFP-10 and Ag85B protein sequences were screened for T cell epitopes using epitope prediction softwares online and 4 polypeptides were chosen based on their scores. According to SYFPEITHI and MHCPred software analysis, there are overlapping H-2K/Ld-binding CD8+T cell epitope and I-Ad binding CD4+T cell epitopes in each of the four polypeptides. Among them, Ag85B141–153 (QQFIYAGSLSALL) and Ag85A124–135 (GLSMAASSALTL) seem to harbor both CD4+ and CD8+ T cell epitopes with higher MHC-binding score than the other two polypeptides. Depending on the functional domains and the three-dimensional structure of HSP65 protein, the 4 T cell epitopes were grafted into 3 inter-domain of the HSP65 scaffold to constitute the “ECANS” chimera gene and then placed into the pcDNA3.1 to construct a plasmid designated as pECANS (Fig. 1A). To verify its transcriptional efficiency in vitro, lipofectamine-aided transfection assays were conducted on 293T cells. Western Blot assay showed that a 65 kDa protein and a 73 kDa protein were present in the cell lysis, which were in accordance with the molecular weights of HSP65 and ECANS proteins, indicating that these plasmids are efficiently expressed in vitro (Fig. 1B).
3.2. Specific antibody response elicited by pECANS immunization
To assess if the engineering of HSP65 would influence its ability to induce HSP65-specific antibody, female BALB/c mice were vaccinated intramuscularly with 50 μg pECANS 4 times biweekly. Two weeks after the final immunization, the levels of serum IgG specific to a range of defined mycobacterial antigens (PPD, HSP65) were detected with ELISA assay. As shown in Fig. 2 , high levels of IgG specific to PPD and HSP65 proteins were detected in pECANS-immunized mice, with OD values reaching 0.94 and 1.38 respectively, indicating induction of specific humoral immunity. However, they seemed lower than those elicited by pHSP65 vaccination (OD value equaled to 1.21 and 1.86) (P < 0.05). When IgG subclasses were concerned, significantly elevated IgG2a/IgG1 ratio was observed following pECANS immunization; indicating that casting T cell epitopes into HSP65 scaffold impairs but not abrogates its immunogenicity to generate TB-specific antibodies. Meanwhile, a more Th1-polarized response is initiated by this strategy.
Fig. 2.
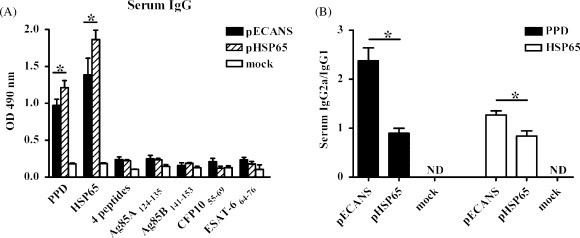
TB-specific antibody responses raised by pECANS intramuscular immunization. Mice were immunized with 4 doses of 50 μg pECANS, pHSP65, or mock vaccine at 2 weeks intervals. (A) Two weeks after the final immunization, TB-specific serum IgG responses were analyzed by ELISA assays. (B) Subclasses of serum IgG were detected at the same time. Each bar represents average values of 6 mice, measured induplicate. *P < 0.05; ND, not detected.
3.3. Enhanced TB-specific IFN-γ+T cell responses following pECANS vaccination
T cells play a critical role in protective immunity against mycobacterial infection. IFN-γ ELISPOT assays were performed on the splenocytes isolated from immunized mice 2 weeks after the final immunization to analyze whether pECANS can induce specific T cell responses. As shown in Fig. 3 , pECANS injection generated significantly increased number of IFN-γ-secreting T cells separately responding to inactivated H37Rv, PPD or HSP65 protein in comparison to mice treated with pHSP65 (P < 0.05), suggesting that pECANS immunization markedly augmented the splenic functional T cell response. Meanwhile, the magnitude of single epitope-specific T cell responses was evaluated and compared. It demonstrated that all the 4 epitopes within pECANS effectively induced specific IFN-γ secreting T cells (Fig. 3B) with Ag85B141–153 to be the most immunogenic epitope which is consistent with the predicted MHC binding score. No epitope-specific T cells were achieved by pHSP65-immunization.
Fig. 3.
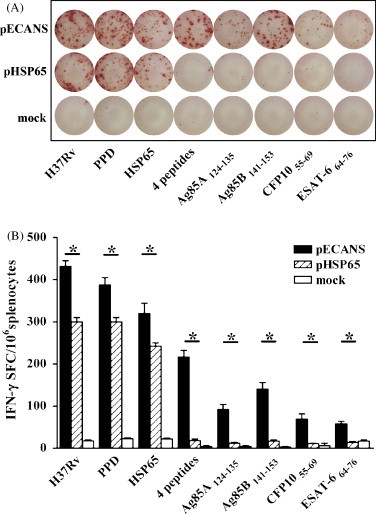
Specific IFN-γ ELISPOT responses elicited by pECANS immunization. Two weeks after the final immunization, splenocytes were collected and stimulated with various TB antigens for 48 h, and then IFN-γ-secreting lymphocytes were quantified by ELISPOT assays. (A) Representative images of splenic ELISPOT responses in the immunized mice. (B) The frequency of IFN-γ-secreting cells in spleen following TB antigen-specific stimulation in vitro. Data are from one representative experiment of three performed and presented as the mean value ±SD (n = 6). *P < 0.05.
To further investigate the contributions of CD4+ and CD8+ T cells to the IFN-γ-production, intracellular cytokine staining assays were performed. Consistent with the ELISPOT data, compared to no response by pHSP65 immunization (Fig. 4A), 2.39% splenic IFN-γ+ CD4+ and 0.90% IFN-γ+ CD8+ T cells specific to the mixed epitopic peptides were evidenced following pECANS injection. And the frequencies of IFN-γ+CD4+ and IFN-γ+CD8+ T cells specific to the inactivated H37Rv increased to 3.50% and 1.71% in pECANS immunized mice, remarkably higher than those in pHSP65-treated mice (1.62% and 0.64%) (P < 0.05, Fig. 4B), indicating the multi-epitopes casting into HSP65 strategy effectively enhanced TB-specific CD4+ and CD8+ T cells responses concurrently than the single use of HSP65 DNA vaccine.
Fig. 4.
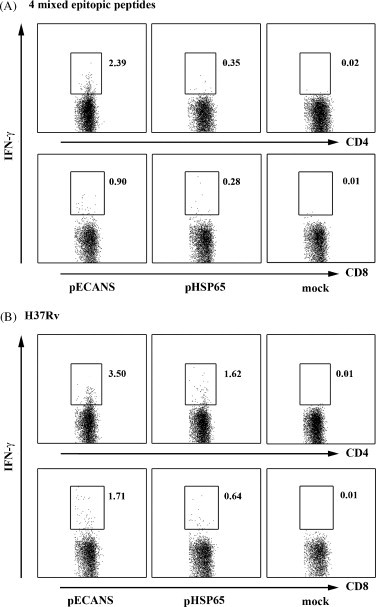
Increased frequencies of IFN-γ-secreting CD4+ and CD8+ T cell by intramuscular immunization with pECANS. Two weeks following the final immunization, splenocytes were collected and stimulated with the pool of 4 epitopes (A) or inactivated H37Rv (B) for 48 h, then the percentages of CD4+IFN-γ+ and CD8+IFN-γ+ T cells were analyzed by flow cytometry. Individual experiments were conducted three times, with one representative shown for each group.
3.4. Augmented CTL activity elicited by ECANS strategy
To assess the cytolytic function of pECANS-induced antigen-specific T cells, an in vivo cytotoxicity assay was performed in which CFSE-labeled splenocytes from naive mice, unpulsed or pulsed with the pool of mixed 4 T cell epitopes, were adoptively transferred to the immunized mice. Epitope-specific lysis of the transferred cells was then investigated by flow cytometric analysis. Compared to the very low levels of killing detected in the control and pHSP65-immunized mice (Fig. 5 ), a significantly increased lysis was observed in the spleens of mice receiving pECANS (P < 0.05), indicating a greatly enhanced TB-specific CTL activity achieved by pECANS immunization.
Fig. 5.
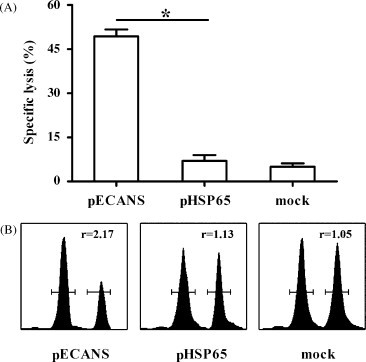
TB-specific CTL activity elicited by pECANS. Unpulsed splenocytes (CFSElow) and peptides-pulsed splenocytes (CFSEhigh) from naive mice were transferred to the immunized mice. The percentages (A) and representative histograms (B) of splenic TB-specific lysis in the immunized mice were compared. Data are from one representative experiment of three performed and presented as the mean ± SD (n = 6). *P < 0.05.
3.5. Promoted Th1 polarization by pECANS immunization
Significantly enhanced serum IgG2a and CTL activity after pECANS immunization indicated the induction of Th1 immune response, the cytokine profiles produced by lymphocytes after specific stimulation were then analyzed. As shown in Fig. 6 , the levels of IFN-γ and IL-12 (Th1 cytokines) in the supernatant of splenocytes were significantly higher in the pECANS-immunized mice than in the pHSP65-treated mice (P < 0.05). While the level of IL-4 and IL-10 (Th2 cytokines) was less in pECANS-treated mice, further confirming a promoted Th1 immune response.
Fig. 6.
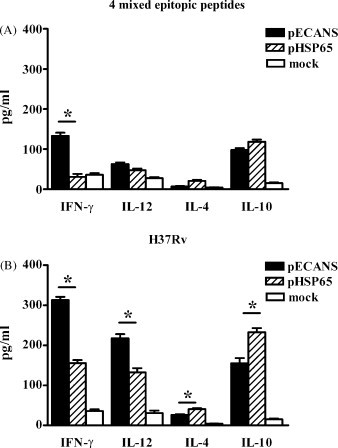
Promoted Th1 immune responses by pECANS immunization. The concentrations of Th1/Th2 cytokines in the cell culture supernatant were determined by ELISA after incubation for 48 h with the pool of 4 mixed T cell epitopes (A) or with the inactivated H37Rv bacteria (B). Data are from one representative experiment of three performed and presented as the mean ± SD (n = 6). *P < 0.05.
3.6. Enhanced protection from intranasal BCG challenge and alleviated tuberculosis pathology by pECANS immunization
To determine the protective potential of the pECANS vaccination, 4 weeks after the final immunization mice were intranasally challenged with 1 × 107 CFU BCG, the bacterial burdens in the lungs and spleens as well as the pathologic pulmonary injury were examined 6 weeks post-challenge. As shown in Fig. 7A and B, immunization with pHSP65 provided a modest level of protection from BCG challenge; the reduction of bacteria burden calculated as log10 CFU reduction were 0.46 and 0.65 in the lung and spleen, respectively. In contrast, pECANS immunization significantly increased the protection by giving a significant reduction of bacteria in the lung (0.72 log10 CFU) and in the spleen (1.15 log10 CFU), demonstrating that the TB-specific immune responses elicited by intramuscular injection of pECANS led to an enhanced host defense at local and systemic tissue compartments.
Fig. 7.
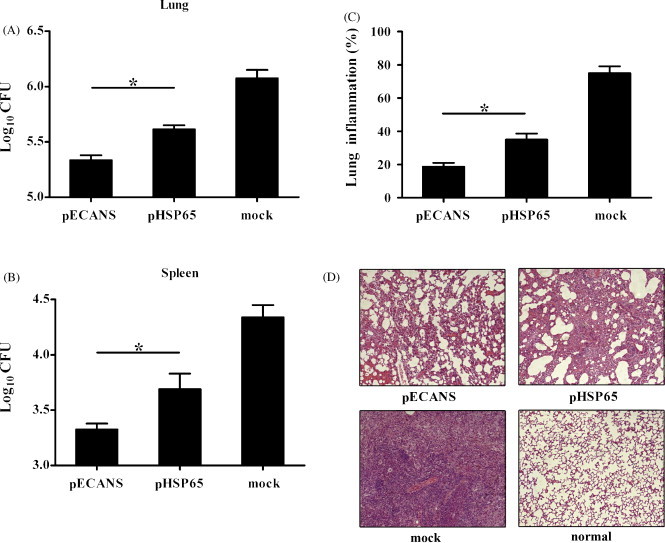
Protection against mycobacterial infection by pECANS immunization. Four weeks following the last immunization, mice received an intranasal 1 × 107 CFU of BCG. Six weeks post-challenge, the bacterial loads in the lungs (A) and spleens (B) were measured. The data are presented as the mean ± SD (n = 6) and are one representative of three separate experiments. *P < 0.05. Paraffin sections from lung tissues were stained with HE, and evaluated for the level of lung inflammation (C). Representative histology sections depicted the lung tissue of the immunized mice, using lung section of normal mice as a negative control (D). Magnification: 100×. Individual experiments were conducted three times, with one representative shown.
When the tissue pathology was observed, it was seen that lung tissues from mock mice showed widespread and severe interstitial pneumonia, intense inflammation and diffuse granuloma response after BCG infection, displaying large clusters of lymphocytes and macrophages infiltration (Fig. 7C and 7D), while mice immunized with pHSP65 showed moderate damage in alveolar tissues with relatively fewer lymphocyte infiltration and smaller granulomas. Mice given pECANS immunization had the intact alveolar tissue with very mild lung inflammation, indicating dramatically attenuated tuberculosis pathology. The histological evidence, together with bacterial burden reduction, corroborated the protection effect achieved by the multi-epitope-based pECANS DNA immunization.
4. Discussion
Up to now, great progresses have been achieved in the development of novel TB vaccines, using various regimens such as attenuated live or inactivated whole bacterium, subunit vaccine or DNA vaccine [17]. Compared with conventional vaccine platforms, DNA vaccine represents a novel approach that is good at generating T cell response, and holds a number of advantages including safety, stability, as well as ease of preparation and manipulation [18]. DNA vaccine preferentially generates Th1 and CTL immune responses which may play meaningful roles in eliminating intracellular pathogen including M. tb. Development of TB DNA vaccine has been benefited tremendously from the sequencing of the M. tb genome [19] and the accelerated identification of novel protective antigens [20]. TB DNA vaccines are reported to have comparable protective efficiency in mice against M. tb infection to BCG, and are currently at some step in the process of clinical trials, highlighting their promising potentials as a new generation of TB vaccine [21].
Selection of appropriate antigens is a prerequisite for the design of TB DNA vaccines. However, the criterion for antigen selection remains vague due to the complexity of the host immune response and the vast repertoire of TB proteome (approximately 4000 proteins). Secreted TB antigens such as Ag85 have been proved to induce stronger immune responses than those of cytosolic proteins in humans [22]. The Ag85 complex constituting of Ag85A, Ag85B and Ag85C proteins is also very important because of its involvement in the synthesis of M. tb cell wall. DNA vaccines encoding Ag85A and Ag85B stimulate strong cell-mediated immune responses and confer significant protection against aerosol or intravenous challenge with live H37Rv, indicating their significance [23], [24], [25]. In addition, RD-1-encoded proteins such as ESAT-6 and CFP-10, which lack in all BCG strains and present in pathologic M. tb, have been identified as potent T cell antigens in both animals and humans and are strongly recognized in the first and memory phases of M. tb infection [26], [27]. Adding these deleted antigens back to TB vaccines has been respected as an important strategy to improve the effects of TB vaccines [28]. Therefore in this study, we tried to identify multiple previously uncharacterized T cell epitopes from ESAT-6, Ag85A, CFP-10 and Ag85B antigens and cast them into H37Rv-dervied HSP65 to integrate multiple T cell epitopes into an immunogenic carrier to achieve the best protective effect. We demonstrated that this multi-epitope DNA vaccine efficiently elicited potent T cell immunity in the systemic lymphoid organs and gave significant protections against mycobacterial challenge which was evidenced by the greatly reduced bacterial burden and lung pathology.
Despite of the progressive understanding of the immune protection factors against M. tb infection, induction of CD4+ Th1 and CD8+ Tc1 cells remains a cornerstone of protective immunity. Evidence from correlative studies indicates that the presence of functional T cells may be associated with improved clinical outcomes in human tuberculosis [29]. Th1 cytokines appear to be also necessary to control the growth of M. tb and for the establishment of protective immune responses in animals and in humans, as deficiency in IL-12 or IFN-γ results in higher susceptibility to M. tb infection [30]. In the current study, significantly higher IFN-γ+ T cell responses, whether to the inactivated H37Rv or the mixed epitopes, were elicited by pECANS-immunization than pHSP65 vaccination. And CD4+ T cells contributed more to the IFN-γ production than CD8+ T cells. Meanwhile, a robust CTL response was noticed, indicating the induction of integrated T cell immune responses by pECANS. Strikingly higher amounts of IL-12 and IFN-γ, and lower amounts of IL-4 and IL-10 were observed in pECANS-immunized mice, suggesting a predominant Th1 immune response. Altogether, CD4+ Th1 and CD8+ CTLs as well as Th1 cytokines accounted for the ideal protection effect seen in pECANS immunized mice.
In summary, here we assessed the feasibility and immunological efficacy of an HSP65-vectorized multi-epitope-based DNA vaccine against TB. Intramuscular immunization of pECANS predominantly induced TB-specific Th1 immune response and CTL activity which confers enhanced protection against mycobacterial infection. This strategy might be helpful for the design of DNA vaccines to control M. tb infection.
Acknowledgements
This work was supported in part by National 11.5 grant (2008ZX10003011, 2008ZX10003013-2), 973 grant (2007CB512401), 863 grant (2006AA02z403), NSFC grant (30772020) and grant (07QA14005, 064319024) from Science and Technology Commission of Shanghai Municipality, P.R. China.
References
- 1.Maartens G., Wilkinson R.J. Tuberculosis. Lancet. 2007;370:2030–2043. doi: 10.1016/S0140-6736(07)61262-8. [DOI] [PubMed] [Google Scholar]
- 2.Young D.B., Perkins M.D., Duncan K., Barry C.E., 3rd Confronting the scientific obstacles to global control of tuberculosis. J Clin Inves. 2008;118:1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thom M., Howard C., Villarreal-Ramos B., Mead E., Vordermeier M., Hope J. Consequence of prior exposure to environmental mycobacteria on BCG vaccination and diagnosis of tuberculosis infection. Tuberculosis (Edinb) 2008;88:324–334. doi: 10.1016/j.tube.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Al-Attiyah R., Mustafa A.S. Characterization of human cellular immune responses to novel Mycobacterium tuberculosis antigens encoded by genomic regions absent in Mycobacterium bovis BCG. Infect Immun. 2008;76:4190–4198. doi: 10.1128/IAI.00199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brave A., Boberg A., Gudmundsdotter L., Rollman E., Hallermalm K., Ljungberg K. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Mol Ther. 2007;15:1724–1733. doi: 10.1038/sj.mt.6300235. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T., Pendleton C.D., Sarobe P., Thomas E.K., Iyengar S., Harro C. Epitope enhancement of a CD4 HIV epitope toward the development of the next generation HIV vaccine. J Immunol. 2006;176:3753–3759. doi: 10.4049/jimmunol.176.6.3753. [DOI] [PubMed] [Google Scholar]
- 7.Song S., Wang F., He X., He Y., Li D., Sun S. Evaluation of antitumor immunity efficacy of epitope-based vaccine with B16 cell line coexpressing HLA-A2/H-2 kb and CTL multiepitope in HLA transgenic mice. Vaccine. 2007;25:4853–4860. doi: 10.1016/j.vaccine.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Depla E.A., Van der Aa B.D., Livingston C., Crimi K., Allosery V., De Brabandere J. Rational design of a multiepitope vaccine encoding T-lymphocyte epitopes for treatment of chronic hepatitis B virus infections. J Virol. 2008;82:435–450. doi: 10.1128/JVI.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Xu W., Tong D., Ni J., Gao H., Wang Y. A chimeric multi-epitope DNA vaccine elicited specific antibody response against severe acute respiratory syndrome-associated coronavirus which attenuated the virulence of SARS-CoV in vitro. Immunol Lett. 2008;119:71–77. doi: 10.1016/j.imlet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson C.C., McKinney D., Anders M., MaWhinney S., Forster J., Crimi C. Development of a DNA vaccine designed to induce cytotoxic T lymphocyte responses to multiple conserved epitopes in HIV-1. J Immunol. 2003;171:5611–5623. doi: 10.4049/jimmunol.171.10.5611. [DOI] [PubMed] [Google Scholar]
- 11.Joyce J.G., Krauss I.J., Song H.C., Opalka D.W., Grimm K.M., Nahas D.D. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc Natl Acad Sci USA. 2008;105:15684–15689. doi: 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neeson P., Pan Z.K., Paterson Y. Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma. Cancer Immunol Immunother. 2008;57:493–505. doi: 10.1007/s00262-007-0388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolhassani A., Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev Vacc. 2008;7:1185–1199. doi: 10.1586/14760584.7.8.1185. [DOI] [PubMed] [Google Scholar]
- 14.Yang B.F., Zhao H.L., Xue C., Xiong X.H., Zhang W., Yao X.Q. Recombinant heat shock protein 65 carrying hepatitis B core antigen induces HBcAg-specific CTL response. Vaccine. 2007;25:4478–4486. doi: 10.1016/j.vaccine.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Perraut R., Lussow A.R., Gavoille S., Garraud O., Matile H., Tougne C. Successful primate immunization with peptides conjugated to purified protein derivative or mycobacterial heat shock proteins in the absence of adjuvants. Clin Exp Immunol. 1993;93:382–386. doi: 10.1111/j.1365-2249.1993.tb08189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick S., Santosuosso M., Small C.L., Shaler C.R., Zhang X., Jeyanathan M. Mucosally delivered dendritic cells activate T cells independently of IL-12 and endogenous APCs. J Immunol. 2008;181:2356–2367. doi: 10.4049/jimmunol.181.4.2356. [DOI] [PubMed] [Google Scholar]
- 17.Hoft D.F. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet. 2008;372:164–175. doi: 10.1016/S0140-6736(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 18.D'Souza S., Rosseels V., Romano M., Tanghe A., Denis O., Jurion F. Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect Immun. 2003;71:483–493. doi: 10.1128/IAI.71.1.483-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jonge M.I., Brosch R., Brodin P., Demangel C., Cole S.T. Tuberculosis: from genome to vaccine. Expert Rev Vacc. 2005;4:541–551. doi: 10.1586/14760584.4.4.541. [DOI] [PubMed] [Google Scholar]
- 20.Bertholet S., Ireton G.C., Kahn M., Guderian J., Mohamath R., Stride N. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta U.D., Katoch V.M., McMurray D.N. Current status of TB vaccines. Vaccine. 2007;25:3742–3751. doi: 10.1016/j.vaccine.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 22.Romano M., Roupie V., Hamard M., Huygen K. Evaluation of the immunogenicity of pBudCE4.1 plasmids encoding mycolyl-transferase Ag85A and phosphate transport receptor PstS-3 from Mycobacterium tuberculosis. Vaccine. 2006;24:4640–4643. doi: 10.1016/j.vaccine.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 23.D'Souza S., Rosseels V., Denis O., Tanghe A., De Smet N., Jurion F. Improved tuberculosis DNA vaccines by formulation in cationic lipids. Infect Immun. 2002;70:3681–3688. doi: 10.1128/IAI.70.7.3681-3688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano M., Roupie V., Wang X.M., Denis O., Jurion F., Adnet P.Y. Immunogenicity and protective efficacy of tuberculosis DNA vaccines combining mycolyl-transferase Ag85A and phosphate transport receptor PstS-3. Immunology. 2006;118:321–332. doi: 10.1111/j.1365-2567.2006.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulmer J.B., Liu M.A., Montgomery D.L., Yawman A.M., Deck R.R., DeWitt C.M. Expression and immunogenicity of Mycobacterium tuberculosis antigen 85 by DNA vaccination. Vaccine. 1997;15:792–794. doi: 10.1016/s0264-410x(96)00255-1. [DOI] [PubMed] [Google Scholar]
- 26.Ganguly N., Siddiqui I., Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis (Edinb) 2008;88:510–517. doi: 10.1016/j.tube.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Goletti D., Butera O., Bizzoni F., Casetti R., Girardi E., Poccia F. Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J Infect Dis. 2006;194:984–992. doi: 10.1086/507427. [DOI] [PubMed] [Google Scholar]
- 28.Ciabattini A., Pettini E., Andersen P., Pozzi G., Medaglini D. Primary activation of antigen-specific naive CD4+ and CD8+ T cells following intranasal vaccination with recombinant bacteria. Infect Immun. 2008;76:5817–5825. doi: 10.1128/IAI.00793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scriba T.J., Kalsdorf B., Abrahams D.A., Isaacs F., Hofmeister J., Black G. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland S.M. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res. 2007;38:342–346. doi: 10.1007/s12026-007-0045-8. [DOI] [PubMed] [Google Scholar]


