Abstract
Yellow fever vaccine-associated viscerotropic disease (YEL-AVD) is a rare complication of yellow fever (YF) vaccination. A previously healthy 22-year-old female died following YF vaccination despite aggressive measures. Serial viral load titers, cytokine levels and host genetic factors were evaluated in an attempt to understand this unusual and lethal outcome. The patient's high-titer vaccine viremia and possibly related minor genetic anomalies provide clues to exploring the etiology of YEL-AVD.
Keywords: Yellow fever, Yellow fever vaccine-associated viscerotropic disease, Yellow fever vaccination
1. Introduction
Since 1937, the live attenuated YF 17D virus vaccine has protected about 400 million humans from YF, a mosquito-transmitted disease with a case fatality rate of 20% [1], [2]. Since the late 1990s, 36 cases of YEL-AVD, characterized by multiorgan failure, have been recorded worldwide following administration of vaccine manufactured in the United States, France, Brazil and China (Centers for Disease Control and Prevention [CDC], unpublished data) [3], [4]. The risk of YEL-AVD is about three per million doses administered, but is highest among people over 60 years of age and those with a history of thymic disease [3], [5], [6]. The incidence of YEL-AVD is 1.1 per 100,000 doses in people aged 60–69 years and 3.2 per 100,000 doses in people 70 years and older [5]. However, nine of the recorded cases were less than 30 years of age and none had thymic disease (CDC, unpublished data) [7], [8], [9]. The recognition of YEL-AVD coincided with decreased use of pre-travel immunoglobulin to prevent hepatitis A, leading to conjecture that it may have been prevented in the past by the fortuitous coadministration of antibody against YF virus (YFV) [2]. Other hypotheses to explain YEL-AVD include genetic host susceptibility and generation of rare mutations during replication in the host that increase virulence [10], [11], [12], [13].
2. Case report
A healthy 22-year-old student received tetanus-diphtheria, hepatitis A, and typhoid vaccines prior to a trip to Bolivia. Eight days later she received YF vaccine. Two days after vaccination, she noted redness, swelling and pain at the inoculation site. Three days later, she presented to an emergency department with ipsilateral axillary adenopathy, myalgias, fever and vomiting. Her temperature was 103.5 F, pulse 118/min, respirations 24/min and blood pressure 92/54. The physical examination was unremarkable except for an enlarged and tender axillary lymph node.
Her white blood cell count (WBC) was 11,500 cells/mm3 with 92.7% neutrophils, hemoglobin and platelet count were normal, aspartate aminotransferase (AST) was 57 U/L, international normalized ratio (INR) was 1.3, and she had 2+ proteinuria (Table 1 ). Chest radiograph and electrocardiogram were normal. Blood cultures were negative.
Table 1.
Results of hematologic laboratory tests
| Post-vaccination day | WBC | HgB | PLT | HCO3 | Creat | AST | ALT | INR | Bili | AP | Art pH | Lactate | pO2 | pCO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.5–10.5 × 109/L | 12–15.5 g/dL | 150–450 × 109/L | 22–29 mmol/L | 0.6–1.1 mg/dL | 12–31 U/L | 9–29 U/L | 1.0 | 0.1–1.0 mg/dL | 55–142 U/L | 7.35–7.45 | 0.6–2.3 mmol/L | 70–100 Torr | 35–45 Torr | |
| 5a | 11.5 | 13.5 | 230 | 25 | 1.1 | 57 | 27 | 1.3 | 0.9 | 84 | NA | NA | NA | NA |
| 6 | 9 | 13 | 198 | 24 | 0.9 | 67 | 28 | 1.1 | NA | NA | NA | NA | NA | NA |
| 7 | 2 | 11.4 | 43 | 16 | 2.7 | 418 | 178 | 1.4 | 4 | 414 | 7.31 | 4 | 35 | 32 |
| 8 | 4 | 9.3 | 35 | 8 | 5.3 | 987 | 322 | 1.5 | 7.4 | 157 | 7.15 | 3.9 | 59 | 40 |
| 9 | 2.3 | 7.7 | 18 | 18 | 3.7 | 616 | 223 | 1.4 | 6.7 | 111 | 7.24 | 6.2 | 49 | 48 |
| 10 | 5.2 | 10 | 16 | 20 | 1.9 | 459 | 160 | 1.3 | 7 | NA | 7.20 | 6.4 | 34 | 48 |
| 11 | 0.8 | 10.4 | 14 | 19 | 1.8 | 353 | 103 | 1.5 | 7.3 | 125 | 7.16 | NA | 39 | 56 |
WBC: white blood cell count; HgB: hemoglobin; PLT: platelets; HCO3: serum bicarbonate; Creat: creatinine; AST: aspartate aminotransferase; ALT: alanine aminotransferase; INR: international normalized ratio; Bili: bilirubin; AP: alkaline phosphatase; Art pH: arterial pH; pO2: arterial oxygen partial pressure; pCO2: arterial carbon dioxide partial pressure; NA: not available. Lab value reported for each 24-h period is most abnormal.
Lab value reported is from either transferring hospital or receiving quaternary care hospital.
She was admitted and given intravenous fluids, analgesics and anti-emetics. She improved initially, but 24 h later developed abdominal pain, loose stools and recurrent fever. Her WBC dropped to 2000 cells/mm3, hemoglobin to 11.5 g/dL and platelets to 73,000 per mm3. Her AST increased to 181 U/L, total bilirubin to 1.8 mg/dL and INR to 1.4. Renal function was initially preserved.
On transfer to the intensive care unit (ICU) of a quaternary care hospital 7 days after YF vaccination, she complained of pleuritic chest pain, dyspnea and worsening abdominal pain. Chest radiograph showed pleural effusions (Fig. 1 ). Transthoracic echocardiogram revealed tamponade-like physiology secondary to the surrounding pleural effusions. Pleural fluid cultures and HIV serology were negative. Anuric renal failure ensued requiring hemodialysis, but labile blood pressure precluded fluid removal. A single 45 g dose of intravenous immunoglobulin (IVIG) was administered. Hydrocortisone was initiated at 50 mg intravenously every 6 h. Laboratory parameters progressively worsened (Table 1). Nine days after vaccination, she developed hypoxemic respiratory failure requiring mechanical ventilation. Repeat chest radiograph showed dense bilateral consolidation. Nitric oxide therapy was initiated. The following day, vasopressors were started for hypotension unresponsive to fluid resuscitation. High frequency oscillatory ventilation, followed by extracorporeal membrane oxygenation, was initiated for refractory hypoxemia. Repeat transthoracic echocardiogram revealed a markedly depressed ejection fraction of 15%. Broad-spectrum antibiotics were started in consultation with the infectious disease service with convincing evidence of a bacterial infection in the pleural fluid. Eleven days after YF vaccination, the patient died. Blood cultures drawn immediately prior to death grew group C beta-hemolytic Streptococcus. Pleural fluid samples grew the same Streptococcus and Staphylococcus aureus. Post-mortem examination revealed multiorgan failure and diffuse hemorrhage consistent with disseminated intravascular coagulation (DIC) (Fig. 2 ). Microscopy of the lungs showed diffuse alveolar damage with hemorrhage and acute necrotizing bronchopneumonia. Post-mortem blood cultures grew S. aureus.
Fig. 1.

Chest radiograph on admission to quaternary care hospital.
Fig. 2.
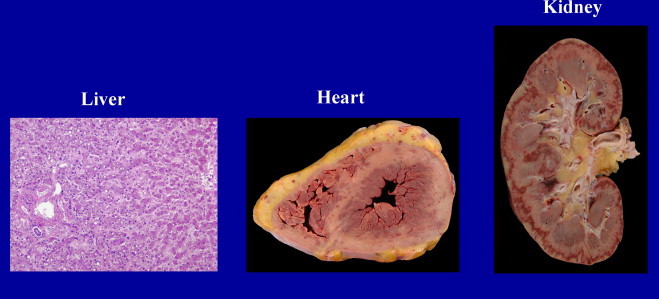
Autopsy specimens liver: midzonal necrosis with sparing of hepatocytes around central vein and portal triad. Heart: circumferential subendocardial hemorrhage involving both ventricles with petechial hemorrhages present over epicardium. Kidney: diffuse cortical hemorrhages with subcapsular infarction.
3. Methods
Formalin-fixed, paraffin-embedded tissues were sent to the CDC for histopathologic and immunohistochemical (IHC) evaluation [4], [14]. Serum and plasma samples were tested for viremia and immunologic response. The consensus sequence of the virus isolate obtained from blood was compared with the lot used for vaccination, three 17D reference strains, and an isolate from a young female who died in Spain following 17D vaccination (GenBank accession numbers: X15062, X03700, U17067 and DQ118157, respectively). Cytokine levels were assessed by the Beadlyte Human Multi-Cytokine Detection System (Cat #48-011, 46-127 and 46-129, respectively; Upstate USA Inc., VA) [15] and the patient's levels were compared with those in previously stored serum from four healthy unvaccinated people. The patient's DNA was isolated from her whole blood and used to analyze the sequences of selected genes that might influence vaccine response.
4. Results
YFV antigens were detected by IHC in the kidney, but not in the liver, spleen, heart or lung. Histopathologic findings in the liver included microvesicular steatosis and Councilman bodies, which are seen in wild-type YF. Panacinar necrosis, presumed secondary to DIC and repeated bouts of hypotension, predominated over the classic finding of midzonal necrosis. Tissue staining revealed abundant colonies of Gram-positive and Gram-variable cocci in the inflammatory infiltrate of the lung. IHC staining identified the colonies as S. aureus.
17D vaccine strain RNA was amplified from plasma and serum samples by RT-PCR. The estimated viral titer was 61,000 plaque-forming units (PFU)/mL on post-vaccination day 5, rising to a peak of 106,500 PFU/mL the following day (Table 2 ). Healthy vaccinated people rarely have greater than 100 PFU/mL [2]. Viremia dropped 8 days after vaccination with the development of YF virus-specific neutralizing and IgM antibody. Viral RNA was thereafter detected at low levels until the day of death. There were elevated levels of several proinflammatory cytokines and chemokines, including IFNγ, IL-6, RANTES, GM-CSF, IL-1a, IL-3, IL-7 and IP-10, during the clinical course but no specific cytokine abnormality that would explain her illness.
Table 2.
Serum levels of viremia, antibody and selected cytokines

Neut Ab: neutralizing antibody; IgM: immunoglobulin M; IFN: interferon; TNF: tumor necrosis factor; IL: interleukin; RANTES: regulated on activation normally T cell expressed and secreted; GM-CSF: granulocyte macrophage colony-stimulating factor; MCP: monocyte chemotactic protein; MIP: macrophage inflammatory protein; IP: interferon-delta-inducible protein; ND: not determined. Cytokine levels are expressed as high, low or baseline compared to four healthy recipients of yellow fever vaccine.
The consensus sequence of viral RNA obtained 5 days post-vaccination showed 100% nucleotide identity to the utilized vaccine lot. These sequences differed from a reference strain of 17D-204 at A1431C (Asn to Thr), C5362T (silent), A5641G (silent), T7496C (silent), A10,243G (silent) and A10,722G (3′ non-coding). Identical changes at positions 5641, 10,243 and 10,722, but not at 1431, 5362, and 7496, were detected in viral RNA from a recent fatal case in Spain [8]. The Spanish isolate also had a unique silent mutation at 6418.
Comparison of the patient's OAS1 gene promoter and exons 1, 2, 4, and 5 sequences with the GenBank human genome assemblies NT_009775 or NW_925395 revealed no differences. However, the minor allele was detected at three previously identified single nucleotide polymorphisms (SNPs) in the OAS1 gene: GG-homozygous for SNP rs3741981 in exon 3 (Gly162Ser); GG-homozygous for SNP rs10774671 in intron 5 (splicing acceptor site); and AA-homozygous for the SNP rs11352835 A/- indel in 3′ terminal exon. The frequency of SNP rs3741981 GG-homozygotes varies from 0.133 in Europeans to 0.767 in Sub-Saharan Africans.1 The frequency of SNP rs10774671 GG-homozygotes is 0.195,1 and of SNP rs11352835 AA-homozygotes is 0.111.2
No variations in the sequences of the regulatory elements of the OAS2 gene promoter were observed, but there was a single variation within the OAS2 exons; the patient was AA-homozygous for SNP rs15895, which causes a premature stop-codon in exon 11. The frequency of AA-homozygotes varies from 0.00 in African-Americans to 0.208 in Caucasians.1 The patient was homozygous for the minor allele at one of four SNPs [CC of rs12992188 (frequency 0.16)2] in the promoter region and for one of five SNPs in exon 2 [5′ NCR;TT of rs2254958 (frequency 0.217)1] and was heterozygous at SNPs: rs4648174 (intron 4), rs2307483 (intron 6) and rs2307469 (silent, exon 15). No mutations were detected in either the promoter or exons of the toll-like receptor 3 gene. She was homozygous wild-type for the SNPs analyzed in the CCR5 gene, CCR5 promoter, RANTES promoter and DC-SIGN gene [16]. Her DC-SIGNR gene was heterozygous for the number of repeats in exon 4 (6/5) [17].
5. Discussion
Development of YEL-AVD could be due to either viral or host anomalies. The consensus viral RNA sequence obtained from this patient's blood was identical to that of the original vaccine lot, indicating that the primary strain had not mutated after inoculation. Although some of the sequence differences from a reference vaccine strain were also detected in an isolate from a similar case of YEL-AVD in Spain, two of these nucleotide changes were silent and one was in a non-coding region. These changes seem unlikely to affect virulence, and other people vaccinated with the same lots did not develop YEL-AVD. Clonal sequencing of virus from an earlier YEL-AVD case revealed a mutated vaccine substrain, and mutated virus was also isolated from a child with encephalitis following YF vaccination [4], [18]. Since clonal sequencing of the isolate from this patient was not done, it is possible that a minor mutant substrain altered the overall virulence of the vaccination without altering the consensus sequence. However, viral sequences from other YEL-AVD cases have not suggested loss of attenuation, and YEL-AVD has occurred with vaccine from various lots, strain subtypes and manufacturers [3], [8].
One of eight Oas1 genes in mice is associated with susceptibility to flaviviruses [10]. Three SNPs were minor allele homozygous in the patient's OAS1 gene. Two of these were in coding regions, while the other was in an intron splicing site. At least five different OAS1 isoforms are generated by alternative splicing. The p42 and p44 isoforms are produced by all genotypes. The A-allele in OAS1 intron 5 SNP rs10774671 produces p48 and p52, while the G-allele produces p46 [19]. Since the patient was GG-homozygous, she would have produced p46. Computer modeling suggests that p46 may have impaired enzymatic activity [20]. The patient was homozygous for the G-allele of SNP rs3741981 in exon 3 which causes an amino acid substitution in all the OAS1 isoforms. The G-allele of this SNP was previously reported to be associated with severe acute respiratory syndrome in a Vietnamese population [21]. Homozygosity for the OAS1 3′ terminal exon A-insertion (rs11352835) produces a frame shift and premature translation termination of the p44 transcript. Although not currently supported by experimental data, the three minor alleles in the OAS1 gene and the AA-homozygosity in OAS2 SNP rs15895 leading to truncation of the OAS2 protein could have had an adverse effect on possible OAS-mediated anti-flaviviral activities that contributed to the abnormally high virus levels. It is unlikely that the mutations in PKR affected protein function but mutations in other candidate genes not evaluated might also have influenced the response to YF vaccination. Similar host factors may be involved in atypically severe manifestations of different flavivirus infections [22].
Following vaccination, this patient developed notable inflammation at the vaccine site where the virus is thought to replicate in dendritic cells. Local inflammation may occur in up to 30% of vaccines but is rarely severe [2]. It has been described in a prior case report [8]. The significance of severe local inflammation is uncertain but might be consistent with increased viral replication or higher intensity of the local immune response. This patient's viremia peaked on post-vaccination day 6 at 1000 times higher than expected following a brief remission and then intensification of her symptoms [2]. By that time, multiorgan failure had developed, likely mediated by inflammatory cytokines, in a clinical pattern similar to that seen in wild-type YF, except that the virus is usually cleared in wild-type infection by day 6 [2]. One dose of IVIG was given on post-vaccination day 8 without obvious clinical benefit. Further doses were withheld because of volume overload concerns and the general consensus that the patient had developed a protective antibody response. IVIG has been proposed for postexposure prophylaxis against wild-type YF, but has no proven benefit after onset of illness [2].
The patient's clinical course was complicated by bacterial pneumonia and sepsis. Secondary bacterial infections also occur in patients with naturally acquired YF [23]. The use of prophylactic antibiotics may consequently be warranted in persons with YEL-AVD in the future.
YF vaccination is the most effective means of preventing wild-type disease. However, because of the potential for extremely rare severe adverse events, the vaccine should only be given to travelers whose itinerary requires vaccination. The etiologic mechanism of YEL-AVD remains unclear and may vary across cases but a genetic susceptibility based on OAS gene abnormalities might have played a role in this case. Suspected cases of YEL-AVD should be promptly reported to the Vaccine Adverse Event Surveillance System at 1-800-822-7967 or http://vaers.hhs.gov. Collection of whole blood and serum from patients with suspected YEL-AVD will aid future investigations of this puzzling and devastating condition.
Footnotes
Genotype frequencies available at: www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=3741981, www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=10774671, www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=15895, www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs2254958.
Perelygin and Brinton, unpublished data.
Reference
- 1.Pugachev K.V., Guirakhoo F., Monath T.P. New developments in flavivirus vaccines with special attention to yellow fever. Curr Opin Infect Dis. 2005;18(5):387–394. doi: 10.1097/01.qco.0000178823.28585.ad. [DOI] [PubMed] [Google Scholar]
- 2.Monath T.P. 4th ed. Philadelphia; Saunders: 2004. Yellow fever vaccine. [Google Scholar]
- 3.Monath T.P. Yellow fever vaccine. Expert Rev Vaccines. 2005;4(4):553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 4.Martin M., Tsai T., Cropp B., Chang G., Holmes D., Tseng J. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet. 2001;358(9276):98–104. doi: 10.1016/s0140-6736(01)05327-2. [DOI] [PubMed] [Google Scholar]
- 5.Khromava A.Y., Eidex R.B., Weld L.H., Kohl K.S., Bradshaw R.D., Chen R.T. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events. Vaccine. 2005;23(25):3256–3263. doi: 10.1016/j.vaccine.2005.01.089. [DOI] [PubMed] [Google Scholar]
- 6.Barwick R. History of thymoma and yellow fever vaccination. Lancet. 2004;364(9438):936. doi: 10.1016/S0140-6736(04)17017-7. [DOI] [PubMed] [Google Scholar]
- 7.Vasconcelos P., Luna E., Galler R., Silva L., Coimbra T., Barros V. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet. 2001;358(9276):91–97. doi: 10.1016/s0140-6736(01)05326-0. [DOI] [PubMed] [Google Scholar]
- 8.Doblas A., Domingo C., Bae H., Bohórquez C., de Ory F., Niedrig M. Yellow fever vaccine-associated viscerotropic disease and death in Spain. J Clin Virol. 2006;36(2):156–158. doi: 10.1016/j.jcv.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Gerasimon G., Lowry K. Rare case of fatal yellow fever vaccine-associated viscerotropic disease. South Med J. 2005;98(6):653–656. doi: 10.1097/01.SMJ.0000157537.11806.DC. [DOI] [PubMed] [Google Scholar]
- 10.Perelygin A.A., Scherbik S.V., Zhulin I.B., Stockman B.M., Li Y., Brinton M.A. Positional cloning of the murine flavivirus resistance gene. Proc Natl Acad Sci USA. 2002;99(14):9322–9327. doi: 10.1073/pnas.142287799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnevie-Nielsen V., Heron I., Monath T.P., Calisher C.H. Lymphocytic 2′,5′-oligoadenylate synthetase activity increases prior to the appearance of neutralizing antibodies and immunoglobulin M and immunoglobulin G antibodies after primary and secondary immunization with yellow fever vaccine. Clin Diagn Lab Immunol. 1995;2(3):302–306. doi: 10.1128/cdli.2.3.302-306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass W.G., McDermott D.H., Lim J.K., Lekhong S., Yu S.F., Frank W.A. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203(1):35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakub I., Lillibridge K.M., Moran A., Gonzalez O.Y., Belmont J., Gibbs R.A. Single nucleotide polymorphisms in genes for 2′–5′-oligoadenylate synthetase and RNase L inpatients hospitalized with West Nile virus infection. J Infect Dis. 2005;192(10):1741–1748. doi: 10.1086/497340. [DOI] [PubMed] [Google Scholar]
- 14.Guarner J., Bartlett J., Reagan S., Fischer M., Finn S., O’Briain D. Immunohistochemical evidence of Clostridium sp, Staphylococcus aureus, and group A Streptococcus in severe soft tissue infections related to injection drug use. Hum Pathol. 2006;37(11):1482–1488. doi: 10.1016/j.humpath.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Pal S., Schnapp L.M. HIV-infected lymphocytes regulate fibronectin synthesis by TGF beta 1 secretion. J Immunol. 2004;172(5):3189–3195. doi: 10.4049/jimmunol.172.5.3189. [DOI] [PubMed] [Google Scholar]
- 16.Liu H., Hwangbo Y., Holte S., Lee J., Wang C., Kaupp N. Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J Infect Dis. 2004;190(6):1055–1058. doi: 10.1086/423209. [DOI] [PubMed] [Google Scholar]
- 17.Liu H., Carrington M., Wang C., Holte S., Lee J., Greene B. Repeat-region polymorphisms in the gene for the dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin-related molecule: effects on HIV-1 susceptibility. J Infect Dis. 2006;193(5):698–702. doi: 10.1086/499820. [DOI] [PubMed] [Google Scholar]
- 18.Jennings A.D., Gibson C.A., Miller B.R., Mathews J.H., Mitchell C.J., Roehrig J.T. Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J Infect Dis. 1994;169(3):512–518. doi: 10.1093/infdis/169.3.512. [DOI] [PubMed] [Google Scholar]
- 19.Bonnevie-Nielsen V., Field L.L., Lu S., Zheng D., Li M., Martensen P.M. Variation in antiviral 2′,5′-oligoadenylate synthetase (2′5′AS) enzyme activity is controlled by a single-nucleotide polymorphism at a splice-acceptor site in the OAS1 gene. Am J Hum Genet. 2005;76(4):623–633. doi: 10.1086/429391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torshin I.Y. Three-dimensional models of human 2′–5′ oligoadenylate synthetases: a new computational method for reconstructing an enzyme assembly. Med Sci Monit. 2005;11(7):BR235–BR247. [PubMed] [Google Scholar]
- 21.Hamano E., Hijikata M., Itoyama S., Tran Q., Nguyen C.P., Hoang T.L. Polymorphisms of interferon-inducible genes OAS-1 and MxA associated with SARS in the Vietnamese population. Biochem Biophys Res Commun. 2005;329(4):1234–1239. doi: 10.1016/j.bbrc.2005.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paddock C.D., Nicholson W.L., Bhatnagar J., Goldsmith C.S., Greer P.W., Hayes E.B. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42(11):1527–1535. doi: 10.1086/503841. [DOI] [PubMed] [Google Scholar]
- 23.Jones E.M., Wilson D.C. Clinical features of yellow fever cases at Vom Christian Hospital during the 1969 epidemic on the Jos Plateau, Nigeria. Bull World Health Organ. 1972;46(5):653–657. [PMC free article] [PubMed] [Google Scholar]


