Abstract
A new viral sequence likely belonging to a virus of the family Astroviridae was determined using the gut content of chickens affected with the runting–stunting syndrome (RSS) in chickens. Since the appropriate virus could not be isolated in cell culture the open reading frame of the viral capsid protein was cloned to generate a recombinant baculovirus. The protein was purified and used as an experimental vaccine in broiler breeders to provide maternal derived antibodies for the protection of the offspring. The presence of specific antibodies was monitored by an ELISA. The offspring of vaccinated breeder hens were partially protected in a RSS challenge model.
Keywords: Astrovirus, Recombinant protein, Runting–stunting syndrome
1. Introduction
Runting and stunting syndrome (RSS) has been recognized since the late 1970s. RSS has also been referred to as malabsorption syndrome (MAS), infectious stunting syndrome, broiler runting syndrome, pale bird syndrome and helicopter syndrome. All associations to this disease manifestation have been based on clinical signs and microscopical lesions in organs of the small intestine. Clinical signs are growth retardation (stunted chickens), ruffled feathers, and slight diarrhea. Histopathologic changes of the stunted chicks include villous atrophy of the small intestine and a distention of crypts of Lieberkühn [1], [2]. Although environmental, nutritional and management issues might play an important role in the manifestation of this syndrome, infectious agents, namely viruses have been implicated as etiologic agent(s). Specifically, reoviruses, rotaviruses, enteroviruses, astroviruses, and other small round viruses have been observed in the intestines and contents of clinically affected birds by virus isolation and/or electron microscopy [3], [4], [5], [6], [7]. Recently a novel parvovirus was described which might be associated with RSS [8].
A similar enteric syndrome has been described for young turkey poults, Poult Enteritis and Mortality Syndrome (PEMS). Viral etiologies associated with this disease are turkey coronavirus (TCV), turkey astrovirus (TAstV) and turkey reovirus [9], [10], [11], [12], [13].
The isolation of viruses from samples associated with RSS in chickens resulted in isolation of avian nephritis virus, enterovirus, and reovirus [6], reovirus and infectious bronchitis virus [14], reovirus [15]. In other cases, a combination of ultracentrifugation and electron microscopy has been used for the detection of viruses which might be associated with RSS [2], [7], [14], [15]. But isolation of viruses in cell culture was mostly only possible for reovirus. However, advancements in molecular technology have made RT-PCR and PCR valuable tools for detecting these viruses which are typically difficult to culture. Previously a multiplex RT-PCR has been developed for detection of TAstV, coronavirus and reovirus [16], [17] in feces of turkey poults experiencing PEMS. On the other hand, viruses which have been described in RSS and PEMS (rotavirus, reovirus, astrovirus, and coronavirus) were also detected by molecular methods in the gut of clinically healthy chicken and turkey flocks [18]. This indicates that the etiology of RSS and its related disease in turkeys is more complex.
Although avian coronavirus might play a role in RSS and PEMS, avian reovirus and avian astrovirus has been shown to partially induce the clinical picture in both diseases, either alone or in combination [14], [19]. Avian reoviruses are commonly isolated from healthy chickens but are also associated with several diseases, namely viral arthritis and MAS. The relationship of reoviruses in cases of MAS is not well understood. Reoviruses isolated from cases of MAS have been shown to cause lesions in the duodenum, jejunum and ilieum with [15], [20] or without an associated weight suppression [21]. In addition, reovirus antigen staining was observed at the tip of the ileal villi of broilers experimentally infected with homogenized intestines of MAS affected flocks [22]. However, in the intestinal lesions associated with avian reovirus MAS isolates no antigen staining was present in the crypt of the Lieberkühn, where numerous cysts have been observed in affected birds [22]. Other studies by researchers in Europe [23], [24] and Australia [25] found little evidence that reovirus played a significant role in RSS.
Avian nephritis virus, a recently classified astrovirus has also been implicated in RSS. This virus was initially classified as a picornavirus (entero- and virus), based on morphological features, until the full length genome was sequenced in 2000 [26]. Early investigations into the etiologies of RSS identified entero-like viruses in young chicks. A crude inoculum containing this virus and a reovirus consistently produced slow feathering, fecal changes and depressed weight gains in broilers inoculated at one day of age [3]. Co-infection with avian reovirus and avian nephritis virus isolated from broilers exhibiting a runting syndrome has been examined in two lines of SPF chickens [19]. Infection with the avian reovirus resulted in weight suppression in both lines of chickens. However, infection with the avian nephritis virus resulted in growth depression and nephritis in only one of the two lines. Challenge of the same line with avian nephritis virus at one day of age and reovirus at three days of age resulted in growth depression less severe than the single reovirus infection. These results suggest a breed-related susceptibility for avian nephritis virus.
In this paper we describe the identification of a new viral sequence likely belonging to a virus of the family Astroviridae. The open reading frame (ORF) of the putative capsid protein was used to generate a recombinant baculovirus expressing the ORF. The protein was purified and used as an experimental vaccine in broiler breeders. The offspring of vaccinated breeder hens were partially protected in a RSS challenge model.
2. Material and methods
2.1. Establishment of a challenge model for RSS
Experiment 1: Chicken litter was collected and transported from a farm with clinical signs of RSS to experimental isolation houses (colony houses). The chicken litter was distributed onto the floor of a colony house with an approximate area of 10 m2 (RSS+). In parallel, fresh shavings were distributed onto the floor of another colony house (RSS−). In each house, 150 one-day-old broiler chickens from a commercial hatchery were raised. Water and feed was provided ad libitum. 12 days after placing the birds in the colony houses, the birds were removed and humanely euthanized with CO2 and body weights recorded. The first thirty birds per group were randomly selected and necropsy was performed, the small intestine was removed and tissue samples from the duodenal loop were harvested, and fixed in neutral buffered formalin for subsequent histological examination. The taken tissue sample was a cross section of the duodenal loop just above the tip of the pancreas including the ascending and descending part of the loop. The remaining parts of the small intestine were homogenized in a Waring blender, diluted 1:1 with sterile PBS, and aliquots were stored frozen at −80 °C.
Experiment 2: In subsequent experiments, one-day-old commercial broiler chickens were inoculated with 1 ml of the homogenized gut of either RSS+ or RSS− birds by feeding tube. The used gut samples were obtained from the 1st animal experiment. One group was left untreated to serve as true negative control. All three groups were held in HEPA filtered Horsfal Bauer units with forced air positive pressure. Water and feed was provided ad libitum. 12 days after inoculation the birds were euthanized with CO2, the duodenal loop was harvested as described above, fixed in neutral buffered formalin and examined for histological lesions.
Experiment 3: Based on the assumption that RSS has a viral etiology, 15 one-day-old commercial broiler chickens were inoculated with 1 ml of three different inocula, non-treated gut content, filtered gut content, and filtered/chloroform treated gut content. The used gut content was from RSS+ chickens obtained from the 1st experiment. Before filtration, the gut content was centrifuged at 3500 × g for 30 min at 4 °C. The supernatant was harvested and centrifuged at 16,000 × g for 10 min at 4 °C, filtered through a 0.45 μm filter and the obtained filtrate was filtered again using a 0.22 μm filter. The final filtrate was left untreated or was mixed with 0.5 volume of chloroform, repeatedly vortexed for 15 s in a 1 min interval over 15 min. The suspension was centrifuged at 3500 × g for 30 min at 4 °C, the upper phase was removed and used for inoculation of commercial one-day-old broiler chickens held in Horsfal Baur units with positive pressure. Water and food was supplied ad libitum. The chickens were inoculated with one of the following treatments: (1) 1 ml of the non-treated RSS+-gut content, (2) 1 ml of the filtered RSS+-gut content, (3) 1 ml of the filtered/chloroform treated RSS+-gut content or (4) no treatment. The chickens were monitored daily. At day 12 after infection, the chickens were euthanized with CO2 and body weights recorded. During necropsy, the duodenal loop was harvested as described above, fixed in neutral buffered formalin and examined histologically. The tissue samples of all experiments were fixed in 10% buffered formalin and routinely processed, embedded, sectioned and stained with hematoxylin and eosin (H&E). The obtained data were statistically validated using the one-way ANOVA analysis from summary data (http://www.danielsoper.com/statcalc/calc43.aspx).
2.2. Delineation of oligonucleotides
Based on the results from the experiments described above and the hypothesis that the infectious agent is a small round virus, available sequences of RNA-dependent RNA polymerases (RdRp) of the virus family Picornaviridae were aligned using the ClustalW (http://www.ebi.ac.uk/Tools/clustalw2/index.html) due to a possibly existing higher similarity between nucleotide sequences of the RdRp. Based on the sequence similarities, two groups of viruses were formed 1) polioviruses, coxsackieviruses including duck picornaviruses (DuckPico) and 2) aphtoviruses (FMDV). The sequences were analyzed based on the requirement for a possible primer pair that would result in a RT-PCR fragment ≤350 bp. The resulting primer pairs (FMDVFP1/FMDVRP1; DuckpicoFP1/DuckpicoRP1) were used for RT-PCR (see Table 1 ).
Table 1.
Oligonucleotides used for cloning of the astrovirus sequences.
| Name | Sequence | Locationa | Orientation | Genbank no.b | Virus |
|---|---|---|---|---|---|
| FMDVFP1 | GGGTTTTACAAACCTGTGAT G | 7786-7806 | Sense | DQ248888 | FMDV |
| FMDVRP1 | CCGCACACGGCGTTCACCC | 7982-7920 | Antisense | DQ248888 | FMDV |
| FMDVFP2 | TAAGGACTTTGTGGTCTATG | ||||
| FMDVFP2Rev | CATAGACCACAAAGTCCTTA | ||||
| FMDVRP2 | GGCCTCGATGCTTGGGAGCC | Sense | |||
| FMDVRP2Rev | GGCTCCCA AGCATCGAGG CC | Antisense | |||
| DucPicoFP1 | AGATTGATTGAAGCCTCCAGTTTG | 6505-6528 | Sense | AY278552 | Human poliovirus 2 |
| 7113-7136 | Sense | AY563023 | Duck picornavirus | ||
| DucPicoRP1 | ATGSWDGTNCCHGARCABCC YGADGGCAT | 6841-6869 | Antisense | AY278552 | Human poliovirus 2 |
| 7458-7486 | Antisense | AY563023 | Duck picornavirus | ||
| DucPicoFP2 | GGAAAGGAAGATGAGGGCATTG | Sense | |||
| DucPicoFP2rev | CAATGCCCTCATCTTCCTTTCC | Antisense | |||
| DucPIcoRP2 | GCCAGTTTGGAGAGTATTTAC | Antisense | |||
| DucPicoRP2Rev | GTAAATACTCTCCAAACTGGC | Sense | |||
| CapFP | EcoR I | Sense | |||
| ccGAATTCATGGCCGATAAGGCTGGGCCGC | |||||
| polyT-GC | GCGCGCGCTTTTTTTTTTTTTTTTTT | Antisense | |||
| CapRP | Not I | Antisense | |||
| ggGCGGCCGCTAGTGATGGTGATGGTGATGCTCGGCGTGGCCGCGGCTGCTAGCAGG | |||||
Location of the oligonucleotides in agreement with the sequences of the food and mouth disease virus (FMDV), duck picornavirus, and human poliovirus 2, respectively.
NCBI Genbank.
2.3. RNA purification and RT-PCR
10 ml of the homogenized gut tissue sample of RSS+ chickens and RSS− chickens from the first experiment were clarified by low speed centrifugation (3500 × g for 20 min) and the supernatant was filtered using a 0.45 μM filter. The resulting filtrate was ultracentrifuged at 174,899 × g for 1 h. The resulting pellet was resuspended in 200 μl sterile PBS. The RNA was purified by using the High-Pure-RNA-Isolation-Kit (Roche, Applied-Science). Reverse transcription-polymerase chain reaction (RT-PCR) was performed using SuperScript™ III One-Step RT-PCR System with Platinum® Taq (Invitrogen) following the standard protocol as provided by the manufacturer. In reactions were the RT step was not performed the reaction mixture was immediately incubated at 97 °C for 1 min to inactivate the reverse transcriptase. For the amplification of the 3′end of the genomic RNA a RT-RAMP/PCR was performed. To this end the time from the annealing step to the extension step during PCR was set with an increment of 30% of the given RAMP using the Eppendorf Mastercycler ep (Eppendorf, Hamburg, Germany).
2.4. Cloning and sequence analysis
Amplified PCR fragments were cloned into the vector pCR2.1 using the TopoTA cloning kit (Invitrogen, Carlsbad, CA, USA). Purified plasmid DNA was sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Lincoln, CA, USA). Resulting sequences were compared using the computer program Gene-Runner Version 3.1 (Hastings Software, Hudson, NY, USA). Subsequent data analysis was performed using online computer programs (http://www.expasy.org/tools/dna.html; http://www.ebi.ac.uk/Tools/clustalw2).
2.5. Generation of recombinant baculovirus expressing the astrovirus capsid protein
For expression of the astrovirus capsid protein a recombinant baculovirus was generated based on the baculovirus transfer vector pFastBac™ Dual and the Bac-to-Bac® Baculovirus Expression System (Invitrogen, Carlsbad, CA, USA). To construct an appropriate plasmid, a 2267 bp fragment was amplified by RT-PCR using a pair of oligonucleotides (CAP-FP; CAP-RP, see Table 1) and purified RNA from the gut sample (see above). The resulting RT-PCR fragment was eluted from a 1% agarose gel using the QIAquick gel extraction kit (Qiagen), cloned into pCR2.1 (CAP-pCR2.1) using the TopoTA cloning Kit (Invitrogen). After verification by sequencing, CAP-pCR2.1 was cleaved with EcoR I/Not I, the eluted DNA fragment encompassing the capsid protein coding region and a 6×His encoding region at its C-terminus was ligated into EcoR I/ Not I cleaved baculovirus transfer vector pFastBac™ Dual to obtain pFAST-CAP. After verification of the nucleotide sequence a recombinant bacmid containing the ORF of the astrovirus capsid protein was generated using the Bac-to-Bac® Baculovirus Expression System following the protocols as provided by the manufacturer. Transfection and subsequent propagation of recombinant baculovirus was performed in Sf9 cells as recommended by the manufacturer using Cellfectin (Invitrogen). Sf9 cells were cultivated in serum-free medium (HyClone SFX-Insect, ThermoFisher) containing ampicillin (100 IU/ml) and streptomycin (100 μg/ml). The baculovirus expressing the astrovirus capsid protein (Cap-Bac) was used for infection of Sf9 cells at a multiplicity of infection of 1. Seventy-two hours after infection cells were harvested by centrifugation at 1000 × g for 10 min at 4 °C. The obtained sedimented cells were used either immediately for protein purification or stored at −20 °C until purification. Protein purification was performed as previously described by Letzel et al. [27].
2.6. Determination of the baculovirus titer
The TCID50 was determined by indirect immunofluorescence. To this end, tenfold dilutions of the viral stock were performed in HyClone SFX-Insect serum-free medium. 100 μl of Sf9 cells (300 000 cells/ml) were seeded into each well of a 96-well cell culture plate and infected with 100 μl of the appropriate virus dilution. Three days after inoculation, the cells were fixed with ice-cold ethanol for 10 min and indirect immunofluorescence was performed using a monoclonal antibody (mAb) directed against Baculovirus V5 protein (mAb-V5, Sigma–Aldrich, St. Louis, MO) as primary antibody in a dilution of 1:1000. As a secondary antibody, a FITC labeled goat anti-mouse IgG antibody (Jackson ImmunoResearch, West Grove, PA) was used in a dilution of 1:200. The TCID50 was calculated using the formula of Reed and Muench [28].
2.7. Detection of recombinant protein
For Western blot analysis, samples were separated by sodiumdodecylsulphate-12% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. The membrane was incubated with blocking solution using 5% non-fat dry milk in TBST (150 mM NaCL, 10 mM Tris, pH 8.0, 0.05% Tween 20) for 1 h. After washing in TBST, membranes were incubated with the anti 6×His mAb (Clone His-1, Sigma–Aldrich, ST. Louis, MO, USA) as primary antibodies (dilution 1:2000) and peroxidase-labeled goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) as secondary antibody (dilution 1:15000). The binding of the antibodies was monitored using a chemiluminescent substrate, Immobilon Western (Millipore, Billerca, MA) and Gel Logic 2200 (Carestream Health, New Haven, CT). For monitoring of the recombinant protein purification, samples were separated by SDS-PAGE and stained with Imperial Protein Stain (ThermoScientific, Rockford, IL, USA) following the manufacturers instructions.
2.8. Generation of astrovirus capsid protein specific antisera
To obtain a standard serum from chickens, five 21-day-old SPF leghorn chickens were bled and vaccinated as described below. Per chicken, 500 μl of the purified protein (250 μg) were mixed with 500 μl of Freund's incomplete adjuvant and a stabile emulsion was obtained by ultrasonic using Branson Sonifier 150 (Branson, Danbury, CT, USA) at level 2. Appropriate controls were left unvaccinated. The chickens were vaccinated three times at intervals of four weeks. At each time point prior vaccination and three weeks following final vaccination, the chickens were bled and the serum was tested for reactivity in the described ELISA system (see below). Four weeks following the final vaccination, a final bleed was obtained from the chickens and euthanized with CO2.
2.9. Development of an ELISA for the detection of astrovirus capsid specific antibodies in chicken sera
For ELISA, the protein detector ELISA kit (KPL, Gaithersburg, MD) was used. The purified recombinant capsid protein was diluted in coating buffer to a final concentration of 500 ng/ml and 50 μl were added in each well of a 96-well flat bottom plate (FisherBrand, Santa Clara, CA). After incubation over night at 4 °C, non-bound antigen was removed, wells were rinsed three times with wash solution (KPL) and 100 μl blocking solution was added (KPL). The plate was incubated for 45 min at 37 °C. Following the washing procedure, 50 μl of diluted serum (1:100) was pipetted into the wells and incubated for 1 h at 37 °C and rinsed three times with wash solution. Finally, 50 μl of 1:1000 diluted peroxidase conjugated goat anti-chicken serum (KPL) was added. After incubation for 1 h at 37 °C, the plate was washed three times, 50 μl of peroxidase substrate solution (KPL) was added and the plate incubated for 15 min at room temperature. The enzymatic reaction was stopped by adding 50 μl of stop solution per well (KPL). The optical density (OD) values were measured at 405 nm using an ELISA reader (ELX 808, BioTek, Winooski, VT, USA).
2.10. Vaccination of broiler breeders
One group of fifteen 21-week-old commercial broiler breeder hens was vaccinated three times (weeks 22, 26, and 30) intramuscularly in the breast muscle with 1 ml of the experimental vaccine. The vaccine was prepared as described above. A second group of fifteen 21-week-old commercial broiler breeder hens was left non-vaccinated. In both groups three broiler roosters were present. The roosters were not vaccinated and held as sentinel controls. No adverse effect in the breast muscle was observed during the experiments. Before vaccination and two weeks after each vaccination, blood was drawn from the brachial vein to obtain serum samples. Serum samples were monitored by the recombinant protein ELISA.
2.11. Challenge experiments in commercial broiler chickens
Starting at 14 days until 24 days after the second vaccination (2nd vac/1, see Fig. 7), eggs were collected from both groups and chickens were hatched separately for challenge experiments. In addition, eggs were collected from 25 days until 28 days after the second vaccination, chickens were hatched and the obtained sera were analyzed for the presence of antibodies raised against the recombinant protein (2nd vac/2, see Fig. 7). Furthermore, 14 days until 28 days (3rd vac/1, see Fig. 7) and 29 days until 42 days (3rd vac/2, see Fig. 7) after the third vaccination eggs were collected, chickens were hatched and the sera were analyzed for the presence of antibodies raised against the recombinant protein (Gr 2 2nd, see Fig. 7). The testing for the presence of antibodies directed against the recombinant protein was performed using the above described indirect ELISA. After hatch (2nd vac/1, 3rd vac/1, 3rd vac/2) the two groups of chickens were weighed and placed into Horsfal Bauer isolation units and given water and food ad libitum. One group of chickens from each vaccination group was challenged by inoculation of filtered gut content from RSS affected chickens obtained from the 1st experiment. One group was mock-challenged with PBS. The chickens were monitored daily. 12 days after infection chickens were euthanized by CO2 and weighed. At necropsy the duodenal loop was taken for histopathological examination. Chickens not used for the challenge experiments were bleed and the obtained serum was used for detection of antibodies using the recombinant protein based ELISA.
Fig. 7.
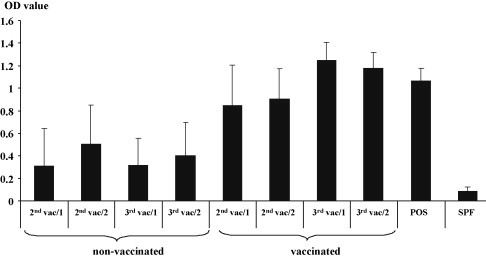
Maternal derived antibodies raised against the astrovirus protein are transferred to the progeny of vaccinated hens. The progeny from vaccinated and non-vaccinated broiler hen groups were hatched two times (Gr 1, Gr 2) after each vaccination. The collection of eggs started 14 days after the 2nd and 3rd vaccination. Blood samples were taken at day of hatch and serum samples were investigated using the astrovirus capsid protein ELISA. Positive and negative (SPF) control serum was analyzed in parallel. The extinction at a wavelength of 405 nm is shown at the left axis.
3. Results
3.1. Runting–stunting syndrome is caused by non-enveloped viruses
In order to establish a challenge model for RSS in commercial broilers under our conditions, chicken litter from a farm showing the typical signs of RSS was transported to a colony house and 150 one-day old commercial broiler chickens were placed on that litter (RSS+exp1). In parallel, 150 one-day-old commercial broiler chickens were placed on fresh shavings (RSS−exp1). During the 1st experiment RSS+ exp1 chickens developed slight diarrhea, ruffled and dirty feathers, and lassitude. The control chickens (RSS−exp1) showed no clinical signs. 12 days after infection, all 300 chickens were euthanized and weighed. On 30 randomly selected chickens the macroscopic inspection of the RSS+exp1 chickens revealed a difference in size compared to the RSS−exp1 control chickens. The ventral abdomen of the RSS+exp1 chickens was swollen in a number of chickens. Macroscopic lesions during necropsy of the RSS+exp1 chickens consisted of a thinning of the small intestine, gaseous ceacae, swollen and dark liver, massive filled bile, and enlarged spleen. Comparison of body weights showed that RSS+exp1 chickens showed a significant different weight depression (p < 0.001) of approximately 50% in comparison to the RSS−exp1 chickens (Fig. 1 ). For histological examination one section of the ascending and one section of the descending part of the duodenal loop was cut and following H&E staining, scored for the presence and number of lesions per section. In the gut of 20 out of the 30 birds, cystic formation of the crypt of Lieberkühn (further designated as cystic lesions) in the RSS+exp1 group were observed (Fig. 1). The number of lesions per bird varied between 1 and 16.
Fig. 1.

RSS caused a severe weight depression and cystic enteropathy in young broiler chickens. (A) 150 one-day-old commercial broiler chickens were placed on chicken litter from a RSS affected farm (RSS+) or on fresh shavings (RSS−). 12 days after placement chickens were weight and average of the body weights in gram (g) is shown. The standard deviation is depicted by bars. (B) The presence of cystic lesions was histologically examined by the analysis of two cross sections of the duodenal loop of 30 birds randomly selected during necropsy. The lesions were counted per bird. The number of birds which showed lesion out of the total number of analyzed birds is shown in parenthesis. The number of lesions per bird is indicated at the left axis.
Based on the results obtained, we performed the next experiment (experiment 2) where 15 one-day-old commercial broiler chickens were inoculated with minced gut content of either RSS+exp1 chickens (RSS+exp2) or RSS−exp1 chickens (RSS−exp2). One group was mock inoculated with PBS (Con-exp2). 12 days after inoculation the chickens were evaluated. The macroscopic inspection of the RSS+exp2 chickens showed distention of the abdomen, retarded growth, dirty feathers, and signs of diarrhea. RSS−exp2 chickens as well as Con-exp2 chickens showed no clinical signs. The average weight of the RSS+exp2 chickens was 53% below the control chickens (Con-exp2) and 45% below the chickens of RSS−exp2 chickens group (Fig. 2A). Both differences were statistically significant [p < 0.01 (RSS−exp2) and 0.001 (Con-exp2)]. In contrast, the weight of the RSS−exp2 chickens was not significant reduced (p < 0.135) in comparison to the controls Con-exp2 (Fig. 2A). But the body weights from both groups (both where significant different from Macroscopic lesions of the abdominal organs of RSS+exp2 chickens were consistent with the signs of RSS+exp1 chickens. Abdominal organs of the RSS−exp2 and the control chickens (Con-exp2) showed no macroscopic lesions. The histological examination of the duodenal loop showed the presence of cystic lesions of RSS+exp2 chickens in comparison to RSS−exp2 and control chickens (Con-exp2) where no microscopical lesions in the gut were observed (Fig. 2B). The obtained results showed that using a small group of chickens the macroscopic and microscopic lesions could be observed in chickens which have been infected with the gut content of RSS+exp1 chickens.
Fig. 2.
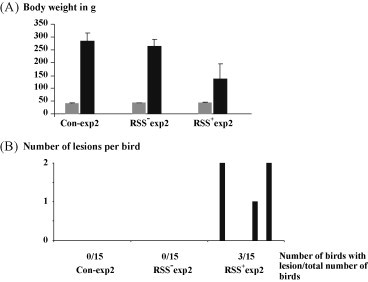
Gut content of RSS negative chickens did not induce lesion or weight depression. One-day-old chickens (15 in each group) were inoculated either with PBS (Con/2) or gut content of RSS negative chickens (RSS−/2). As positive control the same numbers of chickens were inoculated with gut content of chickens showing retarded growth and typical lesions in the gut (RSS+/2). (A) The body weight of the chickens was determined before (grey columns) or after (black columns) inoculation. The standard deviation is depicted by bars. (B) The duodenal loop was evaluated for the presence of cystic lesions. The number of lesions per bird is shown at the left axis. The total number of affected birds is shown below the horizontal axis.
This challenge model was further refined. To this end the gut content of RSS+exp1 chickens was prepared as described in Section 2. Fifteen one-day-old broiler chickens were orally inoculated with either the untreated gut content (RSS+exp3), the filtered gut content (RSS+exp3filt), or the filtered and chloroform treated gut content (RSS+exp3filtCF). One group was mock inoculated with PBS (Con-exp3). The chickens were weighed before inoculation and 12 days after inoculation (Fig. 3A). The average weights at day 12 p.i. of the groups receiving RSS+ material (RSS+exp3, RSS+exp3filt, RSS+exp3filtCF) was significant different from in comparison to the non-treated control group (p value <0.001). In the group of RSS+exp3 three out of 15 chickens died during the experiment at day 5 (2 birds) and 7 (1 bird) likely due to RSS, since they had severe diarrhea (line 342). The difference in body weight between the RSS+exp3filt group and the RSS+exp3filtCF group was statistically not significant (p value: 0.054). It needs to be mentioned that the average weights of both groups, RSS+exp3filt and RSS+exp3filtCF, where also statistically different from RSS+exp3 group (p value <0.001). Macroscopic lesions were not observed in the control group whereas in the group inoculated with the non-treated material, the filtered, and the filtered/chloroform treated material, macroscopic lesions were observed as described above. The data resulting from the histology showed that microscopic lesions in the duodenal loop were observed even after treatment with chloroform (Fig. 3B). Based on this data it was concluded that possible causative agents for RSS belong to non-enveloped viruses whereas the presence of other agents (such as bacteria, fungi) might have an impact on the severity of the disease as indicated by the higher weight depression in chickens infected with the non-filtered RSS+ material. This has been described before [20].
Fig. 3.
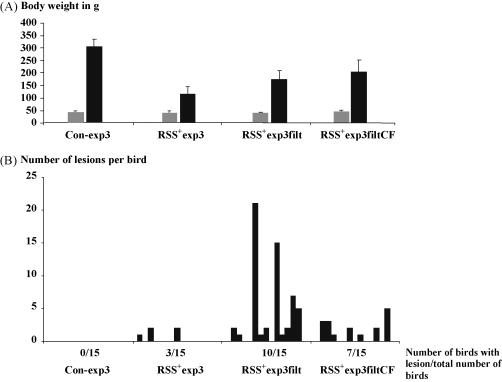
RSS is caused by a non-enveloped virus. (A) 15 one-day-old commercial broiler chickens were inoculated either with PBS (Con), with either gut content of RSS+ affected chickens (RSS+/3) or filtered gut content (RSS+/3filt) or filtered and chloroform treated gut content (RSS+/3filtCF). Before (grey box) and after the experiment (black box) the chickens were weight and the average of the weight in gram is shown. The standard deviation is shown by error bars. (B) Two cross sections of the duodenal loop of each bird were histologically examined for the presence of signs of cystic lesions. The number of birds which showed lesion out of the total number of analyzed birds is shown in parenthesis. Each column represents one chicken. The number of lesions per bird is indicated at the left axis.
3.2. Cloning of the open reading frame of the astrovirus capsid protein
Based on the obtained results experiments were performed to clone nucleotide sequences which might be represent the genomic information of a virus which causes the clinical picture of RSS. Since it has been described that small round viruses were found consistently in the gut content of RSS affected chickens oligonucleotides were delineated focusing on RdRp sequences of picornaviruses. For RNA purification filtered gut content of RSS+exp1 and RSS−exp1 was used in standard RT-PCR using two primer sets of primer pairs (FMDVFP1/FMDVRP1; DucPicoFP1/DucPicoRP1). The RT-PCR using the primer pair FMDVFP1/FMDVRP1 revealed a cDNA fragment of approximately 280 bp (FMDV-frag1) whereas by using the second primer pair a PCR fragment of approximately 500 bp was amplified (DucPic1-frag1). The RT-PCR using RNA from the gut content of RSS−exp1 resulted in no PCR fragment (data not shown). In addition, a PCR was performed without an RT step. In all four reactions no fragment was amplified which indicated that the template for the cDNA fragments was likely RNA. Both fragments were eluted from the gel and cloned using the Topo TA cloning system. Sequence analysis followed by a blastn search (NCBI database) showed no similarities with any nucleotide sequence present in the database. The next experiments were performed to elucidate if the sequence located between the initial used primers of both RT-PCR fragments was indeed present in the RSS+exp1 sample. To this end nested primers (see Table 1) were delineated and RT-PCR was performed using RNA obtained from the RSS+exp1. The primer pairs FMDVFP2/FMDVRP2 and DucPicoFP2/DucPicoRP2 resulted in RT-PCR fragments of the expected size, 232 bp (FMDV-frag2) and 418 bp (DucPic-frag2), respectively. Sequence analysis confirmed the identity of the sequence of the amplified fragments with the previous sequences of the appropriate fragments DucPic1-frag1 and FMDV-frag1. Next, a number of RT-PCR's were performed to elucidate if the obtained sequences from both RT-PCR fragments belonged to one RNA molecule or to different RNA molecules. Each primer of each fragment was used for an RT-PCR using the primer of the other fragment. In addition, the reverse-complementary sequences of each primer (see Table 1) were also used since the location of the RNA sequence to each other was unknown. Only four combinations resulted in amplification of fragments (FMDVFP2/DucPicoFP2Rev, FMDVFP2/DucPicoRP2; FMDVRP2rev/DucPicoFP2Rev, FMDVRP2rev/DucPicoRP2). All fragments were cloned and sequenced. The sequence of the longest fragment (FMDVFP2/DucPicoRP2; Astro-frag1) comprised all sequences of the other three fragments. Based on this result it was concluded that both sequences of the RT-PCR fragments were part of one RNA molecule. The sequence was used for a blastn search in the NCBI database, but no similarities were found. The nucleotide sequence was now in silico translated in all six open reading frames using an online available software program (http://www.expasy.org/tools/dna.html) and analyzed using blastp. The highest similarity (53%) at the amino acid level was found with the capsid precursor protein of a turkey astrovirus 2 (Genbank accession number AAV37186) and the RNA-dependent RNA polymerase of turkey astrovirus 2 (61%, Genbank accession number ABX46565). Thus the amplified sequence likely belonged to an astrovirus which has not been described yet. At this stage, the probable start codon of the open reading frame (ORF) of the capsid protein was already determined. To amplify the complete ORF a RAMP–RT-PCR was performed. Since it is known that the genomic RNA of astroviruses contains a poly-A tail at its 3′end an oligonucleotide was delineated which contained a polyT tail with a GC-clamp sequence (see Table 1, polyT-GC). The RAMP–RT-PCR was performed with a primer pair (DucPicoRP2Rev, polyT-GC) using RSS+ material and yielded a faint fragment of 1846 bp. The fragment was cloned, sequenced and a probable ORF was determined by the using the previously determined nucleotide sequences (Astro-frag1). Location of the single RT-PCR fragments is depicted in Fig. 4 . A blast search of the ORF using blastn (NCBI database) showed no similarities with any nucleotide sequence. Similarities were found by using the in silico translated amino acid sequence of the ORF in a blastp search in the NCBI database. The similarities with amino acid sequences of turkey astrovirus (TAV) 2 (Genbank accession number AAV37186, similarity: 46.2%), TAV 3 (AAV37187, 38.5%), TAV (ABX46578, 37.5%), duck astrovirus (YP_002728003, 36.3%), avian nephritis virus 1 (BAA92849, 33.0%), avian nephritis virus 2 (BAB21617, 30.8%), human astrovirus (BAA33721, 29.8%), bat astrovirus (ACN88712, 29.0%), ovine astrovirus (NP_059946, 28.6%), mink astrovirus (NP_795336, 28.5%), feline astrovirus (AAC13556, 28.0%), and porcine astrovirus (CAB95000, 27.5%). This showed that the new astrovirus had a limited similarity to other astroviruses and seemed indeed a new member of the family Astroviridae. The low similarities to other chicken astroviruses were surprising since avian nephritis virus 1 showed a similarity of 70% to avian nephritis virus 2. Also the similarities between turkey astroviruses were comparable high. The amino acid sequence of TAV showed a similarity to TAV 2 and TAV 3 of 85% and 81%, respectively.
Fig. 4.

Cloning of the open reading frame of the astrovirus capsid protein. The obtained RT-PCR fragments and the determined astrovirus sequence are shown. The determined astrovirus sequence is shown as a striped box. The initially amplified RT-PCR fragments are shown as black boxes. RT-PCR fragments amplified with different primer pair combinations (in parenthesis) are depicted as open box. The amplified RT-PCR fragment encompassing the open reading frame of the astrovirus capsid protein with a 6× His at its C-terminus are indicated as dotted box.
3.3. Generation of a recombinant baculovirus expressing the astrovirus capsid protein
Using the determined nucleotide sequence of 2951 nucleotides an ORF of 2250 nucleotides was deduced which encoded a protein of 743 aa. The ORF of the capsid protein was amplified by RT-PCR using two oligonucleotides (CapFP, CapRP) and the purified RNA from the RSS+exp1 sample. CapRP encoded for a 6×His sequence to obtain a His-tag at the C-terminus of the capsid protein for subsequent purification procedures. The obtained RT-PCR fragment (FragAstroCap-ORF) was cloned, sequenced, and the fragment was cleaved from the cloning vector pCR2.1 with EcoR I/Not I and ligated into the appropriately cleaved baculovirus transfer vector pFASTDUAL. The resulting plasmid (pFAST-AstroBac) was used for the generation of a recombinant baculovirus using the Bac-to-Bac system as described by the manufacturer. The existence of recombinant baculovirus DNA was confirmed by PCR using the primer pair which was used for the amplification of the ORF from the RNA (CapFP, CapRP). This PCR fragment was cloned and the identity of the sequence was verified by sequencing. The recombinant virus (AstCap-Bac) was propagated in Sf9 cells and used for infection of Sf9 cells for subsequent protein purification. Three days after infection the protein was purified. The protein purification was monitored by SDS-PAGE followed by staining of the proteins and Western blot (Fig. 5 ). The stained protein gel showed no prominent band in the lysate of infected cells. In the eluate, a single band with an approximate molecular weight of 80 kDa was observed. This molecular weight was in accordance with the theoretical molecular weight of 82.91 kDa of the capsid protein. By Western blot analysis using a 6×His specific mAb, a band with a similar molecular weight was observed in the lysate before and after centrifugation and after elution of the protein from the Talon matrix. The identity of the protein was confirmed by MALDI-TOF MS/MS analysis (Chemical and Biological Sciences Mass Spectrometry Facility, Department of Chemistry, University of Georgia) using the single stained protein band after the purification process.
Fig. 5.

Purification of the recombinant astrovirus capsid protein. Samples of lysed cells (1), of a lysate after centrifugation (2), of the centrifugation supernatant incubation with Talon (3), the flow through after the wash (4) and the eluate (5) were separated in 12% polyacrylamide gel by SDS-PAGE. The gels were either stained or a Western blot analysis was performed using an anti His mAb. The binding of the mAbs was visualised by chemoluminiscence using a peroxidase-labeled anti-mouse goat serum. A molecular mass marker (M) is shown and the position of the recombinant protein is marked by an arrow.
3.4. Generation of tools for the detection of antibodies
For the development of an ELISA, defined chicken serum samples (Ck-Cap+, Ck-CapCon) were used. Since the SPF status of the chickens does not include the absence of antibodies directed against the cloned astrovirus capsid protein sera of both groups of chickens were tested for their reactivity with the recombinant astrovirus capsid in Western blot using lysates of AstCap-Bac Sf9 cells as well as uninfected cells. Only the serum of the group which has been immunized with the recombinant protein reacted with a band of the appropriate size (data not shown). The serum samples form Ck-CapCon and Ck-Cap+ were considered as negative and positive control, respectively. First a checker-board titration was performed to obtain the best proportion between the reactivity of the positive and negative chicken serum at a certain antigen dilution. The antigen was diluted 1:10 in coating buffer and the chicken serum was diluted 1:100 in dilution buffer prior adding to the plate. Using these parameters we used 100 sera from one-day-old SPF chickens and determined the cut-off point as defined by the average of the adsorption plus three times the standard deviation. The mean and standard deviation of the normalized ELISA absorbance value of the investigated chicken sera were 0.064 and 0.009, respectively. The mean + three standard deviation cut-off value was 0.091. The cut-off point used for the ELISA was set to an adsorption of 0.1.
3.5. Vaccination of broiler breeders and challenge of the progeny
For the study, 30 broiler breeder hens and six broiler breeder rooster were obtained at 21 weeks of age. The animals were divided into two groups of 15 breeder hens and three roosters each. After one week of adaptation to the new facility, hens and rooster were bled to obtain a pre-immunserum. One group of hens was vaccinated with the experimental vaccine consisting of Incomplete Freud's adjuvant and the recombinant protein. The roosters were not vaccinated and served as sentinel controls in each group. Development of the serum antibody titer was measured by an indirect ELISA using the recombinant antigen (Fig. 6 ). Two weeks after each immunization the birds were bled and the serum antibody level specific for this antigen was determined. At 21 weeks of age both groups showed a similar antibody titer as measured by ELISA. In the non-vaccinated group the antibody titer declined over the duration of the experiment with the lowest titer at the time of the third bleed. Interestingly, a high standard deviation was observed which indicates that the antibody titers varied between the single breeder hens. In contrast in the group of hens vaccinated with the recombinant antigen the average antibody titer increased and reached a plateau 14 days after the second vaccination. Moreover, the standard deviation within this group was lower than in the non-vaccinated group. The non-vaccinated roosters were bled in parallel with the breeder hens. The ELISA data showed that the titer of the rooster's sera fluctuated over the tested time independent of whether or not the serum was taken from roosters in the non-vaccinated group or the vaccinated group.
Fig. 6.

Vaccination of broiler breeders with the recombinant astrovirus capsid protein induced an immune response in broiler breeders. A group of fifteen 22-week-old commercial broiler breeder hens were vaccinated with an oil-emulsion vaccine containing the recombinant protein intramuscularly. One group of 15 hens and three roosters in both groups served as non-vaccinated control. All chickens were bled before the first (0-B) and 14 days after each of the three vaccinations (1st B, 2nd B, and 3rd B). The serum samples were analyzed in the astrovirus capsid protein ELISA along with the positive and negative (SPF) control serum. The extinction at a wave length of 405 nm is shown at the left axis.
3.6. Vaccination with the recombinant antigen provides partial protection against RSS
Chickens were hatched after the second and third vaccination and serum samples were obtained at day of hatch. The serum samples were analyzed by ELISA (Fig. 7 ). The antibody titer of chickens in the non-vaccinated group did not increase but antibodies raised against the recombinant capsid protein could be detected by ELISA. This is not surprising since the non-vaccinated breeder hens also showed reactivity with this particular antigen. Is has to be mentioned that two chicken sera of group 2nd vac/1 and three sera of group 2nd vac/2 showed an ELISA OD of >1, but most sera were below an OD of 0.5 (data not shown). In the group of progeny from vaccinated breeder hens the titer was higher after the second vaccination which is in agreement with the ELISA data obtained from the sera of the broiler breeders. The titer in the group of broiler chickens obtained from breeder hens after the third vaccination (3rd vac/1) elevated and declined slightly 14 days later (3rd vac/2). But it needs to be mentioned that ELISA titer in the broiler breeder group increased not significantly after the third vaccination probably because the immune response likely reached a plateau against this particular antigen. The increase in the titer of the offspring might be explained by a more efficient transfer of the maternal derived antibodies via the egg. Furthermore it was observed that the standard deviation in the offspring of the non-vaccinated group was very high, but was comparably low in the vaccinated group indicating a more uniform antibody titer in the latter group.
To test if this antibody response was sufficient to protect from RSS challenge, experiments were performed using 12 chickens per group hatched from eggs collected between 14 and 28 days following the 2nd vaccination (2nd/vac1). The offspring from both groups were randomly divided into two groups each and weighed individually before inoculation. The chickens were either inoculated with filtered gut content or mock-infected with PBS to test if a protection could be obtained against the viral component of this disease syndrome. The results of the experiments demonstrated that two vaccinations were not sufficient to prevent the differences in weight 12 d after infection (Fig. 8A) between the challenged and non-challenged group (p value: 0.921). Both challenge groups showed macroscopic as well as microscopic lesions. But it was observed that microscopic lesions were present in fewer gut sections in the vaccinated group in comparison to the off spring of the non-vaccinated group (Fig. 8B). The first infection experiment using 14 one-day-old chickens in each group after the third vaccination (3rd vac/1) indicated that presence of maternal derived antibodies were able to reduce the difference in weight between the non-inoculated control birds and the challenged birds (Fig. 9A). Statistical analysis revealed that the differences in weight were statistically significant (p < 0.001) between the challenged and non-challenged within one group (offspring of vaccinated and non-vaccinated breeder hens). But it needs to be mentioned that the weights between both challenged groups were also statistically different (p < 0.001). During the experiment the chickens of the non-vaccinated/challenged group showed slight diarrhea and lassitude. Chickens of the other three groups showed no signs of disease. In addition, only the chickens in the non-vaccinated/challenged group showed macroscopic lesions of RSS. The weight difference between non-challenged control birds and their challenged hatch mates was 26% in the vaccinated group whereas a difference of 46% was observed between the non-challenged and challenged chickens of the non-vaccinated group. To obtain a more objective picture the average weight gain was calculated. To this end for each group the average weight at day one and day 12 was calculated and the average weight of day one from the average weight of day 12 was subtracted. Chickens of the vaccinated/challenged group gained in average 94 g more weight during the 12 days period of the experiment in comparison to the progeny of the challenged/non-vaccinated group. Histological examination of the duodenal loop indicated that vaccination of breeder hens with the recombinant protein mitigated the number of affected guts as well as the severity of the lesions in the challenged chicken (Fig. 9B). But it needs also be considered that during the experiments a relatively low number of chickens were analyzed. In both non-challenged control groups no cystic lesions were found. To investigate if the observed differences in weight gain was a random result a second experiment (3rd vac/2) with 12 chickens per group was performed using the same parameters as in the previous experiment. The average weight difference between challenged and the non-challenged group was 45% of the progeny from the non-vaccinated breeder hens. Again, the progeny of the vaccinated group which was challenged showed only 25% weight difference to the non-challenged hatch mates. The calculation of the weight gain showed clearly that vaccinated broiler chickens gained 83% more weight (288 g) than the non-vaccinated broiler chickens (157 g). The evaluation of the histological lesions showed that a lower number of chickens (3/12) in the group of vaccinated and challenged chickens showed cystic lesions. The number of lesion per chicken ranged between two to five lesions. In contrast, in the duodenal loop of the non-vaccinated/challenged group more chickens (9/12) showed cystic lesions and the number of lesions per affected chicken ranged from 2 to 14.
Fig. 8.

Two vaccinations with the recombinant astrovirus capsid protein decreases lesion in the gut. (A) One-day-old progeny from the vaccinated (VACC) and non-vaccinated (NON-VAC) broiler breeders were divided randomly in groups 12 chickens each. The body weight was determined before (grey column) and after challenge (black column). The bodyweight is shown in gram (g) and the scale is shown at the left axis. The standard deviations are indicated by error bars. (B) 12 days after challenge the duodenal loop was taken during necropsy from chickens of all groups and a cross section was histologically examined. The number of cystic lesions per bird in the non-vaccinated/challenged and vaccinated/challenged groups is shown by columns. Each column represents one chicken. The number of lesions per bird is indicated at the left axis.
Fig. 9.
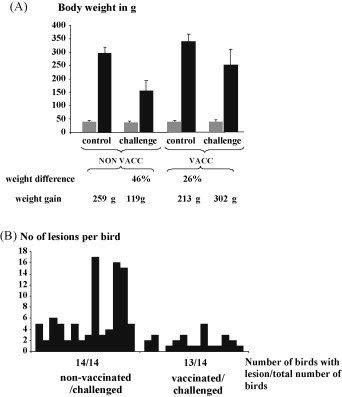
Three vaccinations with the recombinant astrovirus capsid protein decreases lesion in the gut and mitigates weight depression. (A) One-day-old progeny from vaccinated (VACC) and non-vaccinated (NON-VAC) broiler breeders were divided randomly into two groups of 15 chickens each. The body weight was determined before (grey column) and after challenge (black column) and is shown in gram (g) as indicated at the left axis. Calculated standard deviations are indicated by error bars. The weight difference was calculated between the control group and the challenged group within the non-vaccinated and vaccinated hatch mates. The weight gain was calculated between day of hatch and day 12 within each group. (B) 12 days after challenge the duodenal loop was taken during necropsy from all chickens and a cross section was histologically examined. The presences of cystic lesions in the non-vaccinated/challenged and vaccinated/challenged groups are shown by columns. Each column represents one chicken. The number of lesions per bird is indicated at the left axis.
4. Discussion
RSS in chickens is an important problem in the poultry industry especially in the broiler production. The etiological agent has not been described so far likely due to the fact that the agent, or several agents, can not be isolated in cultural systems such as cell culture or embryonated eggs. To develop and subsequently test a vaccine candidate an appropriate challenge model needs to be in place. Since no agent was isolated initially an approach was applied which resembled possible field condition. The approach using non-treated and/or filtered gut content to reproduce the disease has been described before [1], [14], [22]. Our experimental animal model resulted in typical signs for RSS such as growth retardation, and cystic lesions in the gut as observed during necropsy and by histopathology. These findings for RSS have been reported previously by other research groups [1], [4], [7], [14], [20], [29], [30]. The results obtained during the experiments using gut content of non-clinical diseased chickens versus the gut content of clinical diseased chickens provided additional evidence to earlier described experiments [31] that rather an infectious agent than the gut content induced the weight depression. Since in our experiment the 0.22 μm filtrate induced also a significant reduction in weight and the presence of gut lesions a viral etiology is very likely. But it needed to be mentioned that the body weight in the group inoculated with the unfiltered gut content showed a difference in weight in comparison to the group inoculated with the filtrate. These results are in agreement with Nili et al. [1] and suggest that the presence of infectious agents other than viruses might also play a role for the severity of the disease. The presence of both, gut lesions and reduction in body weight, after treatment of the filtrate with chloroform supported the hypothesis that the agent(s) responsible for RSS are non-enveloped viruses. Rotavirues [2], astrovirus [32], reoviruses [21], [33] were isolated from RSS affected chickens but the disease could not be reproduced or only reproduced partially. Interestingly Songserm et al. [21] described that infection of broiler with reovirus in combination with the gut content of clinically healthy chickens was able to induce the clinical picture whereas the gut content of healthy chickens alone did not induce any signs of disease. On the other hand the presence of enteric virus has been shown by amplification of the genomic material in healthy as well affected flocks [34] which indicated additional underlying factors leading to the clinical disease. Based on the description that small round virus might play an important role in the genesis of RSS we decided to amplify the genomic sequence of a picornavirus. The use of a combination of degenerated primers and primers containing sequences of conserved regions based on nucleotide sequences of picornaviral RdRP resulted in the discovery of a sequence of an astrovirus which has not been described jet. The closest relative amino acid sequence was a turkey astrovirus 2 sequence whereas the nucleotide sequence showed no similarities to any sequences present in the NCBI database.
Several attempts in primary chicken liver cells and primary chicken kidney cells failed to isolate a virus which reacted with the anti-capsid protein serum from chickens (Ck-Cap+) in immunofluorescence (data not shown). Thus, the baculovirus-vector approach was chosen to develop a possible vaccine candidate. The expression of viral proteins by using recombinant baculoviruses for the use as vaccine is not a new and has been shown for several viral antigens, e.g. human paramyxovirus 3 [35], dengue virus [36], West Nile virus [37], influenza A virus [38]. The presented data showed clearly that the purified protein was able to induce an immune response in broiler breeders. It needs to be mentioned that the broiler breeders showed already the presence of antibodies raised against the recombinant protein, although at a low level, as measured by the indirect ELISA. This finding indicated the presence of this virus or an antigenic similar virus in the field, thus the vaccination procedure used in the described experiments could also be considered as a boost vaccination to an existing immunity. In addition, field sera obtained from 20 breeder flocks (layers and broiler, 10 sera per flock) from different states in the USA (GA, DE, AL) were investigated for the presence of antibodies against the recombinant protein using the indirect ELISA. The results indicated that this virus or an antigenic related virus was present in the field (data not shown). Due to the prime/boost scheme the level of antibodies were elevated in the vaccinated birds whereas the level of antibodies declined in the non-vaccinated group. After the third vaccination the antibody level in the breeder hens was further elevated but with a lower standard deviation. A similar behavior of the antibody levels was observed in the appropriate serum samples of the progeny. This might explain the results after the challenge experiments. Infected chickens obtained after the second vaccination of the breeder hens showed no difference in weight gain to their non-vaccinated/challenged control chickens. The only observed difference was that a lower number of chickens of the vaccinated hens showed gut lesions in comparison to the chickens of the non-vaccinated controls. These results can be interpreted that the antibody level was not sufficient to provide protection from retarded growth of the chickens. After the third vaccination the challenged offspring showed a further elevated antibody titer and both, the presence of gut lesions and the differences in weight gain between the non-challenged and challenged hatch mates declined. This result was confirmed by a second experiment performed 14 days later. These results showed that a certain level of antibodies needs to be present to induce a partial protection against the differences in weight gain and gut lesions. It needs to be mentioned that the number of cystic lesions in the small intestine can only used as a measurement within one experiment. But it needs to be considered as an important characteristics of the disease since the cystic lesions in the small intestine were always present in the infected group but not in the controls.
In conclusion the recombinant astrovirus capsid protein provided partial protection to the offspring of breeder hens three times vaccinated with a recombinant astrovirus capsid protein. This is the first report for a vaccine candidate able to induce partial protection against RSS in chickens. The partial protection using this vaccine candidate might also be attributed to the fact that other agents play a role in this disease. The feasibility needs to be determined by a cost benefit analysis for this vaccine for chickens.
References
- 1.Nili H., Jahantigh M., Nazifi S. Clinical observation, pathology, and serum biochemical changes in infectious stunting syndrome of broiler chickens. Comp Clin Pathol. 2007;16:161–166. [Google Scholar]
- 2.Otto P., Liebler-Tenorio E.M., Elschner M., Reetz J., Löhren U., Diller R. Detection of rotaviruses and intestinal lesions in broiler chicks from flocks with runting and stunting syndrome (RSS) Avian Dis. 2006;50:411–418. doi: 10.1637/7511-020106R.1. [DOI] [PubMed] [Google Scholar]
- 3.McNulty M.S., Allan G.M., Connor T.J., McFerran J.B., McCracken R.M. An entero-like virus associated with the runting syndrome in broiler chickens. Avian Pathol. 1984;13:429–439. doi: 10.1080/03079458408418545. [DOI] [PubMed] [Google Scholar]
- 4.Reece R.L., Frazier J.A. Infectious stunting syndrome of chickens in Great Britain: field and experimental studies. Avian Pathol. 1990;19:723–758. doi: 10.1080/03079459008418727. [DOI] [PubMed] [Google Scholar]
- 5.Frazier J.A., Reece R.L. Infectious stunting syndrome of chickens in Great Britain: intestinal ultrastructural pathology. Avian Pathol. 1990;19:759–777. doi: 10.1080/03079459008418728. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin M.A., Davis J.F., Player E.C. Reovirus-associated enteritis in Georgia broiler chicks. Avian Dis. 1993;37:229–233. [PubMed] [Google Scholar]
- 7.Goodwin M.A., Davis J.F., McNulty M.S., Brown J., Player E.C. Enteritis (so-called runting stunting syndrome) in Georgia broiler chicks. Avian Dis. 1993;37:451–458. [PubMed] [Google Scholar]
- 8.Zsak L., Strother K.O., Kisary J. Partial genome sequence analysis of parvoviruses associated with enteric disease in poultry. Avian Pathol. 2008;37:435–441. doi: 10.1080/03079450802210648. [DOI] [PubMed] [Google Scholar]
- 9.Guy J.S. Virus Infection of the Gastrointestinal Tract of Poultry. Poultry Sci. 1998;77:1166–1175. doi: 10.1093/ps/77.8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heggen-Pey C.L., Quereshi M.A., Edens F.W., Sherry B., Wakenell P.S., O’Connell P.H. Isolation of a Reovirus from poult enteritis and mortality syndrome and its pathogenicity in turkey poults. Avian Dis. 2002;46:32–47. doi: 10.1637/0005-2086(2002)046[0032:IOARFP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Kapczynski D.R., Sellers H.S., Simmons V., Schultz-Cherry S. Sequence analysis of the S3 gene from a turkey reovirus. Virus Genes. 2002;25:95–100. doi: 10.1023/A:1020130410601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellers H.S., Pereira L., Linnemann E., Kapczynski D.R. Phylogenetic analysis of the sigma 2 protein gene of turkey reoviruses. Avian Dis. 2004;48:651–657. doi: 10.1637/7181-032304R. [DOI] [PubMed] [Google Scholar]
- 13.Spackman E., Pantin-Jackwood M., Day J.M., Sellers H. The Pathogenesis of turkey origin reoviruses in turkeys and chickens. Avian Pathol. 2005;34:291–296. doi: 10.1080/03079450500178501. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery R.D., Boyle C.R., Maslin W.R., Magee D.L. Attempts to reproduce a runting/stunting-type syndrome using infectious agents isolated from affected Mississippi broilers. Avian Dis. 1997;41:80–92. [PubMed] [Google Scholar]
- 15.Decaesstecker M., Charlier G., Meulemans G. Significance of parvoviruses, entero-like viruses and reoviruses in the aetiology of the chicken malabsorption syndrome. Avian Pathol. 1986;15:769–782. doi: 10.1080/03079458608436339. [DOI] [PubMed] [Google Scholar]
- 16.Sellers H.S., Koci M.D., Kelley L.A., Schultz-Cherry S. Development of a multiplex RT-PCR diagnostic test specific for turkey astrovirus and coronavirus. Avian Dis. 2004;48:531–539. doi: 10.1637/7128. [DOI] [PubMed] [Google Scholar]
- 17.Spackman E., Kapczynski D., Sellers H. Multiplex real-time RT-PCR for the detection of three viruses associated with poult enteritis complex: turkey astrovirus, turkey coronavirus, and turkey reovirus. Avian Dis. 2004;49:86–91. doi: 10.1637/7265-082304R. [DOI] [PubMed] [Google Scholar]
- 18.Pantin-Jackwood M.J., Day J.M., Jackwood M.W., Spackman E. Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006. Avian Dis. 2008;52:235–244. doi: 10.1637/8174-111507-Reg.1. [DOI] [PubMed] [Google Scholar]
- 19.Shirai J., Obata H., Nakamura K., Furuta K., Hihara H., Kawamura H. Experimental infection in specific-pathogen-free chicks with avian reovirus and avian nephritis virus isolated from broiler chicks showing runting syndrome. Avian Dis. 1990;34:295–303. [PubMed] [Google Scholar]
- 20.Kouwenhoven B., Vertommen M., Goren E. Investigations into the role of reovirus in the malabsorption syndrome. Avian Pathol. 1988;17:879–892. doi: 10.1080/03079458808436510. [DOI] [PubMed] [Google Scholar]
- 21.Songserm T., van Roozelaar D., Kant A., Pol J., Pijpers A., ter Huurne A. Enteropathogenicity of Dutch and German avian reoviruses in SPF white leghorn chickens and broilers. Vet Res. 2003;34:285–295. doi: 10.1051/vetres:2003004. [DOI] [PubMed] [Google Scholar]
- 22.Songserm T., Pol J.M.A., van Roozelaar D., Kok G.L., Wagenaar F., ter Huurne A. A comparative study of the pathogenesis of malabsorption syndrome in broilers. Avian Dis. 2000;44:556–567. [PubMed] [Google Scholar]
- 23.Meulemanns C., Decaesstecker M., Charlier G. Runting syndrome in broiler chickens. Experimental reproduction studies. In: McFerran J.B., McNulty M.S., editors. Acute virus infections of poultry. Martinus Nijhoff; Dordrecht, The Netherlands: 1986. pp. 115–124. [Google Scholar]
- 24.McFerran J.B., McNulty M.S. Recent advances in enterovirus infections of birds. In: McFerran J.B., McNulty M.S., editors. Acute virus infections of poultry. Martinus Nijhoff; Dordrecht, The Netherlands: 1986. pp. 195–201. [Google Scholar]
- 25.Pass D.A., Robertson M.D., Wilcox G.E. Runting syndrome in broiler chickens in Australia. Vet Rec. 1982;110:386–387. doi: 10.1136/vr.110.16.386. [DOI] [PubMed] [Google Scholar]
- 26.Imada T., Yamaguchi S., Mase M., Tsukamoto K., Kubo M., Morooka A. Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. J Virol. 2000;74:8487–8493. doi: 10.1128/jvi.74.18.8487-8493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letzel T., Mundt E., Gorbalenya A.E. Evidence for functional significance of the permuted C motif in Co2+-stimulated RNA-dependent RNA polymerase of infectious bursal disease virus. J Gen Virol. 2007;88:2824–2833. doi: 10.1099/vir.0.82890-0. [DOI] [PubMed] [Google Scholar]
- 28.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 29.Smart I.J., Barr D.A., Reece R.L., Forsyth W.M., Ewing I. Experimental reproduction of the runting stunting syndrome of broiler chickens. Avian Pathol. 1988;17:617–627. doi: 10.1080/03079458808436481. [DOI] [PubMed] [Google Scholar]
- 30.Bayyari G.R., Huffe W.E., Balog J.M., Rath N.C., Beasly J.N. Experimental reproduction of proventriculitis using homogenates of proventricular tissue. Poultry Sci. 1995;74:1799–1809. doi: 10.3382/ps.0741799. [DOI] [PubMed] [Google Scholar]
- 31.Songserm T., Zekarias B., van Roozelaar D.J., Kok R.S., Pol J.M.A., Pijpers A. Experimental reproduction of malabsorption syndrome with different combinations of reovirus. Escherichia coli, and treated homogenates obtained from broilers. Avian Dis. 2002;46:87–94. doi: 10.1637/0005-2086(2002)046[0087:EROMSW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Zavala G., Sellers H. Runting–stunting syndrome. Poult Inform Profess. 2005;85:1–10. [Google Scholar]
- 33.Rosenberger J.K. Proc. No. 66 annual meeting of the International Union of Immunological Societies. University of Sydney; New South Wales, Australia: 1983. Characterisation of reoviruses associated with stunting syndrome in chickens; pp. 141–152. [Google Scholar]
- 34.Pantin-Jackwood M.J., Spackman E., Woolcock P.R. Molecular characterization and typing of chicken and turkey astroviruses circulating in the United States: implications for diagnostics. Avian Dis. 2006;50:397–404. doi: 10.1637/7512-020606R.1. [DOI] [PubMed] [Google Scholar]
- 35.van Wyke Coelingh K.L., Murphy B.R., Collins P.L., Lebacq-Verheyden A.M., Battey J.F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987;160:465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y.M., Hayes E.P., McCarty T.C., Dubois D.R., Summers P.L., Eckels K.H. Immunization of mice with dengue structural proteins and nonstructural protein NS1 expressed by baculovirus recombinant induces resistance to dengue virus encephalitis. J Virol. 1988;62:3027–3031. doi: 10.1128/jvi.62.8.3027-3031.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonafé N., Rininger J.A., Chubet F R.G., Foellmer H.G., Fader S., Anderson J.F. A recombinant West Nile virus envelope protein vaccine candidate produced in Spodoptera frugiperda expresSF+ cells. Vaccine. 2009;27:213–222. doi: 10.1016/j.vaccine.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmood K., Bright R.A., Mytle N., Carter D.M., Crevar C.J., Achenbach J.E. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]


