Abstract
Recently, an outbreak of fatal infection caused by a pantropic variant (strain CB/05) of canine coronavirus (CCoV) has been reported. In this study, evidence is provided that immunity induced by natural exposure to enteric CCoV is not fully protective against strain CB/05. Twenty-two, 10-week-old beagles with a recent natural infection by enteric CCoV were randomly distributed in two experimental groups of eight (groups A and B) and one control group of six (group C) dogs. Dogs in groups A and B were inoculated oronasally with different doses (4 × 105 or 4 × 103 TCID50) of the pantropic strain CB/05, whereas dogs in group C were used as negative controls. Clinical, post-mortem and virological investigations showed that, despite the high serum antibody titres induced by the prior natural infection with enteric CCoV, dogs were susceptible to experimental infection with strain CB/05. This was shown by the occurrence of faecal shedding, and dogs displaying moderate clinical signs, mainly vomiting and diarrhoea. Involvement of the lymphoid tissues was evident as demonstrated by the acute lymphopenia (below 70% of the initial counts), gross lesions in spleen and lymph nodes and detection of CB/05 RNA in thymus, spleen and lymph nodes of some infected dogs. The presence of viral RNA in lymphoid tissues was observed only in dogs euthanised in the early stages of infection and the clinical course of the infection was unrelated to the viral dose administered. The present study demonstrates that strain CB/05 is able to induce infection and disease in dogs seropositive to enteric CCoV, thus highlighting the need for extensive epidemiological investigation and for the possible development of novel antigenically relevant vaccines.
Keywords: Pantropic canine coronavirus, Enteric canine coronavirus, Cross-protection
1. Introduction
Canine coronavirus (CCoV) belongs to group 1 coronaviruses, along with feline coronaviruses (FCoVs) type I and type II, transmissible gastroenteritis virus (TGEV) of swine, porcine respiratory coronavirus (PRCoV), porcine epidemic diarrhoea virus (PEDV) and human coronavirus 229E (HCoV 229E) [1]. Coronaviruses are single-stranded, positive-sense RNA viruses, whose replication system is responsible for mutations, deletions and recombinations in the viral genome. These mechanisms of genetic evolution may account for the emergence of new genotypes with different biological patterns with particular regards to tissue tropism, in vitro and in vivo host range and interaction with the immune system [2].
To date, two different genotypes of CCoV are known, CCoVs type I and type II [3]. Additionally, a group 2 coronavirus, the canine respiratory coronavirus (CRCoV), has been detected recently in the respiratory tract of dogs [4], [5].
CCoV is generally recognised as an aetiological agent of self-limiting infections of the intestinal epithelium, which can lead to the onset of clinical signs typical of the gastroenteric involvement, such as inappetance, diarrhoea and vomiting [6], [7]. Recently, a highly virulent variant of CCoV type II (strain CB/05) was reported that caused a systemic disease, followed by fatalities in pups [8]. The virus was isolated from the internal organs (lungs, spleen, liver and kidney), that had severe gross lesions. CCoV type II RNA was also detected in the brain, thus accounting for the neurological signs observed in the affected pups. Sequence analysis of the 3′ genome end of the pantropic CCoV strain, including ORFs 2 (S gene), 3a, 3b, 3c, 4 (E gene), 5 (M gene), 6 (N gene), 7a and 7b, showed that strain CB/05 has a high degree of aa identity to the cognate ORFs of CCoV type II, although the S protein displayed the highest identity to FCoV type II strain 79–1683. A genetic marker was identified in the CB/05 genome, consisting of a 38-nt deletion in ORF3b which is responsible for a predicted truncated nonstructural protein 3b [9]. Experimental infection of seronegative pups with strain CB/05 reproduced the disease with occurrence of severe clinical signs, including pyrexia, anorexia, depression, vomiting, diarrhoea and leukopenia [10]. A different clinical course was observed according to the age of the infected pups. The older dogs (6 months of age) slowly recovered from the disease, whereas two of three 2.5-month-old dogs were sacrificed due to the severity of the CB/05-induced disease [10].
The aim of the present study was to evaluate the pathogenic potential of different infectious doses of strain CB/05 in CCoV-seropositive dogs following a recent natural infection caused by an enteric CCoV type II strain in order to assess whether the immunity induced by enteric CCoV is cross-protective against the new pantropic CCoV variant.
2. Materials and methods
2.1. Origin of the challenge virus
Strain CB/05 was isolated from the lungs of a dead pup (117/05-C) and adapted to growth on A-72 canine cells [9]. The challenge virus was titrated on cell cultures and inocula that contained 105 or 103 TCID50/ml of viral suspension were prepared and stored at −70 °C.
2.2. Dogs
Twenty-two beagles, 10 weeks of age, belonging to four different litters in the same kennel, were used throughout the study. All dogs tested repeatedly positive for CCoV RNA in their faeces by real-time RT-PCR [11] and for CCoV antibodies in their sera using an ELISA test [12]. Additional pooled faecal samples were also collected from other pups in the kennel which housed the dogs that were used for the experimental infection with CCoV strain CB/05. These dogs also tested positive for CCoV by real-time RT-PCR, thus accounting for the purchased dogs being infected in the kennel.
At day 10 before inoculation (day −10), 13/22 pups were still shedding enteric CCoV RNA in their faeces, albeit at low titres, and all pups had high CCoV antibody titres in their sera. Sequence analysis of the 5′-end of the S gene and the full-length ORF3b showed that the coronavirus strain detected in their faeces was an enteric CCoV strain (data not shown). However, from day −5 to day −1, no CCoV shedding was observed in any pup, whereas CCoV antibody titres remained constantly high. The dogs were grouped randomly at day −1. Grouped dogs were housed separately in three different isolation rooms on the basis of their treatment and provided water and fed ad libitum with a commercial dry dog food for pups (Purina, Italy).
2.3. Experimental design
The experimental study was performed at the isolation unit of the Animal Hospital, Faculty of Veterinary Medicine of Bari, according to the animal health and well-being regulations and was authorised by the Ministry of Health of Italy (authorization no. 57/2006-C).
Dogs of group A (n = 8) were administered strain CB/05 oronasally in two doses (4 ml each, 3 ml orally and 1 ml nasally), 12 h apart, with viral suspensions containing 105 TCID50/ml. Dogs of group B (n = 8) were administered two doses (4 ml each, 3 ml orally and 1 ml nasally), 12 h apart, with a viral suspension that contained 103 TCID50/ml. Dogs of group C (n = 6) were maintained as controls by administration of the cryolysate of the same passage of A-72 cells used for the preparation of the stock virus (two doses, each of 4 ml, 3 ml orally and 1 ml nasally, 12 h apart).
2.4. Necropsies
At 7 and 14 days post-inoculation (dpi), 2 control dogs (group C), 3 dogs of groups A and 3 dogs of group B chosen on the basis of the clinical signs (mild gastroenteritis) were chosen and euthanised by intravenous administration of 10 mg/kg of body weight of Zoletil 100 (Virbac S.r.l., Italy) followed by 0.5 ml/kg body weight of Tanax (Intervet Italia, Italy). Complete post-mortem examinations were carried out. The remaining pups (2 dogs per group) were necropsied at 28 dpi. The following organs were examined macroscopically and virologically: brain, thymus, lungs, liver, spleen, mesenteric lymph nodes, gut, kidney and bone marrow.
2.5. Clinical and general health observations
Clinical examinations were performed on all dogs, once daily starting from day −1 as long as an animal remained on the study, taking into account the occurrence of abnormal clinical signs, dehydration, lethargy and loss of appetite. General health observations were performed on each animal twice daily from day −1 to day 7 dpi and once daily from 8 dpi until the day before necropsy for the remaining observation period. Body weights were recorded on days −1, 3, 5, 7, 14, 21 and 28, whereas rectal temperatures were registered daily from days −1 to 7 and on alternate days from days 9 to 27.
2.6. Sample collection
Blood samples were collected on days −1, 3, 5, 7, 14, 21 and 28 into two vials, one with EDTA for whole blood (haematological and virological examinations) and another without anticoagulant for serum preparation (serology). In addition, nasal and faecal swabs were collected on the aforementioned days for virological investigations.
2.7. Virological assays
Tissue samples were collected at post-mortem examination, while blood samples, nasal and faecal swabs were collected intra-vitam. Subsequently, these samples were tested for CCoV by virus isolation on A-72 cells and real-time RT-PCR [11].
For virus isolation, samples were homogenised (10%, wt/vol) in Dulbecco's minimal essential medium (D-MEM), treated with antibiotics (penicillin 5000 IU/ml, streptomycin 2500 μg/ml, amphotericin B 10 μg/ml) and inoculated in cell cultures. Cells were grown in D-MEM supplemented with 10% foetal calf serum (FCS). When monolayers were confluent, the medium was removed and the cells were washed twice with FCS-free medium and inoculated with the homogenates. After an adsorption of 60 min at 37 °C, the inoculum was replaced with fresh serum-free medium. Infected cells were monitored daily for the occurrence of cytopathic effect (CPE) and 3 days later they were tested for CCoV antigen by an immunofluorescence (IF) assay using a monoclonal antibody targeting the N protein (courtesy of Dr. G. Chappuis, Merial, Lyon, France).
For real-time RT-PCR, RNA was extracted with the commercial kits, QIAamp® Viral RNA Mini Kit (Qiagen S.p.A., Milan, Italy) from 140 μl of homogenates of nasal and faecal swabs, QIAamp® RNeasy Mini Kit (Qiagen S.p.A.) from 25 mg of tissue samples and with QIAamp Blood Mini Kit from 1 ml of EDTA-treated blood. RNA extracts were subjected to a real-time RT-PCR assay for detection and quantitation of CCoV RNA [11]. Reverse transcription was carried out using GeneAmp® RNA PCR (Applied Biosystems, Applera Italia, Monza, Italy), following the manufacturer's recommendations. Real-time PCR was performed in a 50 μl-reaction mixture containing 25 μl of IQ™ Supermix (Bio-Rad Laboratories Srl, Milan, Italy), 600 nM of primers CCoV-For (TTGATCGTTTTTATAACGGTTCTACAA) and CCoV-Rev (AATGGGCCATAATAGCCACATAAT), 200 nM of probe CCoV-Pb (FAM-ACCTCAATTTAGCTGGTTCGTGTATGGCATT-TAMRA) and 20 μl of c-DNA. The thermal cycle protocol used was the following: activation of iTaq DNA polymerase at 95 °C for 10 min and 45 cycles consisting of denaturation at 95 °C for 15 s and primer annealing-extension at 60 °C for 1 min.
2.8. Serological assays
Serum samples from inoculated dogs were tested in parallel by virus neutralisation (VN) and ELISA tests [12].
For VN tests, serial twofold dilutions of heat-inactivated sera were mixed with 100 TCID50 of the isolated strain CB/05 in 96-well microtitre plates. After preincubation at room temperature for 90 min, approximately 20,000 A-72 cells were added to each well. Plates were read after 4 days of incubation at 37 °C. VN titers were expressed as the highest serum dilution neutralising the virus.
For ELISA tests, microtitre plates were coated with CCoV antigen and, after treatment with blocking solution and repeated washing, the 1:50 dilutions of the serum samples were added to each well. The plates were incubated for 90 min at 37 °C, washed four times and incubated for 60 min at 37 °C with anti-dog IgG-goat peroxidase conjugates (Sigma-Aldrich srl, Milan, Italy). After another washing cycle, 10 mg of freshly prepared substrate, 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]diammonium salt (ABTS, Sigma-Aldrich srl) in 50 ml of 0.05 M phosphate citrate buffer (pH 5.0) was placed in each well and the optical density at 405 nm (OD405) was determined.
Antibody titres <1:2 and OD values <0.040 were regarded as negative for VN and ELISA tests, respectively.
2.9. RT-PCR assay for specific detection of strain CB/05
An RT-PCR assay was developed for specific detection of strain CB/05 in biological samples from dogs infected experimentally. Taking into account the presence of the 38-nt deletion in ORF3b of strain CB/05, primers VNV-F (5′-ACTTGTGTGTATAGGTTTTGGTGA-3′) and VNV-R (5′-TAAGTGTCATTGATACAATCTTAAACA-3′) were designed to amplify a fragment within ORF3b with a size of 492 and 454 bp for enteric and pantropic CCoVs respectively. Reverse transcription and PCR amplification were carried out using SuperScript™ One-Step RT-PCR for Long Templates (Life Technologies, Invitrogen. Milan, Italy), according to the manufacturer's instructions. RT-PCR products were detected by electrophoresis through a 2% agarose gel and visualisation under UV light after bromide ethidium staining.
3. Results
3.1. Clinical signs and WBC counts
Dogs of groups A and B had intermittent diarrhoea from 2–3 to 11–13 days post-inoculation with strain CB/05. Episodes of diarrhoea in pens were observed for 8 and 6 days in dogs of groups A and B, respectively. Only dog A3 showed lethargy in dpi 2–3 and 5–7. No other clinical signs were evident in animals in groups A and B. Animals in group C remained healthy throughout the in vivo phase.
Body weights and temperatures did not vary considerably on the basis of the titre of the inoculum administered to the groups. However, two dogs in group A and one in group B showed limited growth (dogs A4 and B3) and weight loss (dog A3). Peaks in increased rectal temperatures (normal ranges = 38.5–38.8 °C) were observed in groups A and B, with some dogs (especially in group B) displaying temperatures between 39.3 and 39.5 °C (Fig. 1 ).
Fig. 1.
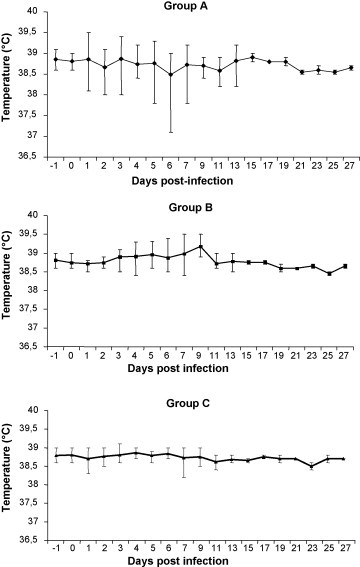
Rectal temperatures (means ± SE) in experimentally infected (groups A and B) and control dogs (group C).
Total and differential WBC counts in infected and control dogs are summarised in Fig. 2 . In group A, after a mild leukocytosis (114% of the initial mean values) at 5 dpi, a moderate leukopenia appeared starting from 7 dpi, with total WBC counts decreasing progressively until the end of the in vivo phase and dropping below 70% of the baseline mean values from 21 dpi. Lymphopenia and neutropenia (below 70% of the baseline counts) were evident at 14–21 and 21–28 dpi, respectively. In group B, leukocytosis appeared at 3 dpi and persisted until 7 dpi, with WBC counts reaching values of 140% of the initial counts, whereas only mild leukopenia was observed at 21 and 28 dpi, with WBC counts remaining over 80% of the baseline values. These dogs displayed moderate leukopenia (59–72% of the initial values) at 21–28 dpi and mild neutropenia (86% of the baseline counts) at 28 dpi. Neither clinical signs nor leukopenia were observed in control dogs (group C), whose total and differential WBC counts remained at the baseline values throughout the experiment. In these dogs, lymphocytosis was evident constantly, whereas neutrophil counts fluctuated with values below the initial counts only at 14 and 21 dpi.
Fig. 2.
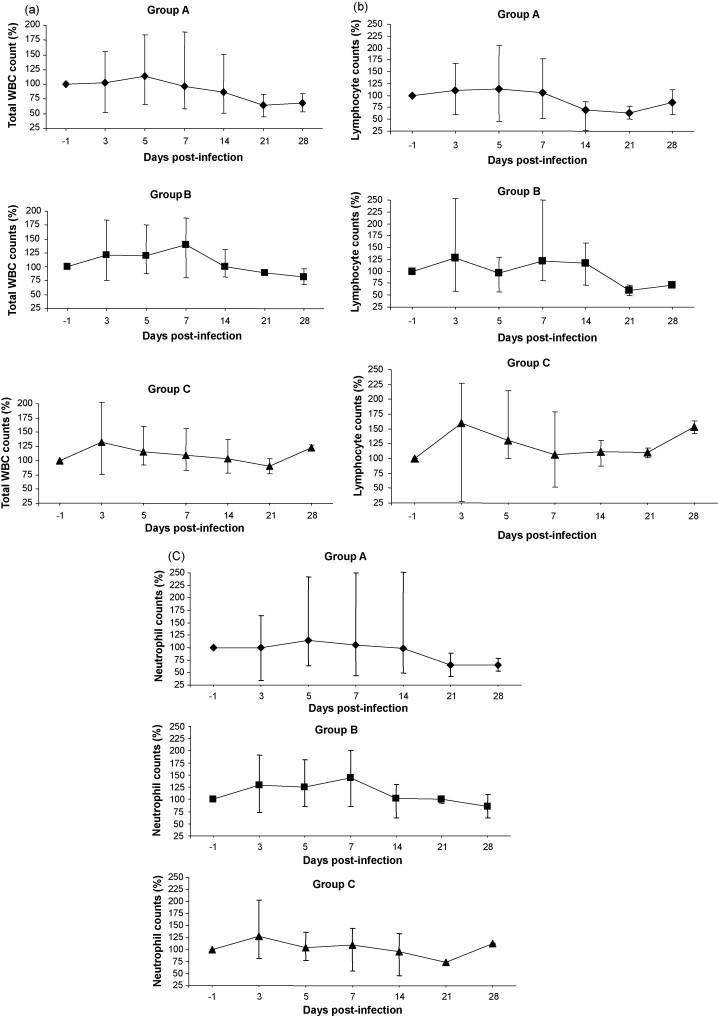
Total WBC (a), lymphocyte (b) and neutrophil (c) counts (means ± SE) in experimentally infected (groups A and B) and control dogs (group C). Counts are presented as percentages of the cell counts determined at day −1.
3.2. Necropsies
One of the most important features of strain CB/05 is its ability to infect organs other than the gut epithelium. In this experiment, the distribution and pathology of the pantropic CCoV strain CB/05 were investigated to demonstrate its ability to induce disease in animals already exposed to classical enteric coronaviruses. At post-mortem examination, the 6 animals of group C (control dogs), euthanised at 7 dpi (two dogs), 14 (two dogs) and 28 (two dogs), did not show any gross lesions. The group A dogs euthanised at 7 dpi (A3, A4, A6) showed enteritis with liquid content (3/3) and mucosal haemorrhages (2/3), enlargement of lymph nodes (3/3) with haemorrhages (2/3), and moderate to remarkable enlargement of spleen (3/3). Only 2/3 of groups B dogs necropsied at 7 dpi (B2, B3, B5) presented fluid or liquid content in the gut lumen; lymph nodes and spleens were enlarged in all 3 dogs, with scattered haemorrhages in lymph nodes of dog B5. No signs of enteritis were observed in groups A and B dogs, euthanised at 14 dpi, whereas enlargement of lymph nodes was observed in 3/3 (A5, A7, A8) and 2/3 (B1, B4) dogs of groups A and B, respectively, and enlargement of spleen was evident in 2/3 (A7, A8) and 1/3 (B1) dogs of groups A and B, respectively. No macroscopic lesions were observed in dog B7. At 28 dpi, enlargements of lymph nodes and spleen were observed in 2/2 animals of group A (A1, A2), whereas, of 2 group B dogs, 1 dog (B6) showed enlargement of lymph nodes and the other dog showed enlargement of spleen.
3.3. Virus detection in biological samples
All dogs of groups A and B became infected with strain CB/05 as shown by the results of real-time RT-PCR and virus isolation on cell cultures. By real-time RT-PCR (Fig. 3 ), faecal shedding was observed at 3–14 dpi, whereas no viral RNA was detected in the faeces of inoculated dogs at 21 and 28. CCoV faecal shedding peaked at 5 dpi in dogs of both groups A and B, with mean values of 3.86 × 105 and 7.12 × 104 RNA copies μl−1 of template, respectively. CCoV RNA was also detected in the intestine of all infected dogs euthanised at 7 dpi with viral titres ranging from 2.63 × 104 to 1.46 × 105 RNA copies μl−1 of template. Low CCoV titres (from 1.17 × 101 to 3.00 × 102 RNA copies μl−1 of template) were detected in the lymphoid organs (thymus, spleen and mesenteric lymph nodes) of 4/6 dogs euthanised at 7 dpi. In dogs euthanised at 14 and 28 dpi, viral RNA was not found in any organs with the exception of the intestines of some dogs that displayed traces of the virus.
Fig. 3.
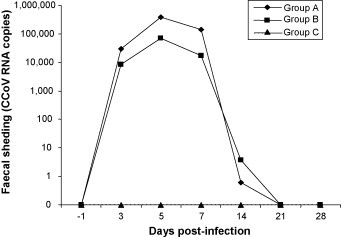
CB/05 RNA titres (means) in the faeces of experimentally infected (groups A and B) and control dogs (group C). Viral RNA titres as determined by real-time RT-PCR are expressed as log copy numbers per μl of template.
Virus isolation on A-72 cells gave positive results only for some faecal samples collected from group A (2/8 dogs) at 3 dpi and from groups A (4/8 dogs) and B (2/8 dogs) at 5 dpi. Due to the low titres of CCoV RNA detected by real-time RT-PCR, strain CB/05 was not isolated from lymphoid organs. Both real-time RT-PCR and virus isolation failed to detect the virus in nasal swabs and EDTA-treated blood samples, as well as in any biological samples collected from dogs of group C.
An RT-PCR assay targeting ORF3b showed that the virus detected in both faecal samples and lymphoid tissues of the infected dogs had the 38-nt deletion, thus characterising the virus as strain CB/05 (data not shown).
The presence of other canine pathogens, such as canine parvovirus type 2, canine distemper virus and canine adenoviruses, which could have been potentially involved in the onset of clinical signs in the experimentally infected dogs, were ruled out by specific molecular assays [13], [14], [15].
3.4. CCoV antibody titres
At day −1, all dogs were already highly seropositive to CCoV, as shown by the values obtained using ELISA and VN tests. An increase in the CCoV antibody titres was observed in the infected dogs, whereas the control dogs (group C) displayed a slight decrease in CCoV-antibody titres (Fig. 4 ).
Fig. 4.
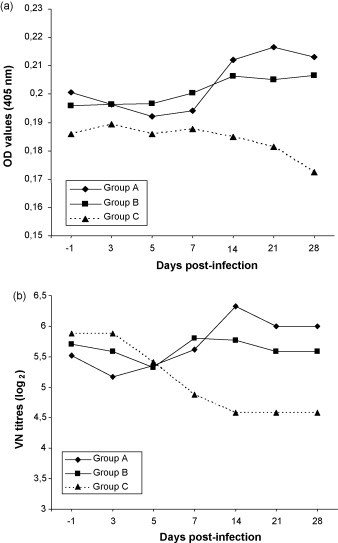
CCoV antibody titres (means) in experimentally infected (groups A and B) and control dogs (group C). Antibody responses are presented as geometric means of ELISA optical density (OD) values (a) or virus neutralising (VN) titres (b).
4. Discussion
Coronavirus strain CB/05, first isolated from the internal organs of pups aged between 45 and 56 days, has been shown consistently to cause systemic disease in young and older pups that were infected experimentally [10]. The present study demonstrates that strain CB/05 is able to infect dogs seropositive to enteric CCoV and induce clinical signs. Although the dogs used in this study had a strong humoral immunity to enteric CCoV at the time of challenge, the experimental infection with strain CB/05 was successful in all pups irrespective of the viral dose administered. It is noteworthy that exposure to even low amounts of virus would have similar infectivity on seropositive animals, since dogs inoculated with different viral loads (4 × 105 or 4 × 103 TCID50/ml of viral suspension) displayed the same duration of viral shedding and there was little difference in the viral titres in the faeces.
In this study, the duration of viral shedding was shorter and the clinical signs were milder with respect to our previous observations in seronegative dogs [10]. This was attributed mainly to the cross-protection induced by antibodies against enteric CCoV. However, the lymphotropism of the strain CB/05 was clearly demonstrated by the occurrence of lymphopenia (albeit moderate) in several infected pups of groups A and B. We hypothesise that the high antibody titres to enteric CCoV could have induced only partial protection against strain CB/05, which infected the dogs, causing enteritis and lymphopenia, but which failed to spread systemically, with the exception of lymphoid tissues where traces of the viral RNA were detected after necropsy. However, despite the occurrence of moderate lymphopenia and the presence of the virus in lymphoid tissues, the viral RNA was not detected in the blood at any time. This finding may be due to very low levels of viremia that were not detected by the molecular assays used. A similar pattern has been described for feline infectious peritonitis virus, that may be undetected in the blood especially during periods of apparent recovery after acute infection [16].
In a previous study [17], inactivated vaccines that are currently used against enteric CCoV have been shown to be poorly effective, as they induced high serum antibody levels but no protection after experimental infection with enteric CCoV. Only an experimental modified-live virus vaccine administered oronasally has been able to induce complete protection from disease as well as from infection. Considering that the immunity induced by natural infection with enteric CCoV is not able to protect pups from challenge with strain CB/05, the efficacy of currently used vaccines prepared with enteric CCoV strains may be poorer against pantropic CB/05-like viruses. According to this scenario, dogs vaccinated with enteric CCoV may acquire subclinical infections with CB/05-like strains resulting in lymphopenia which may represent a predisposing factor for opportunistic pathogens and for a more severe disease induced by “true” pathogens (canine parvovirus, canine distemper virus, etc.).
An extensive epidemiological survey would assess whether the pantropic CCoV infection is widespread in dog populations. Systematic vaccination programmes using homologous live vaccines would seem important in environments such as kennels, shelters and pet shops that are at high risk of exposure to this new pathogen.
Acknowledgements
This work was supported by a Pfizer Animal Health grant. We thank Donato Narcisi, Carlo Armenise and Arturo Gentile for their excellent technical assistance. We also acknowledge Dr. Giuseppe Procino for the supervision and management of the dogs used in this study. We would also like to thank P.J. Collins (Biology Department, Cork Institute of Technology, Ireland) for the English revision of the manuscript.
References
- 1.Enjuanes L., Brian D., Cavanagh D., Holmes K., Lai M.M.C., Laude H. Family Coronaviridae. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., editors. Virus taxonomy, classification and nomenclature of viruses. Academic Press; New York: 2000. pp. 835–849. [Google Scholar]
- 2.Dolja V.V., Carrington J.C. Evolution of positive-strand RNA viruses. Semin Virol. 1992;3:315–326. [Google Scholar]
- 3.Decaro N., Martella V., Ricci D., Elia G., Desario C., Campolo M. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J Virol Methods. 2005;130:72–78. doi: 10.1016/j.jviromet.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decaro N., Desario C., Elia G., Mari V., Lucente M.S., Cordioli P. Serological and molecular evidence that canine respiratory coronavirus is circulating in Italy. Vet Microbiol. 2007;121:225–230. doi: 10.1016/j.vetmic.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tennant B.J., Gaskell R.M., Kelly D.F., Carter S.D., Gaskell C.J. Canine coronavirus infection in the dog following oronasal inoculation. Res Vet Sci. 1991;51:11–18. doi: 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decaro N., Buonavoglia C. An update on canine coronaviruses: viral evolution and pathobiology. Vet Microbiol. 2008;132:221–234. doi: 10.1016/j.vetmic.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C. Canine coronavirus highly pathogenic for dogs. Emerg Infect Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decaro N., Martella V., Elia G., Campolo M., Desario C., Cirone F. Molecular characterisation of the virulent canine coronavirus CB/05 strain. Virus Res. 2007;125:54–60. doi: 10.1016/j.virusres.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decaro N., Campolo M., Lorusso A., Desario C., Mari V., Colaianni M.L. Experimental infection of dogs with a novel strain of canine coronavirus causing systemic disease and lymphopenia. Vet Microbiol. 2008;128:253–260. doi: 10.1016/j.vetmic.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaro N., Pratelli A., Campolo M., Elia G., Martella V., Tempesta M. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J Virol Methods. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pratelli A., Elia G., Martella V., Palmieri A., Cirone F., Tinelli A. Prevalence of canine coronavirus antibodies by an enzyme-linked immunosorbent assay in dogs in the south of Italy. J Virol Methods. 2002;102:67–71. doi: 10.1016/S0166-0934(01)00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu R.L., Huang G., Qiu W., Zhong Z.H., Xia X.Z., Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet Res Commun. 2001;25:77–84. doi: 10.1023/a:1006417203856. [DOI] [PubMed] [Google Scholar]
- 14.Decaro N., Elia G., Martella V., Desario C., Campolo M., Di Trani L. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs. Vet Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E. Detection of canine distemper virus in dogs by real-time RT-PCR. J Virol Methods. 2006;136:171–176. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 16.de Groot-Mijnes J.D., van Dun J.M., van der Most R.G., de Groot R.J. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J Virol. 2005;79:1036–1044. doi: 10.1128/JVI.79.2.1036-1044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratelli A., Tinelli A., Decaro N., Martella V., Camero M., Tempesta M. Safety and efficacy of a modified-live canine coronavirus vaccine in dogs. Vet Microbiol. 2004;99:43–49. doi: 10.1016/j.vetmic.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


