Highlights
-
•
The reverse genetic operating systems of IBV H120 and IBYZ strains were created.
-
•
We constructed rH120-S1/YZ strain, based on the backbone of the H120 vaccine strain, with the S1 gene replaced with that of the QX-like nephropathogenic strain, ck/CH/IBYZ/2011.
-
•
We demonstrated that rH120-S1/YZ had the potential to be a candidate vaccine strain for the prevention and control of the epidemic of renal strains.
Abbreviations: IB, infectious bronchitis; IBV, infectious bronchitis virus; SPF, specific pathogen free; EID50, 50% embryo infectious dose; dpi, days post-infection; dpv, days post-vaccination; dpc, days post-challenge; ELISA, enzyme-linked immunosorbent assay
Keywords: Infectious bronchitis virus, Attenuation, Spike, Vaccine, QX-like
Abstract
Infectious bronchitis (IB) is a highly infectious viral disease responsible for major economic losses in the poultry industry. A reverse genetic vaccine is a safe, rapid, and effective method of achieving IB prevention and control. In this study, we constructed the recombinant strain, rH120-S1/YZ, using a reverse genetic system, based on the backbone of the H120 vaccine strain, with the S1 gene replaced with that of the QX-like nephropathogenic strain, ck/CH/IBYZ/2011, isolated in China. The results of dwarf chicken embryos, growth kinetics, and viral titration in the embryos demonstrated that the biological characteristics of the recombinant virus remained unchanged. Like the rH120-infected group and in contrast to the rIBYZ-infected group, no mortality, clinical signs, or lesions were observed in the lungs or kidneys of young chickens inoculated with rH120-S1/YZ. The viral loads in various tissues, cloacal, and oral swabs was lower in most types of samples, indicating that the rH120-S1/YZ strain was highly safe in chicks. Compared to rH120 vaccination group, when the efficacy of this strain was evaluated against the QX-like IBV strain, better protection, with 100% survival rate and no disease symptom or gross lesion was observed in the chickens vaccinated with rH120-S1/YZ. Increased levels of IBV-specific antibodies were detected in the serum of the rH120-S1/YZ-vaccinated animals 14 days post-vaccination. Collectively, our results suggest that the recombinant strain, rH120-S1/YZ, may represent a promising vaccine candidate against QX-like IBVs.
1. Introduction
Infectious bronchitis (IB) was first described as a respiratory disease affecting chicks in the US in 1931 as an acute and highly contagious viral disease, and continues to cause major economic loss within the poultry industry worldwide [1], [2], [3]. As a result of the damage to the respiratory system, the clinical signs of this disease include depression, coughing, head-shaking, as well as nasal and ocular discharge [1]. Since the virus causing IB replicates in many non-respiratory epithelial surfaces (e.g., kidney and gonads), nephritis, reduced egg production, and egg quality is also observed [2], [3].
The causative agent of IB is infectious bronchitis virus (IBV), a non-segmented, positive-sense, single-stranded RNA virus, which belongs to the genus Gammacoronavirus, family Coronaviridae, in the order Nidovirales [4]. The full length RNA genome of IBV is 27.6 kb in length and encodes non-structural proteins, four accessory proteins, and four structural proteins (spike [S], matrix [M], nucleocapsid [N], and envelope [E]). The S protein is cleaved into S1 and S2 as two subunits at the S1/S2 cleavage site. The S1 protein contains the receptor-binding domain and mediates viral attachment to host cells, whereas S2 is responsible for membrane fusion [5], [6].
Errors generated during the genomic replication of IBV and the selective pressure following the use of live attenuated vaccines or multiple infections with different IBV serotypes has led to the emergence of numerous IBV variants [7], [8]. In particular, small change as little as 5% in the amino acid composition of S1 gene may lead to alteration in cross protection among closely related serotypes [9]. In China, IBV was first observed during the early 1980s, and outbreaks have since been frequently reported [7]. The QX-like genotype is thought to have originated during the mid-1990s and continues to be the predominant strain, whereas the LDT3, 4/91, and Taiwan subtypes have also recently been frequently isolated in China [10], [11], [12], [13]. Severe nephritis, false layer syndrome, and high mortality are observed in chickens infected with this viral genotype, which represents a major problem in the poultry industry, particularly in China.
Using a live attenuated or inactivated vaccine is typically considered to be the most cost-effective means of controlling viral infections [14]. Unfortunately, inactivated IB vaccines are not effective if used alone, which could induce little or no protection against egg loss [15], [16], as well as impaired ciliary activity in the trachea [17]; thus, birds must continue to be given one or a series of vaccinations with live attenuated IB vaccines to provide broad heterologous protection [1]. Commercial attenuated live vaccines used against IBV in China include the H120, LDT3, and 4/91 strains [7]; however, phylogenetic analysis indicates that the QX-like genotype is genetically distant from the strains described above, which may explain the poor cross-protectivity against infection in chickens immunized with these classical vaccines [18], [19]. Recently, there has been increasing research on live attenuated vaccines against the QX-like IBV strains. Different strains of QX-like IBVs were serially passaged in embryonated eggs or primary chicken kidney cells (CK) to obtain vaccines that are less virulent and exhibit high immunogenicity against the QX-like epidemic IBV strains [20], [21], [22], [23], [24]. However, in addition to causing tissue damage or secondary bacterial infections in young vaccinated chicks [25], [26], live attenuated vaccines of RNA viruses may not be genetically stable or they may be associated with the tendency to revert back to a virulent form [9], [14]. Moreover, the potential for recombination between vaccine and virulent strains can lead to the creation of new virulent virus [27]. Furthermore, it takes a substantial amount of time to prepare a extensively passaged attenuated vaccine strain, which may not be able to effectively solved the loss problem caused by the rapid variation exhibited by prevalent strains on the flocks.
In considering the limitations of the live attenuated and inactivated IBV vaccines, reverse genetic IBV vaccines have been developed in recent years as they display increased safety and efficacy [28], [29], [30]. As reverse genetic technology is primarily based on the H120 and Beaudette strains [31], [32], [33], both of which are passage-generated attenuated strains, the mechanism of virus virulence attenuation remains unclear, thereby posing difficulties in the development of attenuated vaccines using this technology. In addition to the function of host invasion and increasing genetic diversity, the IBV S1 subunit can induce virus neutralizing and cross-reactive antibodies [34]. Therefore, some researchers have created an attenuated IBV vaccine by replacing the S1 or S genes from other virulent strains using reverse genetic technology to generate protection against strains of the corresponding serotype [5], [28], [30]. In the present study, we constructed a recombinant strain, using the reverse genetic system. rH120-S1/YZ is based on the backbone of the vaccine strain H120, and the S1 gene was replaced with that of the QX-like nephropathogenic strain, ck/CH/IBYZ/2011, which was isolated in China. Our results demonstrate that this recombinant strain is safe and provides effective protection in young chickens against QX-like IBV challenge.
2. Materials and methods
2.1. Vaccine and viruses
The H120 vaccine strain is currently a widely used vaccine strain, created from 120 serial passages of the H strain isolated in 1956. The ck/CH/IBYZ/2011 strain (referred to as IBYZ, GenBank KF663561.1) used in this study is classified as a QX-like IBV isolated from a flock presenting with IB symptoms by our research group in 2011 in Jiangsu Province, China. Both of these strains were recovered from a full-length clone using reverse genetics, as previously described [35], [36].
2.2. Eggs and animals
Fertilized specific pathogen free (SPF) chicken embryos were obtained from Beijing Merial Vital Laboratory Animal Technology Co., Ltd. (Beijing, China) and hatched in our lab (Lab of Chicken Infectious Disease Protection and Control, Poultry Institute, Chinese Academic of Agriculture Sciences, Yangzhou, China). For hatching, one-day-old SPF chickens from the SPF chicken embryos as described previously were incubated at 37 °C in a relative humidity of 55–65%. All chickens were maintained in isolators under negative pressure, and food and water were provided ad libitum.
2.3. Construction of recombinant rH120-S1/YZ
The rIBV rH120-S1/YZ strain used in this study is described in the schematic illustration presented in Fig. 1 . The genome RNA was synthesized in vitro by T7 RNA polymerase and transfected into BHK-21 cells, as previously described [35], [36]. The cell supernatants were harvested 48 h following transfection and propagated in 10-day-old SPF chicken embryos. The allantoic fluid was harvested for RT-PCR, whole genome sequencing, and was then stored at −80 °C until further use.
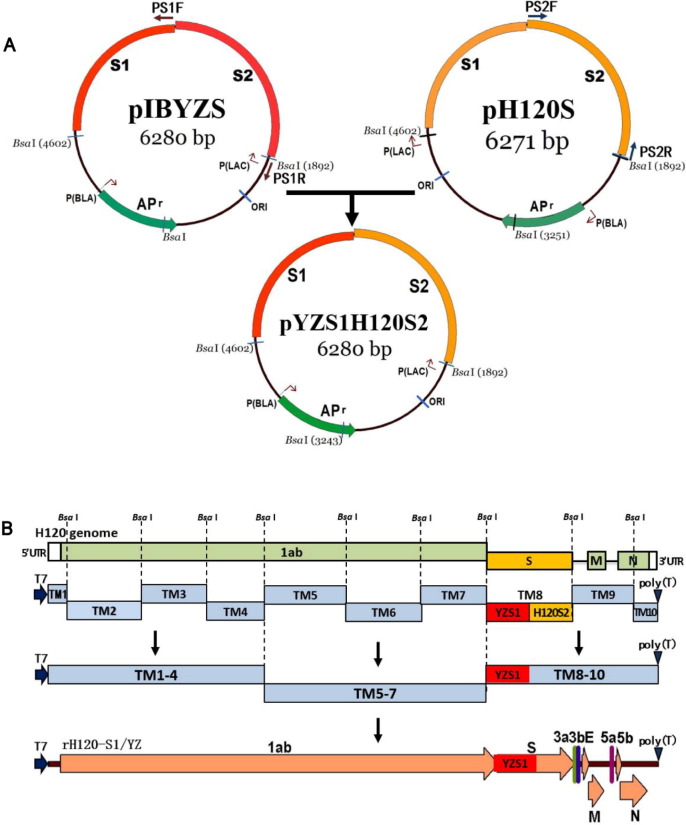
Fig. 1.
Schematic diagram for the construction of the chimeric S gene and production of a full-length cDNA of rH120-S1/YZ. (A) Replacement of the H120 S1 fragment by the corresponding sequence of IBYZ for construction of pYZS1H120S2. The plasmids pIBYZS and pH120S contained the S gene of IBYZ strain and H120 strain, respectively, were constructed during the establishment of reverse genetic system. Primers PS1F and PS1R were used to amplify the S1 fragment of IBYZ and vector fragment, while primers PS2F and PS2R were used to amplify the S2 fragment of H120 strain. By overlapping PCR, the pYZS1H120S2 was constructed which contained a chimeric S gene. (B) Strategy for the construction of full-length cDNA clones of rH120-S1/YZ. Ten cDNA fragments covering the entire genome of H120 strain was amplified by RT-PCR. Unique Bsa I sites were inserted at the junctions between each clone, a unique T7 start site was inserted at the 5′ end of clone TM1, and a 28-nucleotide T tail was inserted at the 3′ end of clone TM10. The S fragment in the pMDTM8 plasmid of H120 strain was replaced by chimeric S gene. By using appropriate ligation strategy, the genomic cDNA of rH120-S1/YZ was assembled by in vitro ligation using appropriate restriction sites as indicated.
2.4. Chicken embryo dwarf assay
To determine the recombinants’ pathogenicity in chicken embryos, 0.2 mL of rH120, rIBYZ, or rH120-S1/YZ virus (diluted to 1:100 in normal saline) was inoculated into the allantoic cavities of five 10-old-day embryonated SPF chicken eggs, respectively. Chick embryo lesions were examined for lesions at 144 h post-inoculation at 37 °C.
2.5. Growth kinetics in chicken embryos
To examine the viral growth ability in chicken embryos, a real-time reverse transcription quantitative polymerase chain reaction (RT-qPCR) method was established. According to the sequences of IBV from GenBank, primers were designed based on conservative area in the 5′-UTR. The upstream primer was 5′-CCGTTGCTTGGGCTACCTAGT-3′, and the downstream primer was 5′-CGCCTACCGCTAGATGAACC-3′. The amplification product was cloned to pMD18-T vector (Takara) as a positive plasmid and its concentration was measured. A gradient dilution of 5 × 102–5 × 108 copies/μL of the plasmid was used as template for quantitation test. By plotting the cycle threshold (CT) values against the copies of the plasmid, the standard curve was generated.
18 10-day-old embryonated SPF chicken eggs (six eggs/group) were inoculated with rH120, rIBYZ, and rH120-S1/YZ at a dose of 107 viral RNA copies/100 μL, and used for growth curve experiments. The allantoic fluids were collected separately by syringe from the six inoculated embryonated eggs of each group at 12, 18, 24, 36, 48, 72, 96 h per inoculation. RNA was extracted from the allantoic fluids using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. cDNA was obtained by reverse transcription using a PrimeScript RT Master Mix Perfect Real Time Kit (TaKaRa, Otsu, Shiga, Japan). The viral copies were measured by absolute quantitative method of Real-time PCR, which was performed using SYBR® Premix Ex Taq™ II (TaKaRa, Otsu, Shiga, Japan) on an Applied Biosystems 7500 Fast Real-time PCR System. The standard curve was plotted against the log of the template copy number. All of the assays were run in triplicate and the copy number of each virus was calculated according to the standard curve.
2.6. Titration of rIBVs in embryos
Serial 10-fold dilutions from 105 copies to 109 copies per 0.1 mL of virus of the recombinant strain, rH120-S1/YZ, and its parental strains (rH120 and rIBYZ) were inoculated into the allantoic cavity of 10-day-old embryonated SPF chicken eggs. For each dilution, 0.1 mL of the virus solution was injected into each egg and 10 eggs were used for each dilution. PBS was used as a negative control. After a 24 h incubation, the dead embryos were considered nonspecific deaths and discarded. The embryos were examined for the presence of specific lesions caused by the virus after a 144-h incubation. Dead and live embryos that displayed IBV infectious signs (e.g., dwarfing, curling, and stunting) were considered positive samples. The 50% embryo infectious dose (EID50) of these rIBVs was calculated using the Reed-Muench method [37].
2.7. Safety testing
2.7.1. Assessment of pathogenicity in one-day-old SPF chickens
The chickens were separately housed in isolators under consistent conditions, and food and water was provided ad libitum. A total of 120 one-day-old SPF chickens were randomly divided into four groups (n = 30 per group). The chickens in the experimental groups were intranasally inoculated with 200 μL allantoic fluid per chick containing 105EID50 of the rH120, rIBYZ, and rH120-S1/YZ strains. The control group (n = 30) was inoculated with 200 μL sterile PBS via the same route. The morbidity and mortality was followed-up for 14 days. All experimental groups were monitored daily for clinical signs related to IB infection for 14 days post-infection (dpi), including coughing, sneezing, and tracheal rales. Dead chickens were examined for gross tracheal, lung, and kidney lesions.
2.7.2. Tissue tropism of early viral infection in vivo
A total of 100 1-day-old SPF chickens were assigned to four groups (n = 25 per group). The birds were inoculated with 200 μL allantoic fluid containing 105 EID50 of the rH120, rIBYZ, or rH120-S1/YZ strains, respectively via eye drop and the intranasal route and 200 μL PBS per chick was administered to the control group (n = 25) via the same route. The tracheas, lungs, kidneys, and bursa from the 5 inoculated birds per group were harvested at 1, 3, 5, and 7 dpi, weighed, and collected into 1 mL PBS per sample and frozen at −80 °C. After grinding the samples, the viral RNA was extracted using TRIzol, and the cDNA was obtained by reverse transcription using a PrimeScript RT Master Mix Perfect Real Time Kit (TaKaRa, Otsu, Shiga, Japan). The viral RNA copies from each of the different samples were detected by real-time PCR as described above. All assays were run in triplicate and the copy number for each virus was calculated according to the standard curve.
2.7.3. Histopathology
After inoculation with 105 EID50 dose of the rH120, rIBYZ, and rH120-S1/YZ strains, three infected chickens per group were randomly selected to be euthanized by bleeding, and the tissue pathology was examined in the different groups at 3, 5, 7, 10 dpi. The tracheas, lungs, kidneys, and bursas of three dead or other randomly-selected chickens from the experimental and control groups (inoculated with PBS) were collected at 5 or 7 dpi and further processed for histopathology. The samples were fixed in formalin, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin. The slides were examined under light microscopy for the presence of lesions.
2.7.4. Viral shedding
To determine the level of viral shedding of the different recombinants, 10 birds per group were inoculated with 200 μL allantoic fluid containing 105 EID50 of the rH120, rIBYZ, or rH120-S1/YZ strains, respectively. 200 μL PBS per chick was administered to the control group via eye drop and the intranasal route. . Oral and cloacal swabs were collected from each bird into 1 mL PBS at 7, 14, 18, 22, 26, and 30 dpi and stored at −80 °C. After freezing and thawing three times and centrifuging at 8,000 × g for 5 min at 4 °C, relative amount of virus present in 200 μL of supernatants of each sample was quantified by RNA extraction, reverse transcription, and real-time PCR as described previously.
2.8. Efficacy of rH120-S1/YZ vaccination
2.8.1. Viral challenge
In total, 30 one-day-old SPF chickens were divided into three groups of 10 chickens. The three groups were intranasally vaccinated with the rH120 and rH120-S1/YZ strains at a dose of 104 EID50/200 μL, and the control group received PBS. Two weeks post-vaccination, the chickens in each group were challenged with the QX-like strain, rIBYZ, at 106 EID50/200 μL via eye drop and the intranasal route. Any clinical signs, as well as the percentage of morbidity and mortality were recorded for 14 days. The dead chickens were also examined for gross tracheal, lung, and kidney lesions.
2.8.2. Antibody response measured by an enzyme-linked immunosorbent assay (ELISA)
45 1-day-old SPF chickens were divided into three groups (n = 15 per group). After vaccinating with the rH120 and rH120-S1/YZ strains at a dose of 104 EID50/200 μL, the sera of 10 birds from each group were randomly collected and antibodies were tested using an ELISA created in our lab at 7, 14, 21, and 28 days post-vaccination (dpv). ELISA plates were coated with antigen of culture supernatants of inactivated IBV rMJ , a vero cells adaption strain domesticated from its parental strain of IBYZ in our lab. Individual chicken sera collected from different groups diluted to 1:200 were loaded and the plates were incubated at 37 °C for 60 min. IBV-specific IgG was detected with anti-chicken IgY (IgG) (whole molecule)-peroxidase antibodies produced in rabbits (Sigma-Aldrich, Germany). After incubated with 3,3′,5,5′-Tetramethylbenzidine(TMB) substrate for 15 min and terminated with 2 mol/L H2SO4 solution, the absorbance at 490 nm was measured using a Model 680 microplate reader (Bio-Rad, USA).
2.9. Statistical analysis
GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA, USA) was used for the statistical analyses. For the antibody test, growth kinetics, tissue tropism, and viral shedding test, the collected data were analyzed using a two-way ANOVA to determine whether there was a significant difference between the different groups. The significance was considered as follows: significant at P ≤ 0.05 (*); highly significant at P ≤ 0.01 (**); and extremely significant at P ≤ 0.001 (***).
3. Results
3.1. Characterization of the rH120-S1/YZ strain
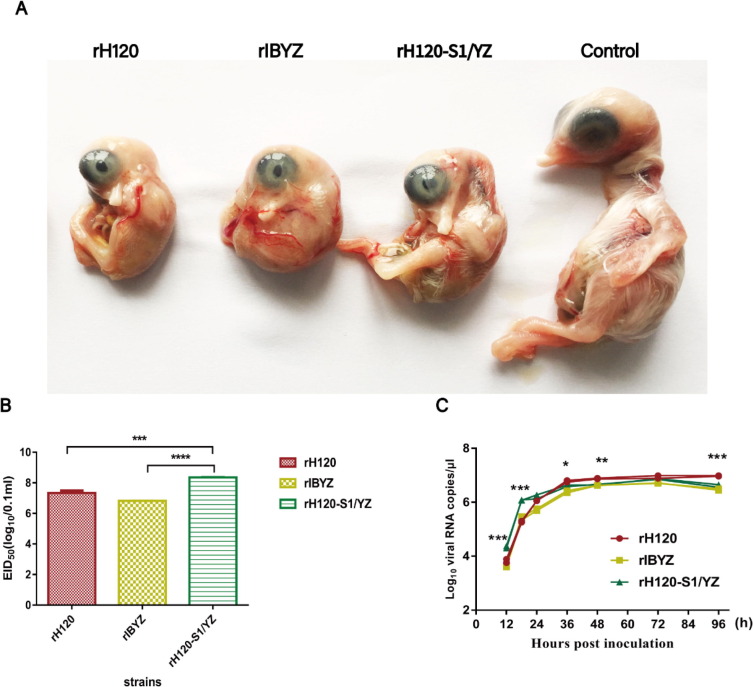
After being inoculated with the recombinant strains, typical IB lesions in the chick embryos from each group were observed, including amniotic membrane thickening, dwarfing, stunted growth, curling, or death of the embryo (Fig. 2 A). The EID50 of the virus was calculated according to the Reed-Muench method. The titers of the rH120, rIBYZ, and rH120-S1/YZ strains were determined to be 107.3 EID50/mL, 106.8 EID50/mL, and 108.4 EID50/mL, respectively (Fig. 2B).
Fig. 2.
The biological characteristics of the rIBVs in SPF chicken embryos. (A) Pathogenicity of recombinant IBVs in infected chick embryos. Five 10-old-day embryonated SPF chicken eggs per group were inoculated with 0.2 mL virus diluent of rH120, rIBYZ, or rH120-S1/YZ. Chick embryo lesions were observed at 144 h post-inoculation at 37 °C. (B) EID50 of the recombinant IBVs, rH120, rIBYZ, and rH120-S1/YZ in SPF chicken embryonated eggs. (C) The multicycle growth kinetics of the recombinant IBVs, rH120, rIBYZ, and rH120-S1/YZ in SPF chicken embryonated eggs. All data are presented as the mean ± standard deviation (SD). Some of the error bars are too small to be seen. The qRT-PCR was carried out with three replicates. Markers of statistical difference were acquired by comparing between rH120 group and rH120-S1/YZ group. ***Indicates an extremely significant difference at P ≤ 0.001; **Indicates a highly significant difference at 0.001 < P ≤ 0.01; *Indicates a significant difference at 0.01 < P ≤ 0.05.
The growth kinetics of the rIBVs were assessed by inoculation with 107 copies of virus per egg in 6 eggs from each group. The relative viral load was determined at 12, 18, 24, 36, 48, 72, and 96 h post-inoculation by quantitative real-time RT-PCR. The growth curves of all three strains reached a relatively stable plateau at 36–48 h after infection; the level of viral RNA for the rH120-S1/YZ strain was significantly higher than that of the rIBYZ strain for the majority of the time points (Fig. 2C).
3.2. Safety
3.2.1. Pathogenicity of the recombinants in one-day-old SPF chickens
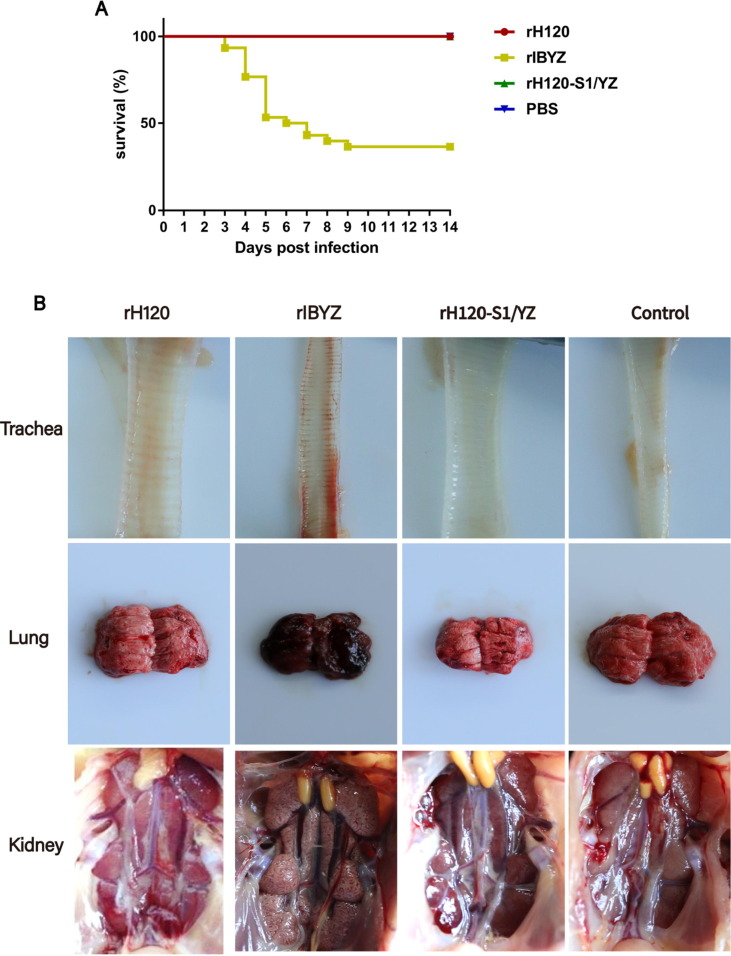
The earliest death was observed at 3 dpi in the QX-like strain rIBYZ group, which virus was highly pathogenic to one-day-old SPF chickens; the final mortality of this group was 63.3% (Fig. 3 A). The morbidity of the infected chickens in the rIBYZ group reached higher than 80%, and the diseased chickens exhibited respiratory symptoms (e.g., sneezing, coughing, as well as tracheal and bronchiolar rales), and the severe cases presented with additional signs of listlessness, huddling, and ruffled feathers. At necropsy, lesions were detected both in the respiratory and urinary system, including mucus, hyperemia, and hemorrhage in the trachea, as well as swelling and urate deposition in the kidney. Edema and congestion were observed in the lungs in 20% of cases (Fig. 3B). However, following inoculation with the rH120 or rH120-S1/YZ strains, the morbidity rates of the chickens was less than 20%, with moderate respiratory signs of coughing in some of the chickens. All of the infected chickens presented no visible lesions in the kidney and the survival rate was 100% through 14 dpi in both the rH120 and rH120-S1/YZ groups (Fig. 3A and B). No obvious clinical signs, gross lesions, or death attributable to IBV was observed in the control group.
Fig. 3.
Pathogenicity of the rIBV rH120, rIBYZ, and rH120-S1/YZ strains. (A) Percent survival of SPF chickens infected at 1-day-of age during the 14-day observation period. No mortality was observed in rH120, rH120-S1/YZ, or the control groups. (B) Gross lesions on the lungs and kidneys of the infected chickens at 5 or 7 dpi.
3.2.2. Tissue pathology
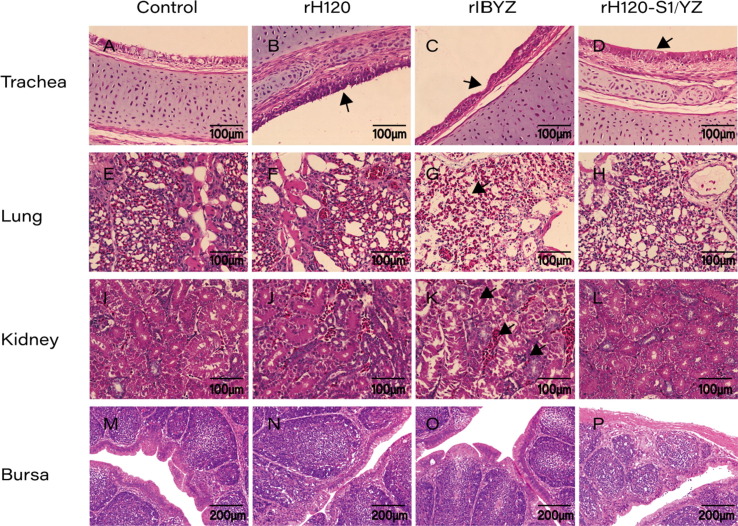
For the chickens inoculated with the QX-like strain rIBYZ, lesions in the trachea at 5 dpi were characterized as cilia necrosis and loss, epithelial cell congestion, hemorrhage, and lymphoid infiltration (Fig. 4 C). In the bronchial and capillary lumen of rIBYZ-infected lungs, congestion, hemorrhage, as well as erythrocyte and inflammatory cell infiltration was observed in some severe cases from 7 to 10 dpi (Fig. 4G). Moreover, erythrocyte and lymphocyte infiltration, as well as tubular dilation was observed in the kidney of rIBYZ-inoculated chickens (Fig. 4K). Similar to the rH120 group, chickens inoculated with rH120-S1/YZ presented with mild pathology in the trachea at the early infection time points of 3, 5 dpi. Lesions in the trachea consisted of the loss of epithelial cells and cilia, decreased number of secretory cells, and thickening of the mucosa (Fig. 4B and D). In contrast, there were no lesions observed in the lungs and kidneys of rH120 and rH120-S1/YZ-infected chickens during the infection period (Fig. F, H, J, and L). No histopathological evidence of damage was detected in the bursa of all the groups and no IBV-associated lesions were found in the tissue samples of the control chickens (Fig. 4A, E, I, M–P).
Fig. 4.
Histopathological changes in the trachea, lungs, kidneys, and bursa of SPF chickens infected with the recombinant rH120, rIBYZ, and rH120-S1/YZ strains at 1-day-of-age. Black arrows in the trachea indicate cilia and necrosis loss. Black arrows in the lungs indicate congestion and hemorrhage. Trachea samples were collected at 3, 5 dpi (A–D); lung and bursa samples were collected at 7, 10 dpi (E–H; M–P); and kidney samples were collected at 5, 7 dpi (I–L). Black arrows in kidneys indicate tubular dilation, congestion, and inflammation. Scale bar = 100 μm or 200 μm.
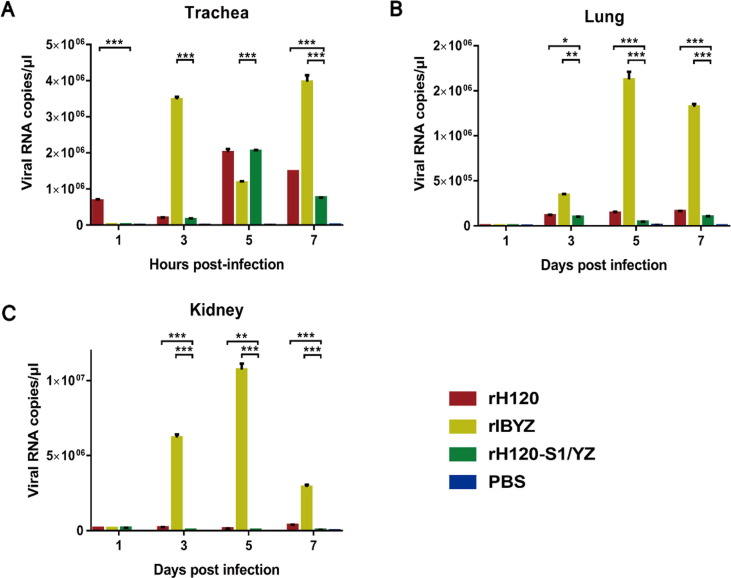
3.2.3. Growth in tracheal, lung, and kidney tissues
The time-dependent levels of viral replication in the different tissues determined by real-time RT-PCR presented in Fig. 5 are consistent with the tissue histopathology results. Compared to rH120 and rH120-S1/YZ groups, one day after inoculation with the QX-like strain, rIBYZ, the viral load in all organs began to rise rapidly, except at 5 dpi in the trachea. The viral load in tracheal samples in the rH120-S1/YZ group increased and peaked at 5 dpi. The level of viral RNA was significantly lower than the rH120 group both at 1 dpi and 7 dpi (Fig. 5A). The viral load in lung samples from all infection groups increased starting at 3 dpi, and the viral RNA in the lungs of the rH120-S1/YZ group was significantly lower compared with both the rH120 and rIBYZ groups (Fig. 5B). The viral load in the kidney of all IBV-inoculated groups was detected at 1 dpi. In contrast to the rH120 and rH120-S1/YZ groups, the viral growth in kidney of rIBYZ-infected chickens exhibited an extremely significant increase at 3 dpi and peaked at 5 dpi. The viral RNA copies of the rH120-S1/YZ group peaked at 1 dpi, after which they gradually decreased (Fig. 5C). No viral load was detected in the control samples at any of the time points.
Fig. 5.
Viral load in the different organs at 1, 3, 5, and 7 dpi of SPF chickens infected with the recombinant strains rH120, rIBYZ, and rH120-S1/YZ at 1-day-of age. The bars indicate means ± standard deviations. ***Indicates an extremely significant difference at P ≤ 0.001; **Indicates a highly significant difference at 0.001 < P ≤ 0.01; *Indicates a significant difference at 0.01 < P ≤ 0.05.
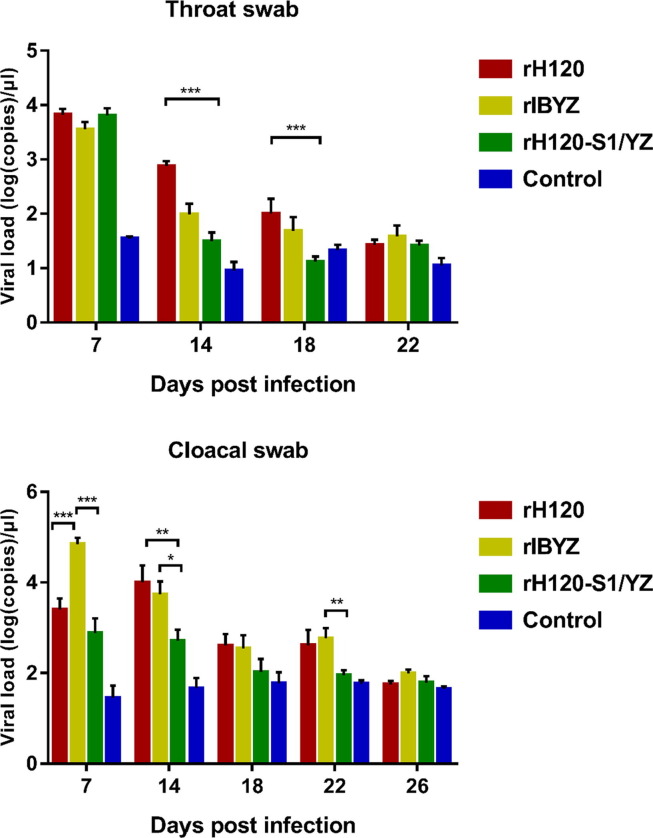
3.2.4. Viral load in the oral and cloacal swabs
In all IBV infection groups, the viral load in both the oral and cloacal swabs generally showed a gradual decline from 7 dpi to 26 dpi. Compared with the rH120 group, The reduction in viral load between 14 or 18 dpi was significantly greater for chickens infected with rH120-S1/YZ than for chickens infected with rH120, and the viral RNA of the QX-like strain rIBYZ was lower than that of the rH120 group at 7, 14, and 18 dpi but with no statistically significance (Fig. 6 A). In contrast, the viral load in the cloacal swab of the rIBYZ group was significantly higher than that of the rH120 and rH120-S1/YZ groups at 7 dpi. Compared with other groups, the viral RNA in the cloacal swab of the rH120-S1/YZ group was significantly lower at 7, 14, and 22 dpi (Fig. 6B). No virus was detected in the control group at any time point.
Fig. 6.
Viral loads at 7, 14, 18, 22, and 26 dpi in the oral and cloacal swabs of chickens infected with the recombinant rH120, rIBYZ, and rH120-S1/YZ strains. All data are presented as the mean ± standard error of the mean (SEM); ***Indicates an extremely significant difference at P ≤ 0.001; **Indicates a highly significant difference at 0.001 < P ≤ 0.01; *Indicates significant difference at 0.01 < P ≤ 0.05.
3.3. Vaccine efficacy
3.3.1. Efficacy of the different vaccines against QX-like IBV challenge in SPF chickens
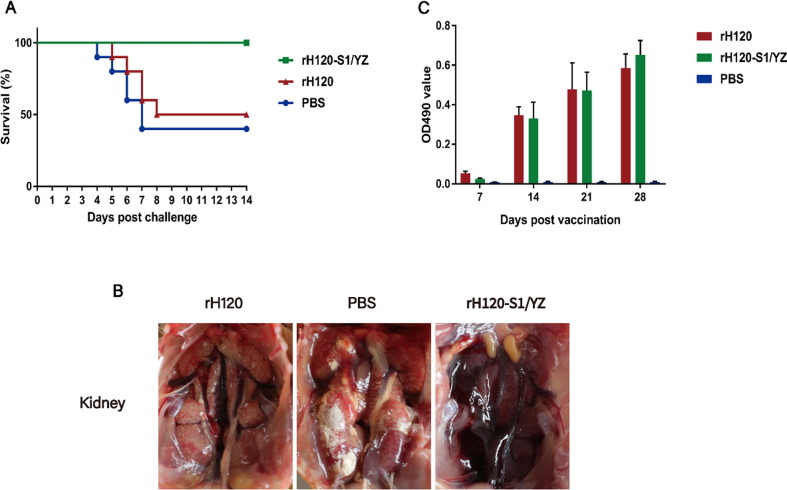
The rH120-S1/YZ group exhibited effective protection against challenge with the QX-like strain rIBYZ. The mortality rate in the rH120-S1/YZ group was 0%, and no clinical signs or lesions were observed during the rIBYZ challenge period (Fig. 7 A). In contrast, chickens vaccinated with the rH120 strain began to exhibit clinical signs (e.g., coughing and nasal discharge) at 3 days post-challenge (dpc), and some chickens showed severe signs of listlessness, huddling, and ruffled feathers, with the mortality rate reaching 50% during the observation period. At necropsy, the dead animals displayed typical kidney lesions characterized by swelling and urate deposition in the tubules and ureters. In contrast to chickens vaccinated with rH120-S1/YZ, the chickens in the unvaccinated control group exhibited clinical signs at 3 dpc, and a 60% mortality rate; the gross lesions of the dead birds in this group were similar to that of the rH120 group (Fig. 7B).
Fig. 7.
Efficacy of the rH120 and rH120-S1/YZ vaccines. (A) Percent survival of the vaccinated chickens challenged with the QX-like IBV rIBYZ strain during the 14-day observation period. No mortality was observed in the chickens vaccinated with the rH120-S1/YZ strain. (B) Gross lesions observed on the kidneys of the vaccinated chickens challenged with the rIBYZ strain at 5 or 7 dpc. (C) The mean antibody OD value at 7, 14, 21, and 28 dpv. All data are presented as the mean ± standard error of the mean (SEM).
3.3.2. Antibody response
Antibodies against the IBVs were measured using an ELISA (Fig. 7C). None of the birds in the rH120 or rH120-S1/YZ groups showed a positive antibody response at 7 dpv, whereas 100% of the birds had an antibody response detectable at 14 dpv, with the antibody level gradually increasing during the observation period. However, there was no significant difference in the level of serum antibodies between rH120-vaccinated chickens and rH120-S1/YZ-vaccinated chickens. No positive serum antibody response was detected in the non-vaccinated controls.
4. Discussion
Vaccines, particularly live-attenuated vaccines, remain the most effective means of protection against IBV challenge (e.g., the Mass serotype strains H120, H52, Ma5, and W93, which are still widely used in the poultry industry) [38], [39], [40]. However, due to the poor cross-protection provided between the different serotypes, these attenuated serotype-specific vaccines cannot provide complete protection, and the IB endemic in China cannot be controlled [22]. Genetic mutations and immune pressure during the replication process of the single stranded RNA virus often results in the emergence of IBV variants [41], [42]. To date, at least six genotypes of IBV strains have been identified in China, of which the QX-type (first isolated in 1999) remains the most prevalent genotype [10], [42], [43]. The poor relationship between the QX-type strains, which are abundant and display variable virulence in various parts of China, and the Mass-type vaccine strains could explain the failure of the Mass-type vaccination programs to control IBV in these flocks [19], [39]. Consequently, the development of novel vaccines against circulating IBV strains in China using local IBV strains is required [20], [43], [44].
Optimal vaccination against circulating IBV strains in China requires the development of attenuated vaccines designed from local strains [20], [21], [22], [45]. However, due to the particularity of RNA virus replication, attenuated vaccine strains generated by continuous passage remain associated with the risk of reversion to virulence or potential recombination between vaccine strains and virulent field strains [27], [46], [47], which represents a substantial hidden concern to the poultry industry. To reduce the problems associated with vaccine reversion, researchers have explored the option of creating vaccine viruses using reverse genetic technology (e.g., Beaudette strains carrying the S1 gene of the H120 vaccine strain, virulent M41 strain, or QX-like strain) [30], [48]. However, the titer of the Beaudette strains carrying the S1 gene of the vaccine H120 strain only reaches 106.13 ± 0.23 EID50 [49], which is lower than that of the H120 backbone strain. Therefore, we aimed to develop a recombinant rH120-S1/YZ strain based on the H120 vaccine strain that carries the S1 gene of the ck/CH/IBYZ/2011 strain (a Chinese QX-like nephoropathogenic strain) using reverse genetic technology [35], [36].
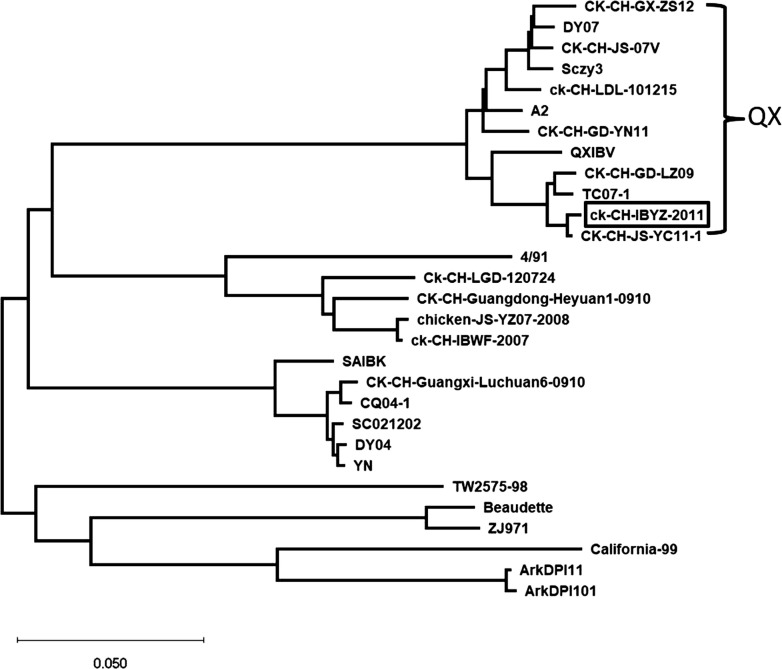
In the present study, we isolated a nephoropathogenic strain (IBYZ) in 2011. This strain was isolated, identified, and preserved from a large-scale chicken farm where an outbreak of IBV emerged in Jiangsu province. Chickens infected with the IBYZ strain showed severe kidney damage and a high mortality rate (Fig. 3B). The complete sequence has been deposited in the GenBank database under the accession number of KF663561. The S1 gene sequencing data indicated that the IBYZ strain belonged to the QX-like genotype (Fig. 8 ), with a nucleotide homology with the attenuated vaccine H120 strain of only 77.4%, and amino acid homology of 77.1%. Moreover, the S1/S2 cleavage site of IBYZ is HRRRR/S, which differed from RRFRR/S of the H120 vaccine strain. After whole-genome sequencing, we constructed a molecular clone strain using reverse genetic technology, termed rIBYZ. In addition, another strain used in this study is the live attenuated IBV vaccine, H120, and the molecular clone strain, rH120, which was constructed using the same method.
Fig. 8.
Phylogenetic tree constructed based on the S1 gene of isolate ck/CH/IBYZ/2011 and 28 published IBV reference strains using the neighbor-joining method (Mega10.1.6).
The spike protein is the coronavirus structural proteins, which plays an important role on pathogenicity in coronaviruses [6], [50]. However, replacing the S gene of Beaudette strain by the virulent strains does not make it virulent [51], [52]. The S protein can be cleaved into S1 and S2 subunits at the S1/S2 cleavage site [53], the S1 subunit is responsible for receptor binding to the host cells [54]. Whether the S1 gene has an effect on the virulence of the virus is unknown. In this study, the recombinant strain, rH120-S1/YZ, expressed the S1 gene of the QX-like strain, which retains the S1/S2 cleavage site of H120, maintained the ability to replicate and exhibited pathogenicity in embryos. However, providing IBYZ S1 sequences to H120 did not increase its virulence to that of rIBYZ. IBVs replicate in many epithelial cells, including respiratory tissues, the alimentary canal, kidney, gonads, and bursa [2], [6]. The QX-like rIBYZ strain is capable of considerable growth in the trachea, lung, and kidney, which leads to severe tissue damage. In addition, this strain caused cilia necrosis and loss, lung congestion and hemorrhage, nephritis, as well as inflammatory cell infiltration in these tissues in infected chickens at different time points post-infection. The ability of IBV to replicate within many respiratory, enteric, and other epithelial surfaces may be partially related to the fact that the attachment of IBV to host cells is dependent on sialic acid on the cell surface being recognized by the S1 subunit [6], [54], [55]. However, the binding of virus to the host cell is the first step in determining tropism and S2 is responsible for membrane fusion [56]. Thus, to further explore the different tissue tropism between rH120-S1/YZ and rIBYZ, we examined the viral loads and histopathology in the tissues of the rH120-S1/YZ-infected group. It was found that rH120-S1/YZ remains moderately pathogenic in the trachea (e.g., some cilia necrosis and loss and decreased secretory cells) but no lesions were observed in the lungs and kidneys of infected chickens, in contrast to chickens infected with the rIBYZ strain. This finding suggests that both the S2 and S1 genes might play an important role in viral tissue tropism as also suggested by others [57]. Another possibility is that the lack of viral replication and damage in kidney following rH120-S1/YZ is due to lower replication ability of backbone except S2 gene from its parental rH120 strain. The viral load of the respiratory-type strain, rH120 in the different tissues indicated that the viral levels in the trachea, early following infection were significantly higher than those of rIBYZ, which indicates that the ability of the virus to attached to trachea epithelial cells of the rH120 strain was stronger than that of the rIBYZ strain. However, during the later time points post-infection, the level of the rH120 strain was significantly lower than rIBYZ, which suggests that the virulence of IBV depends both on tissue invasion and viral replication ability, as well as other functions mediated by other viral proteins (e.g., non-structural, structural, and accessory proteins) [6], [29], [58], [59], [60]. The reduction of the viral load at 5 dpi in the group infected with the rIBYZ strain may be associated with the necrosis and shedding of the trachea epithelium. Moreover, the ability to replication in the different organs of chickens infected with rH120-S1/YZ as reflected by viral loads at multiple time points was lower than that exhibited in the chickens infected with the rIBYZ strain, which demonstrated that the S1 subunit was not the only determinant of viral tropism that was consistent with results by others [57], [61], [62], and the significant difference in viral load was related to differences in the viral backbone. However, when comparing the rH120-S1/YZ and rH120 strains which carry the same backbone, it was found that the viral RNA of rH120-S1/YZ in the lungs and kidneys was significantly lower than that of rH120, which may due to the replacement of the IBYZ S1 gene in the recombinant virus, whose structure on the surface contains some degree of changes, which we speculated that may affects the functionality of the S protein of coronaviruses. Although cryo-EM structure of IBV spike protein has been determined, the relation between structure and function of different IBV S1 genes is unknown. [63]. In addition to the growth in epithelial tissues observed during the early infection period, IBV can establish long-term persistent infections in chicken flocks via oral and cloacal shedding, which represents a substantial challenge to IBV control [1], [6]. In this study, we detected viral RNA in the oral and cloacal swabs isolated from infected chickens at 7, 14, 18, 22, and 26 dpi by real-time PCR. Compared with rH120 and rIBYZ, the viral load in both the oral and cloacal swabs of the rH120-S1/YZ group at different time points after infection was significantly decreased. However, while it has been reported that the persistent virus will be re-excreted at the point of lay [1], the persistence of rH120-S1/YZ strain in the egg-laying chickens needs to be further verified. In a word, due to its low virulence i presented in virus loading and lesion in different tissue, oral and cloacal shedding of host animals, rH120-S1/YZ can be considered a safe attenuated vaccine candidate for young chickens.
In addition to its role in tissue tropism, the S1 glycoprotein also has an important function in inducing a neutralizing antibody response [64], [65], and small differences in the S1 contribute to poor cross protectionby neutralization test [64], [66]. To evaluate the protective efficacy of this candidate vaccine, we selected the QX-like virulent rIBYZ strain to infect chickens at 14 dpv that were vaccinated with either the rH120-S1/YZ strain or rH120 strain. We found that little protection against rIBYZ virulent strain challenge in the rH120 vaccinated group was induced, with a 50% survival rate and severe clinical symptoms associated with IB. This finding could be explained by differences in the antigenicity between rH120 and rIBYZ. No death, clinical signs, or lesions were observed in the rH120-S1/YZ-vaccinated group, indicating that this strain could provide effective protection against rIBYZ challenge in young chickens. Since high humoral antibody titers can contribute to reduced viral replication in various organs and disease recovery [67], [68], the level of specific IgG antibodies remains an important standard for evaluating the immune response to an IBV vaccine. In the present study, the humoral antibody level of chickens vaccinated with the rH120-S1/YZ strain was observed to gradually increase compared to the non-vaccinated group. However, although the rH120 strain was able to induce similar levels of humoral antibodies, it provided poor protection against rIBYZ, suggesting that protection against IBV infection is not only with humoral immunity, but also with both mucosal immunity combined with the local tracheal and cellular immune response [69], [70], [71], [72]. Thus, the detection of mucosal and cellular immunity in rH120-S1/YZ-vaccinated animals requires further exploration. Nevertheless, some studies have shown that a peptide located near the amino terminal end of S2 can be recognized by neutralizing monoclonal antibodies [73], and the S2 domain also plays an important role in inducing protective immunity [28], [61], [74]. Because the S2 domain plays an important role in cell tropism [57], we also considered that expressing the S1 subunit would provide greater safety than the entire S gene, which could lead to further destruction in some organs of the vaccinated chickens; however, further experiments are required.
5. Conclusion
In conclusion, we constructed the recombinant strain, rH120-S1/YZ, which was based on the backbone of the H120 vaccine strain, and replaced the S1 gene with that of the QX-like nephropathogenic strain, ck/CH/IBYZ/2011, isolated from China using the reverse genetic system. The safety results showed that this recombinant strain was not lethal to one-day-old chicks and it had not gained the ability to tissue invasion or replication in the kidneys and lungs of infected animals. Moreover, the viral load of the chickens was significantly decreased compared with the rH120 and rIBYZ strains. Furthermore, rH120-S1/YZ was found to provide effective protection against challenge with the QX-like nephropathogenic strain in young chickens. The level of antibody production in the serum of the vaccinated chickens detected by ELISA continued to increase after 7 dpi. Thus, rH120-S1/YZ may be considered a potential live vaccine candidate for protection against Chinese QX-like nephropathogenic IBV infection.
6. Ethics statement
All of the animals were cared for in accordance with the animal ethics guidelines and approved protocols, and the experimental protocols were performed with the approval of the Animal Welfare and Ethical Censor Committee of the Poultry Institute, Chinese Academy of Agriculture Sciences.
CRediT authorship contribution statement
Yi Jiang: Conceptualization, Methodology, Software, Investigation, Validation, Visualization, Resources, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. Xu Cheng: Investigation, Data curation. Xiumei Zhao: Visualization, Investigation. Yan Yu: Investigation, Software. Mingyan Gao: Investigation. Sheng Zhou: Conceptualization, Validation, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank all members of the Poultry Institute, Chinese Academy of Agricultural Sciences (Yangzhou, China) for their contribution to this study.
Funding
This work was supported by the National Natural Science Foundations of China (31602091, 31101832, 31572524), the National Key Research and Development Program, China (2017YFD0500703), and Jiangsu Province Agricultural Science & Technology Support Program (BE2012369).
Contributors and authorship
Yi Jiang designed the study and conducted all the experiments, analysis and interpretation of the data, and wrote the manuscript. Sheng Zhou helped perform the recombinant IBV construction. Xu Cheng, Xiumei Zhao, Yan Yu, and Mingyan Gao participated in the whole animal experiment in different groups. Xu Cheng conducted the experiments involving viral shedding. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.01.001.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- 3.Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casais R., Dove B., Cavanagh D., Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003;77:9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 7.Lin S.Y., Chen H.W. Infectious bronchitis virus variants: molecular analysis and pathogenicity investigation. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusters J.G., Jager E.J., Niesters H.G., van der Zeijst B.A. Sequence evidence for RNA recombination in field isolates of avian coronavirus infectious bronchitis virus. Vaccine. 1990;8:605–608. doi: 10.1016/0264-410X(90)90018-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bande F., Arshad S.S., Bejo M.H., Moeini H., Omar A.R. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J Immunol Res. 2015;2015:424860. doi: 10.1155/2015/424860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H., Shao Y., Sun C., Han Z., Liu X., Guo H. Genetic diversity of avian infectious bronchitis coronavirus in recent years in China. Avian Dis. 2012;56:15–28. doi: 10.1637/9804-052011-Reg.1. [DOI] [PubMed] [Google Scholar]
- 12.Feng K., Xue Y., Wang F., Chen F., Shu D., Xie Q. Analysis of S1 gene of avian infectious bronchitis virus isolated in southern China during 2011–2012. Virus Genes. 2014;49:292–303. doi: 10.1007/s11262-014-1097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou N.L., Zhao F.F., Wang Y.P., Liu P., Cao S.J., Wen X.T. Genetic analysis revealed LX4 genotype strains of avian infectious bronchitis virus became predominant in recent years in Sichuan area, China. Virus Genes. 2010;41:202–209. doi: 10.1007/s11262-010-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meeusen EN, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. Clin Microbiol Rev. 2007;20:489–510, table of contents. [DOI] [PMC free article] [PubMed]
- 15.Box P.G., Beresford A.V., Roberts B. Protection of laying hens against infectious bronchitis with inactivated emulsion vaccines. Vet Rec. 1980;106:264–268. doi: 10.1136/vr.106.12.264. [DOI] [PubMed] [Google Scholar]
- 16.McDougall J.S. Avian infectious bronchitis: the protection afforded by an inactivated virus vaccine. Vet Rec. 1969;85:378–381. doi: 10.1136/vr.85.14.378. [DOI] [PubMed] [Google Scholar]
- 17.Martins N.R., Mockett A.P., Barrett A.D., Cook J.K. IgM responses in chicken serum to live and inactivated infectious bronchitis virus vaccines. Avian Dis. 1991;35:470–475. [PubMed] [Google Scholar]
- 18.Liu S., Zhang X., Wang Y., Li C., Han Z., Shao Y. Molecular characterization and pathogenicity of infectious bronchitis coronaviruses: complicated evolution and epidemiology in china caused by cocirculation of multiple types of infectious bronchitis coronaviruses. Intervirology. 2009;52:223–234. doi: 10.1159/000227134. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y., Liu X.Y., Cheng J.L., Wu Y.P., Zhang G.Z. Molecular characterization of an infectious bronchitis virus strain isolated from northern China in 2012. Arch Virol. 2014;159:3457–3461. doi: 10.1007/s00705-014-2213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y., Cheng J.L., Liu X.Y., Zhao J., Hu Y.X., Zhang G.Z. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet Microbiol. 2015;180:49–58. doi: 10.1016/j.vetmic.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan S., Zhao J., Xie D., Huang X., Cheng J., Guo Y. Attenuation, safety, and efficacy of a QX-like infectious bronchitis virus serotype vaccine. Vaccine. 2018;36:1880–1886. doi: 10.1016/j.vaccine.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 22.Xia J., He X., Du L.J., Liu Y.Y., You G.J., Li S.Y. Preparation and protective efficacy of a chicken embryo kidney cell-attenuation GI-19/QX-like avian infectious bronchitis virus vaccine. Vaccine. 2018;36:4087–4094. doi: 10.1016/j.vaccine.2018.05.094. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Huang S., Zeng Y., Xue C., Cao Y. Rapid development and evaluation of a live-attenuated QX-like infectious bronchitis virus vaccine. Vaccine. 2018;36:4245–4254. doi: 10.1016/j.vaccine.2018.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geerligs H.J., Boelm G.J., Meinders C.A., Stuurman B.G., Symons J., Tarres-Call J. Efficacy and safety of an attenuated live QX-like infectious bronchitis virus strain as a vaccine for chickens. Avian Pathol. 2011;40:93–102. doi: 10.1080/03079457.2010.542742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarpey I., Orbell S.J., Britton P., Casais R., Hodgson T., Lin F. Safety and efficacy of an infectious bronchitis virus used for chicken embryo vaccination. Vaccine. 2006;24:6830–6838. doi: 10.1016/j.vaccine.2006.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakenell P.S., Sharma J.M., Slocombe R.F. Embryo vaccination of chickens with infectious bronchitis virus: histologic and ultrastructural lesion response and immunologic response to vaccination. Avian Dis. 1995;39:752–765. [PubMed] [Google Scholar]
- 27.Lee S.W., Markham P.F., Coppo M.J., Legione A.R., Markham J.F., Noormohammadi A.H. Attenuated vaccines can recombine to form virulent field viruses. Science. 2012;337:188. doi: 10.1126/science.1217134. [DOI] [PubMed] [Google Scholar]
- 28.Ellis S., Keep S., Britton P., de Wit S., Bickerton E., Vervelde L. Recombinant infectious bronchitis viruses expressing chimeric spike glycoproteins induce partial protective immunity against homologous challenge despite limited replication in vivo. J Virol. 2018;92 doi: 10.1128/JVI.01473-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Beurden S.J., Berends A.J., Kramer-Kuhl A., Spekreijse D., Chenard G., Philipp H.C. Recombinant live attenuated avian coronavirus vaccines with deletions in the accessory genes 3ab and/or 5ab protect against infectious bronchitis in chickens. Vaccine. 2018;36:1085–1092. doi: 10.1016/j.vaccine.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bickerton E., Dowgier G., Britton P. Recombinant infectious bronchitis viruses expressing heterologous S1 subunits: potential for a new generation of vaccines that replicate in Vero cells. J Gen Virol. 2018;99:1681–1685. doi: 10.1099/jgv.0.001167. [DOI] [PubMed] [Google Scholar]
- 31.Casais R., Thiel V., Siddell S.G., Cavanagh D., Britton P. Reverse genetics system for the avian coronavirus infectious bronchitis virus. J Virol. 2001;75:12359–12369. doi: 10.1128/JVI.75.24.12359-12369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y.S., Zhang Y., Wang H.N., Fan W.Q., Yang X., Zhang A.Y. Establishment of reverse genetics system for infectious bronchitis virus attenuated vaccine strain H120. Vet Microbiol. 2013;162:53–61. doi: 10.1016/j.vetmic.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bentley K., Armesto M., Britton P. Infectious bronchitis virus as a vector for the expression of heterologous genes. PLoS ONE. 2013;8:e67875. doi: 10.1371/journal.pone.0067875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ignjatovic J., Galli L. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou S.D.Y., Tang M.J., Liu M., Cheng X., Lv X.J. Construction of an infectious full-length cDNA clone of infectious bronchitis virus H120 vaccine strain. China Poultry. 2010;32(23):22–26. [Google Scholar]
- 36.Zhou S.T.M., Dai Y.B., Liu M., Zhao B.H., Cheng X., Lv X.J. Expression of green fluorescent protein using an infectious cDNA clone of infectious bronchitis virus. Chinese J Virol. 2011;27(1):11–17. [PubMed] [Google Scholar]
- 37.Reed L.J.M.H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27(3):493–497. [Google Scholar]
- 38.Cavanagh D., Elus M.M., Cook J.K. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 1997;26:63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- 39.Liu S., Chen J., Han Z., Zhang Q., Shao Y., Kong X. Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol. 2006;35:394–399. doi: 10.1080/03079450600920984. [DOI] [PubMed] [Google Scholar]
- 40.Gelb J., Jr., Weisman Y., Ladman B.S., Meir R. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000) Avian Pathol. 2005;34:194–203. doi: 10.1080/03079450500096539. [DOI] [PubMed] [Google Scholar]
- 41.Chen L., Zhang T., Han Z., Liang S., Xu Y., Xu Q. Molecular and antigenic characteristics of Massachusetts genotype infectious bronchitis coronavirus in China. Vet Microbiol. 2015;181:241–251. doi: 10.1016/j.vetmic.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia J., He X., Yao K.C., Du L.J., Liu P., Yan Q.G. Phylogenetic and antigenic analysis of avian infectious bronchitis virus in southwestern China, 2012–2016. Infect Genet Evol. 2016;45:11–19. doi: 10.1016/j.meegid.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Z., Sun C., Yan B., Zhang X., Wang Y., Li C. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infect Genet Evol. 2011;11:190–200. doi: 10.1016/j.meegid.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackwood M.W., Hilt D.A., Lee C.W., Kwon H.M., Callison S.A., Moore K.M. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Dis. 2005;49:614–618. doi: 10.1637/7389-052905R.1. [DOI] [PubMed] [Google Scholar]
- 45.Feng K., Xue Y., Wang J., Chen W., Chen F., Bi Y. Development and efficacy of a novel live-attenuated QX-like nephropathogenic infectious bronchitis virus vaccine in China. Vaccine. 2015;33:1113–1120. doi: 10.1016/j.vaccine.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKinley E.T., Hilt D.A., Jackwood M.W. Avian coronavirus infectious bronchitis attenuated live vaccines undergo selection of subpopulations and mutations following vaccination. Vaccine. 2008;26:1274–1284. doi: 10.1016/j.vaccine.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H.J., Youn H.N., Kwon J.S., Lee Y.J., Kim J.H., Lee J.B. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine. 2010;28:2887–2894. doi: 10.1016/j.vaccine.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masters P.S., Rottier P.J. Coronavirus reverse genetics by targeted RNA recombination. Curr Top Microbiol Immunol. 2005;287:133–159. doi: 10.1007/3-540-26765-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei YQ, Guo HC, Wang HM, Sun DH, Han SC, Sun SQ. [Rescue of the recombinant infectious bronchitis virus with the ectodomain region of H120 spike glycoprotein]. Bing Du Xue Bao. 2014;30:668–74. [PubMed]
- 50.Cavanagh D., Davis P.J., Pappin D.J. Coronavirus IBV glycopolypeptides: locational studies using proteases and saponin, a membrane permeabilizer. Virus Res. 1986;4:145–156. doi: 10.1016/0168-1702(86)90038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Britton P., Armesto M., Cavanagh D., Keep S. Modification of the avian coronavirus infectious bronchitis virus for vaccine development. Bioeng Bugs. 2012;3:114–119. doi: 10.4161/bbug.18983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodgson T., Casais R., Dove B., Britton P., Cavanagh D. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J Virol. 2004;78:13804–13811. doi: 10.1128/JVI.78.24.13804-13811.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavanagh D., Davis P.J., Pappin D.J., Binns M.M., Boursnell M.E., Brown T.D. Coronavirus IBV: partial amino terminal sequencing of spike polypeptide S2 identifies the sequence Arg-Arg-Phe-Arg-Arg at the cleavage site of the spike precursor propolypeptide of IBV strains Beaudette and M41. Virus Res. 1986;4:133–143. doi: 10.1016/0168-1702(86)90037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahwan K., Hesse M., Mork A.K., Herrler G., Winter C. Sialic acid binding properties of soluble coronavirus spike (S1) proteins: differences between infectious bronchitis virus and transmissible gastroenteritis virus. Viruses. 2013;5:1924–1933. doi: 10.3390/v5081924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wickramasinghe I.N., van Beurden S.J., Weerts E.A., Verheije M.H. The avian coronavirus spike protein. Virus Res. 2014;194:37–48. doi: 10.1016/j.virusres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wickramasinghe I.N., de Vries R.P., Grone A., de Haan C.A., Verheije M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J Virol. 2011;85:8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Promkuntod N., Wickramasinghe I.N., de Vrieze G., Grone A., Verheije M.H. Contributions of the S2 spike ectodomain to attachment and host range of infectious bronchitis virus. Virus Res. 2013;177:127–137. doi: 10.1016/j.virusres.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laconi A., van Beurden S.J., Berends A.J., Kramer-Kuhl A., Jansen C.A., Spekreijse D. Deletion of accessory genes 3a, 3b, 5a or 5b from avian coronavirus infectious bronchitis virus induces an attenuated phenotype both in vitro and in vivo. J Gen Virol. 2018 doi: 10.1099/jgv.0.001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruch T.R., Machamer C.E. The hydrophobic domain of infectious bronchitis virus E protein alters the host secretory pathway and is important for release of infectious virus. J Virol. 2011;85:675–685. doi: 10.1128/JVI.01570-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armesto M., Cavanagh D., Britton P. The replicase gene of avian coronavirus infectious bronchitis virus is a determinant of pathogenicity. PLoS ONE. 2009;4:e7384. doi: 10.1371/journal.pone.0007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eldemery F., Joiner K.S., Toro H., van Santen V.L. Protection against infectious bronchitis virus by spike ectodomain subunit vaccine. Vaccine. 2017;35:5864–5871. doi: 10.1016/j.vaccine.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shan D., Fang S., Han Z., Ai H., Zhao W., Chen Y. Effects of hypervariable regions in spike protein on pathogenicity, tropism, and serotypes of infectious bronchitis virus. Virus Res. 2018;250:104–113. doi: 10.1016/j.virusres.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shang J., Zheng Y., Yang Y., Liu C., Geng Q., Luo C. Cryo-EM structure of infectious bronchitis coronavirus spike protein reveals structural and functional evolution of coronavirus spike proteins. PLoS Pathog. 2018;14:e1007009. doi: 10.1371/journal.ppat.1007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cavanagh D., Davis P.J., Mockett A.P. Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Res. 1988;11:141–150. doi: 10.1016/0168-1702(88)90039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore K.M., Jackwood M.W., Hilt D.A. Identification of amino acids involved in a serotype and neutralization specific epitope within the s1 subunit of avian infectious bronchitis virus. Arch Virol. 1997;142:2249–2256. doi: 10.1007/s007050050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kant A., Koch G., van Roozelaar D.J., Kusters J.G., Poelwijk F.A., van der Zeijst B.A. Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J Gen Virol. 1992;73(Pt 3):591–596. doi: 10.1099/0022-1317-73-3-591. [DOI] [PubMed] [Google Scholar]
- 67.Alexander D.J., Gough R.E. A long-term study of the pathogenesis of infection of fowls with three strains of avian infectious bronchitis virus. Res Vet Sci. 1978;24:228–233. [PubMed] [Google Scholar]
- 68.Thompson G., Mohammed H., Bauman B., Naqi S. Systemic and local antibody responses to infectious bronchitis virus in chickens inoculated with infectious bursal disease virus and control chickens. Avian Dis. 1997;41:519–527. [PubMed] [Google Scholar]
- 69.Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhinakar Raj G., Jones R.C. Protectotypic differentiation of avian infectious bronchitis viruses using an in vitro challenge model. Vet Microbiol. 1996;53:239–252. doi: 10.1016/s0378-1135(96)01258-8. [DOI] [PubMed] [Google Scholar]
- 71.Kotani T., Wada S., Tsukamoto Y., Kuwamura M., Yamate J., Sakuma S. Kinetics of lymphocytic subsets in chicken tracheal lesions infected with infectious bronchitis virus. J Vet Med Sci. 2000;62:397–401. doi: 10.1292/jvms.62.397. [DOI] [PubMed] [Google Scholar]
- 72.Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev Comp Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 73.Kusters J.G., Jager E.J., Lenstra J.A., Koch G., Posthumus W.P., Meloen R.H. Analysis of an immunodominant region of infectious bronchitis virus. J Immunol. 1989;143:2692–2698. [PubMed] [Google Scholar]
- 74.Ignjatovic J., Sapats S. Identification of previously unknown antigenic epitopes on the S and N proteins of avian infectious bronchitis virus. Arch Virol. 2005;150:1813–1831. doi: 10.1007/s00705-005-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.