Abstract
The induction of antigen specific memory CD8+ T cells in vivo is very important to new vaccines against infectious diseases. In the present study, we aimed to evaluate the immune responses of peptide-specific CD8+ T cells induced by HLA-A*0201 restricted severe acute respiratory syndrome-associated coronavirus (SARS-CoV) S epitopes plus CpG oligodeoxynucleotide (CpG ODN), PolyI:C and R848 as adjuvants. Furthermore, the generation, distribution and phenotype of long-lasting peptide-specific memory CD8+ T cells were assessed by ELISA, ELISPOT and flow cytometry. Our results showed that antigen specific CD8+ T cells were elicited by HLA-A*0201 restricted SARS-CoV S epitopes. Furthermore, the frequency of peptide-specific CD8+ T cells was dramatically increased after both prime and boost immunization with peptides plus CpG ODN, whereas slight enhancements were induced following boost vaccination with peptides plus PolyI:C or R848. SARS-CoV S peptide-specific IFN-γ+CD8+ T cells were distributed throughout the lymphoid and non-lymphoid tissues. Results also demonstrated that the HLA-A*0201 restricted peptide-specific CD8+ T cells induced by peptides plus CpG ODN carried a memory cell phenotype with CD45RB+ and CD62L− and possessed long-term survival ability in vivo. Taken together, our results implied that HLA-A*0201 restricted SARS-CoV S epitopes plus CpG ODN might be the superior candidates for SARS vaccine.
Abbreviations: SARS, severe acute respiratory syndrome; SARS-CoV, SARS-associated coronavirus; CpG ODN, CpG oligodeoxynucleotides; TLR, toll-like receptor
Keywords: SARS-CoV, Epitope, Adjuvant, T cell, Vaccine
1. Introduction
Severe acute respiratory syndrome (SARS) is a life-threatening infectious disease, caused by a novel coronavirus named as SARS-CoV [1], [2]. Although the first SARS epidemic has been successfully controlled, it still remains a potential threat to human beings for the variability of SARS-CoV [3], the possibility of reintroduction of SARS-CoV into humans from animals [4] and the risk of an escape from laboratories [5]. Furthermore, some evidence proved that the humoral immune responses in SARS recovered patients decreased in a time-dependent manner [6], [7], and our previous results showed that cellular immune responses attenuated in recovered individuals [8]. To date, there is no effective drug to prevent or treat SARS.
As all know, vaccines are the most economic and effective strategies to protect bodies against infectious diseases. Recently, epitope vaccines which mainly contain the protective epitopes without pathological peptides have been considered as alternative approaches [9], [10], [11], [12]. With regard to SARS-CoV, there have been many T cell epitopes identified by us and other groups [13], [14], [15], [16], [17], [18]. In our previous study, we have identified five epitopes for CD8+ T cells in HLA-A*0201 transgenic mouse by “DNA prime, peptide boost” strategy [13]. However, their poor immunogenicity emphasizes a major unmet need for immunostimulatory adjuvants to safely boost cellular immune responses. Vaccinations with adjuvants that mimic toll-like receptors (TLR) ligands are advantageous as they are capable of eliciting positive effects across the entire spectrum of innate and adaptive immunity. Our previous data has demonstrated the additive effects of CpG oligodeoxynucleotide (CpG ODN), R848 and PIKA as adjuvants on augmenting immune responses to HBsAg vaccination [19], [20]. CpG, PolyI:C and R848 which are ligands for TLR9, TLR3 and TLR7 respectively can activate antigen-presenting cells and lead to T cell priming and acquired immunity.
In the present study, we evaluated the cellular immune responses induced by HLA-A*0201 restricted SARS-CoV S epitopes and examined whether TLR ligands CpG, PolyI:C and R848 as adjuvants lead to a further enhancement of CD8+ T cell activation to HLA-A*0201 restricted SARS S peptides. Our results demonstrated that antigen specific CD8+ T cells were elicited by HLA-A*0201 restricted SARS-CoV S epitopes. For the first time, we found that CpG, PolyI:C and R848 were different in their ability to prime naïve CD8+ T cells or to boost memory CD8+ T cells. The data provided evidence that efficiency of CTL production in mice after vaccination with HLA-A*0201 restricted SARS S peptides plus CpG was obviously enhanced. Furthermore, the majority of the long-lasting CD8+ T cells specific for SARS S protein in vivo were effector memory phenotype.
2. Materials and methods
2.1. Mice
HLA-A*0201/Kb transgenic (Tg) mice were a gift from Prof. Y.Z. Wu (Institute of Immunology, Third Military Medical University, Chongqing, China). Tg mice were bred in a pathogen-free facility. Cell surface HLA-A*0201 expression was assessed by flow cytometry using FITC-labeled anti-human HLA-A2 Ab (BD Biosciences PharMingen). For experimental purposes, six to eight week-old female mice were used. All experiments were performed according to the guidelines in the Institutional Animal Committee of TMMU.
2.2. Adjuvants and synthesis of HLA-A*0201 restricted peptides
CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was synthesized by Sangon Crop. (Shanghai, China). PolyI:C was purchased from Sigma (USA) and R-848 was a kind gift from 3M Pharmaceutical Corps (St. Paul, USA). The HLA-A*0201 restricted peptides derived from the BJ01 strain of SARS-CoV (GenBank accession no. AY278488) were synthesized by Sangon Crop. (Shanghai, China). These five peptides were named as P19 (S1042-1050 VVFLHVTYV), P20 (S787-795 ILPDPLKPT), P21 (S1167-1175 RLNEVAKNL), P22 (S958-966 VLNDILSRL) and P23 (S411-420 KLPDDFMGCV) were identified by us and other groups before [13], [14], [16], [17], [18]. Peptides were purified to >98% by reverse phase HPLC, as confirmed by mass spectrometry, which were dissolved in PBS at 2 mg/ml and stored in aliquots at −80 °C.
2.3. Immunization
Pooled HLA-A*0201 restricted peptides (P19-23, 20 μg of each) were injected subcutaneously either alone or in a mixture with soluble CpG (25 μg), PolyI:C (5 μg) and R848 (50 nmol) respectively into the base of the tail of HLA-A*0201/Kb Tg mice. One week later, mice were boosted once with the same regimen. PBS was administrated alone as control. Three mice were used in each group.
2.4. Media and antibodies
RPMI-1640 media was purchased from GIBCO and supplemented with 10% heat-inactivated FCS, 0.1% 2-ME, 100 U/ml penicillin and 100 μg/ml streptomycin. Purified anti-mouse CD28, anti-CD8-FITC, anti-CD8-PE, anti-CD8-PerCP, anti-IFN-γ-APC, anti-IFN-γ-PE, anti-TNF-α-APC, anti-CD62L-FITC, anti-CD45RB-FITC and isotype control Abs were purchased from BD Biosciences PharMingen.
2.5. Cell culture and measurement of cytokine by ELISA
Mice were sacrificed. Lymph nodes (popliteal fossa, groin and mesentery), spleens and lungs from individual mouse were harvested 7–10 d or 1 month after the boost vaccination. Single-cell suspensions were prepared and plated in triplicate in 96-well round bottom plates at 4 × 106 cells/200 μl per well. Peptides19–23; 2 μg/ml at the final concentration plus anti-CD28 (1 μ/ml) was added to cultures. In each experiment, cultures in the absence of peptides were as negative controls. Three days after incubation, cell-free culture supernatants were collected and levels of IFN-γ were detected by specific ELISA kit (BD PharMingen) according to the manufacturer's protocol.
2.6. ELISPOT assay
Assay was performed using mouse ELISPOT set (BD PharMingen) according to the instruction provided by the manufacture. Briefly, spleen single-cell suspensions from vaccinated mice were prepared and plated in 96-well micro plates (Millipore) pre-coated with anti-IFN-γ antibody overnight at 4 °C and blocked by RPMI-1640 containing 10% FBS. Cells were incubated for 24 h in the presence or absence of peptides19–23 plus anti-CD28 at 37 °C and 5% CO2. Wells were washed and incubated with biotinylated labeled anti-mouse IFN-γ antibody for 2 h at room temperature. After washing, wells were incubated with streptavidin conjugated horseradish peroxidase for 1 h at room temperature. Wells were extensively washed again, and developed with 3-amino-9-ethyl-carbazole (AEC) substrate solutions. After drying, wells were counted by TCL ELISPOT reader (USA).
2.7. Intracellular cytokine and cell surface staining
Single-cell suspensions from lymph nodes, spleens and lungs of vaccinated mice were co-cultured with or without peptides19–23 plus anti-CD28 for 6 h at 37 °C and 5% CO2. Brefeldin A (10 μg/ml, Sigma) was added in the last 5 h incubation. Cells were washed, fixed with 4% paraformaldehyde and permeabilized in PBS buffer containing 0.1% saponin (Sigma), 0.1% BSA and 0.05% NaN3 overnight at 4 °C. Then cells were stained with conjugated mAbs for surface markers (CD4, CD8, CD62L, CD107a/b and CD45RB) and intracellular cytokine IFN-γ for 20 min at 4 °C in dark. Cells were acquired on flow cytometer (Calibur, BD) and data were analyzed with the program FlowJo version 6.0 (Tree Star Inc., USA). Isotype controls were included in each staining.
2.8. Statistics
Statistical evaluation of differences between means of experimental groups was performed by analysis of variance and a non-parametric two-tailed t-test. A value of P < 0.05 was considered to be significant.
3. Results
3.1. The expression of IFN-γ from HLA-A*0201 restricted peptide-specific CD8+ T cells was enhanced in varying degrees by CpG ODN, PolyI:C and R848
To assess the cellular immune responses elicited by vaccination with HLA-A*0201 restricted SARS S peptides in combinations with CpG ODN, PolyI:C and R848 respectively, HLA-A*0201/Kb Tg mice were randomly divided into five groups to accept immunizations. 7–10 d after prime and boost vaccination, mice was sacrificed and cells were prepared from lymph nodes, spleens and lungs. The cells were stimulated with or without cognate peptides plus anti-CD28 to detect HLA-A*0201 restricted peptide-specific immune responses. The levels of IFN-γ in the cell-free culture supernatants were determined by ELISA. The results revealed that IFN-γ was almost undetectable when lymphocytes were cultured in medium alone and in PBS groups. Following the prime immunization, there was only modest IFN-γ production in spleen and lymph node lymphocytes from peptides alone immunized mice (P < 0.01). However, the production of IFN-γ was markedly enhanced in all of the organs from peptides plus CpG ODN immunized mice (spleen and lymph node P < 0.001, lung P < 0.01). However, only CpG ODN but not PolyI:C and R848 promoted IFN-γ production after the first vaccination. As shown in Fig. 1 , the diamond symbols represented the levels of IFN-γ from lymphocytes in boosted mice. Interestingly, the production of IFN-γ in spleen from boosted mice was much stronger than the one in primed mice. In addition, there was modest increase of IFN-γ in spleen from peptides plus PolyI:C or R848 boosted mice though the level of IFN-γ in lymph nodes and lungs was almost undetectable. Taken together, these data indicated that systemic striking cytokine production was induced in Tg mice following HLA-A*0201 restricted peptides plus CpG ODN vaccination. Furthermore, the results provided evidence that CpG ODN, PolyI:C and R848 which were potent activators of innate immune responses maybe differ in their ability to prime naïve CD8+ T cells or to induce memory CD8+ T cells.
Fig. 1.
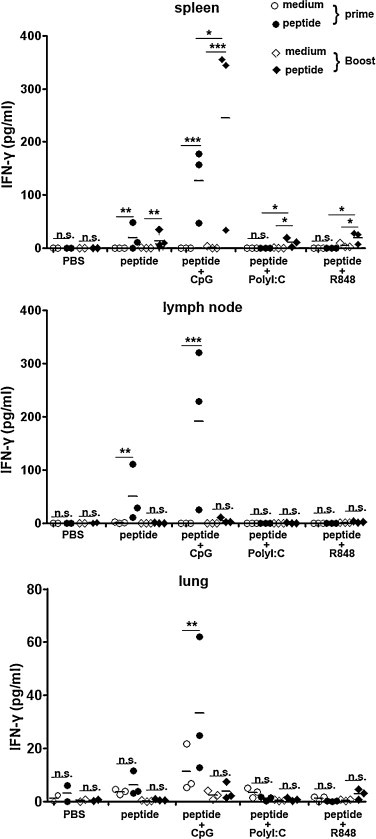
The expression of IFN-γ after prime and boost immunization with peptides plus different adjuvants. HLA-A*0201 Tg mice were vaccinated with different agents in a similar manner as described in the materials and methods. Fresh isolated lymphocytes from spleens, lymph nodes and lungs were cultured with 2 μg/ml cognate peptides plus 1 μg/ml anti-CD28 and in medium alone as controls. The production of IFN-γ in supernatants for the prime and boost immunization. Cultures were set up in triplicate. Each symbol represents one data point from one mouse. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., P > 0.05.
3.2. Frequency of IFN-γ-producing cells from immunized mice following stimulation with HLA-A*0201 restricted peptides
We have detected the production of IFN-γ in total lymphocytes by ELISA. Furthermore, to confirm the distinct ability of CpG ODN, PolyI:C and R848 in the experiment, we next analyzed the frequencies of IFN-γ-producing cells at the single cell level by ELISPOT assay. As shown in Fig. 2 , 7–10 d after prime immunization with HLA-A*0201 restricted peptides alone and peptides plus CpG ODN, cells from spleens produced at an average of 18 spots in 2 × 105 cells and 79 spots in 2 × 105 cells, respectively. The antigen specific cells exhibited 4-fold increase in the presence of CpG ODN as adjuvant. However, there was almost no IFN-γ-producing cells induced in peptides plus PolyI:C or R848 groups. Compared with the prime vaccination, the frequency of IFN-γ-producing cells in spleen from boost immunized mice was dramatically increased to 155 spots per 2 × 105 cells in peptides plus CpG ODN group. Meanwhile, 33 spots and 28 spots per 2 × 105 cells were showed respectively in peptides plus PolyI:C group and peptides plus R848 group. These results were consistent with the data from ELISA and further confirmed the effects of CpG ODN, PolyI:C and R848 as adjuvants in improving the cellular immune responses.
Fig. 2.
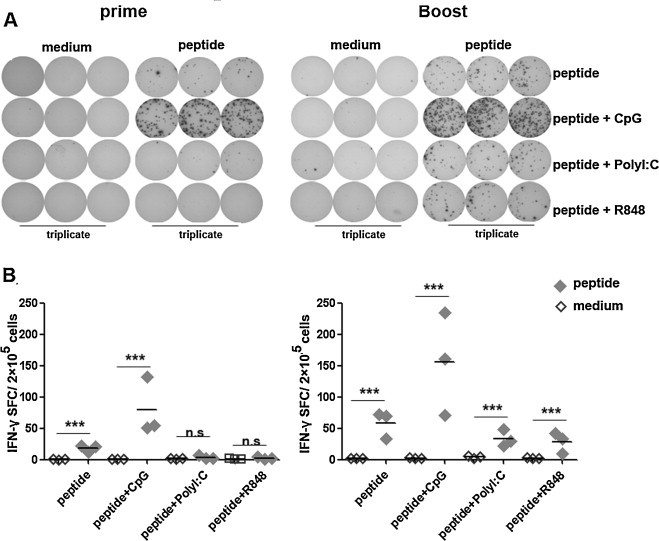
Frequency of HLA-A*0201 restricted peptide-specific IFN-γ producing cells. Splenocytes from HLA-A*0201 Tg mice immunized with peptides plus different adjuvants were cultured at a density of 2 × 105 cells/well in 96 well pre-coated plates. (A) Representation of ELISPOT results. (B) The frequency of IFN-γ producing cell. Cultures were set up in triplicate. Each symbol represents one data point from one mouse. Horizontal bars represent mean values. ***P < 0.001; n.s., P > 0.05.
3.3. The correlation of cytokines and distribution of CTLs induced in different organs
In our previous studies, we have shown that peptide-specific IFN-γ-producing cells induced by peptides19–23 belonged to CD8+ T cells but not CD4+ T cells. Peptide19-23 as HLA-A*0201 restricted peptides could act as stimulators to elicit a striking peptide-specific CTL response [13]. In the present study, we still found that there was no cytokine production in CD4+ T cells following cognate peptides stimulation, which were consistent with the previous results (data not shown). As shown in Fig. 3 , CD8+ T cells were gated after the prime immunization and the production of TNF-α and IFN-γ were detected simultaneously. Results showed that after the first immunization, HLA-A*0201 restricted peptide-specific CTLs could produce IFN-γ and TNF-α at the same time both in peptides alone and peptides plus CpG ODN group (Fig. 3). Furthermore, the overall response obtained after coinjection of CpG ODN was consistently stronger than the CTL yield obtained when using peptides alone. Interestingly, following the boost immunization the percentages of cytokine positive CD8+ T cells from spleen were dramatically increased in peptides plus CpG ODN group (Fig. 4 ). In agreement with Fig. 3, the percentage of splenic peptide-specific CTLs elicited in peptide alone boosted mice was generally lower than the one in coinjection of CpG ODN group, but also showed about two-fold enhancement of the cytokine-producing CD8+ T cells when the mice were boosted. Compared with the first immunization, modest cytokine-positive CD8+ T cells were induced in spleen from peptides in combination with PolyI:C and R848 boosted mice, which conformed to the previous results from ELISA and ELISPOT (Fig. 4).
Fig. 3.
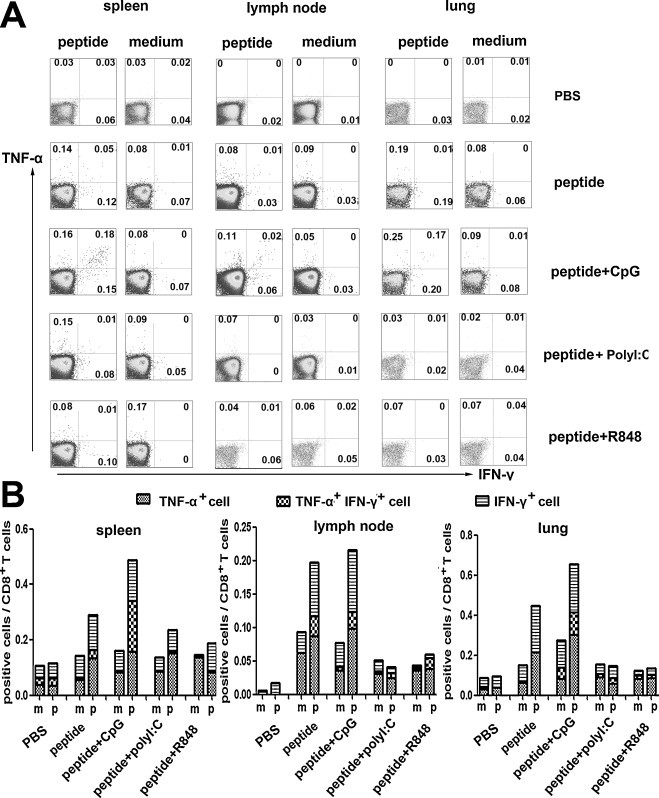
The correlation of cytokines produced by peptide-specific CTLs following the first immunization. Lymphocytes from different organs were cultured with or without peptides plus anti-CD28 for 6 h, and then were harvested and stained for FACS analysis. (A) Representative results from three independent experiments show the expressing of IFN-γ and TNF-α. The numbers in the corner represent the mean percentage of positive cells in gated CD8+ T cells. (B) The statistical analysis of the three independent experiments.
Fig. 4.
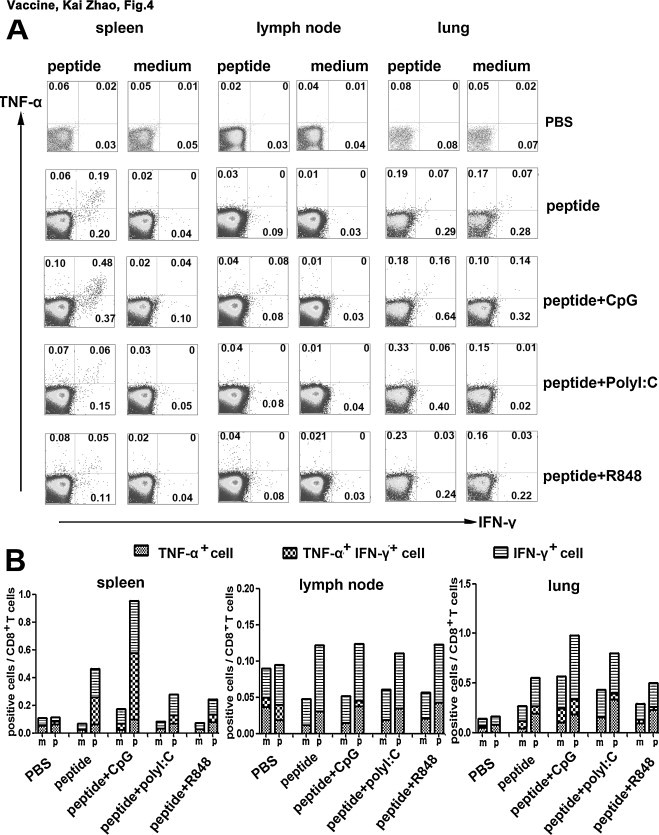
Following the boost immunization, the production of cytokines by peptide-specific CTLs. Tg mice were boosted with HLA-A*0201 restricted peptides plus different adjuvants. 10 d after the vaccination, splenocytes were harvested, stimulated and stained for FACS. Cells were initially gated at CD8+ T cells and positive cells were analyzed by flow cytometry. The expression of TNF-α and IFN-γ in HLA-A*0201 restricted peptide-specific CD8+ T cells were detected. (A) The results of one representative assay from three similar independent experiments are shown. The data in the corner of each panel represents the means of three independent experiments. (B) Summary of the independent experiments.
To further analysis of the relationship of IFN-γ and TNF-α producing CD8+ T cells, we found that most TNF-α+CD8+ T cells expressed IFN-γ simultaneously in both primed and boosted mice, and only small subsets of the cells expressed either IFN-γ or TNF-α alone (Fig. 3, Fig. 4). It implied that peptide-specific CTLs induced in this model had strong ability to secret various cytokines and may be polyfunctional lymphocytes. Besides, according to the expression of IFN-γ and TNF-α, peptide-specific CTLs could be divided into three distinct subpopulations, including IFN-γ+ TNF-α+ cells, IFN-γ+ cells and TNF-α+ cells in the same experiment (Fig. 3, Fig. 4). As shown above, the results from ELISA, ELISPOT and FACS, three different assays from distinct aspects, were coincident and demonstrated that CpG ODN which had significantly additive adjuvant effect could augment the cellular immune responses induced by HLA-A*0201 restricted peptides from SARS S protein.
To further ascertain whether the distributions of HLA-A*0201 restricted peptide-specific CD8+ T cells were distinct in different organs, we detected the percentages of both IFN-γ and TNF-α producing CD8+ T cells in lymph nodes, spleens and lungs. As shown in Fig. 3, Fig. 4, both IFN-γ+CD8+ T cells and TNF-α+CD8+ T cells could be induced in lymph tissues (lymph nodes, spleens) and in non-lymph tissue (lungs). It indicated that peptides plus adjuvants could induce systemic cellular immune responses, suggesting that the CTLs elicited in vivo may have effective function in protecting body against infection.
3.4. Phenotype of CTLs following immunization
To characterize the phenotype of HLA-A*0201 restricted peptide-specific CD8+ T cells on the basis of their ability to produce IFN-γ, splenocytes from peptides alone and peptides plus CpG ODN immunized Tg mice were stimulated with cognate peptides. After 6 h of incubation, cells were harvested, washed and stained for cell-surface markers, including CD45RB as a marker for memory cells in mice and CD62L as effector/central memory T subset marker. Cells were first gated on CD8+ T cells and the phenotype of IFN-γ+CD8+ T cells was analyzed. As shown in Fig. 5 , most of IFN-γ-producing CD8+ T cells from spleens expressed CD45RB in peptides alone and peptides plus CpG ODN groups. According to the expression of CD62L, approximately 54–80% IFN-γ+CD8+ T cells were CD62L− cells which represented the effector memory T cells and a variable fraction of IFN-γ+CD8+ T cells were CD62L+ cells that were considered as typical central memory CD8+ T cells. Thus, on the basis of IFN-γ producing cells, a population of peptide-specific CD8+ T cells that have characteristics of memory T cells has been identified in the present study. And the IFN-γ+CD8+ T cells with CD45RB+CD62L− might indeed belong to an intermediate subset ready to be conscripted into the effector population during reinfection.
Fig. 5.
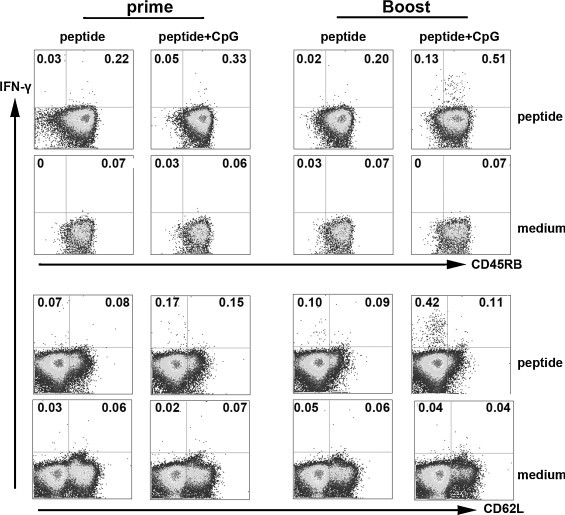
Phenotype of HLA-A*0201 restricted peptide-specific CD8+ T cells. Tg mice were primed or boosted with peptides alone or peptides plus CpG. The splenocytes from Tg mice 10 d after immunization were harvested, stimulated and stained with cell surface anti-CD45RB and anti-CD62L Abs, and then the intracellular cytokine IFN-γ was marked. Based on the expression of IFN-γ, characteristics of HLA-A*0201 restricted peptide-specific CD8+ T cells were analyzed by FACS. The expression of CD45RB and CD62L by IFN-γ+CD8+ T cells are shown. Representative results and the averages in the corner are shown from three independent experiments.
3.5. Long-lived memory CD8+ T cell responses following boost immunization
In the above experiments, we evaluated the peptide-specific CTLs immune responses 7–10 d after prime and boost vaccination. As we all know, antigen-specific memory CD8+ T cells played very important roles in clearing virus, which could be induced in vivo in a fast and specific manner. Here, we were interested in whether long survival memory CD8+ T cells could be elicited following peptides plus CpG ODN vaccination. Therefore, we extended our study to further characterize SARS S HLA-A*0201 restricted peptide-specific long-persistent memory T cell responses 30 d after the boost immunization. According to the data from ELISA and ELISPOT, we found that mice immunized with peptides in combination with CpG ODN still maintained high level of cellular immune responses in splenocytes though 30 d had passed since the second vaccination. As shown in Fig. 6 , splenocytes stimulated by specific peptides in vitro secreted higher level of IFN-γ than controls (P < 0.05). Based on the IFN-γ production, the frequency of peptide-specific CD8+ T cells were ∼58 spots in 2 × 105 cells. The results suggested that peptide-specific CD8+ T cells induced by peptides plus CpG ODN vaccination have a long-time life span in vivo. Compared with the previous data from 7–10 d after boost immunization, though the degree of cellular immune response decreased in part, we still believe that these peptide-specific memory CD8+ T cells long lived in vivo will be quickly conscripted into effector populations to protect body during reinfection.
Fig. 6.
Cellular immune responses were elicited by HLA-A*0201 restricted peptides 30 d after the boost vaccination. Lymphocytes were obtained from Tg mice boosted with peptides plus CpG 30 d later. (A) Expression of IFN-γ was detected by ELISA. (B) The frequency of IFN-γ-producing cells in spleens. (C) Representative results are shown from three independent experiments. Cultures were set up in triplicate. Each symbol represents one data point from one mouse. The bars represent the mean value. *P < 0.05; ***P < 0.001; n.s., P > 0.05.
3.6. The characterization of memory CD8+ T cell
Furthermore, we detected the percentages of cytokine producing persistent peptide-specific memory CD8+ T cells. The results showed that IFN-γ and TNF-α producing CD8+ T cells still could be detected in spleens, though 1 month has pasted since the boost vaccination. The percentage of IFN-γ+CD8+ T (mean value 0.14%) cells and TNF-α+CD8+ T cells (mean value 0.21%) from stimulated splenocytes were 2.3 fold and 10.5 fold higher than cells cultured in medium alone, respectively (Fig. 7 A). Besides, based on IFN-γ production the phenotype of these long-lived peptide-specific CD8+ T cells were characterized with surface marker CD45RB and CD62L. As shown in Fig. 7B, the majority of IFN-γ+CD8+ T cells were CD45RB+ and CD62L−, which carried the memory T cell phenotypes. Taken together, the results provide evidence that long-lasting peptide-specific memory CD8+ T cells can be induced by SARS S HLA-A*0201 restricted peptides plus CpG ODN and they have the ability to produce IFN-γ and TNF-α in the same time. In addition, these results are consistent with the previous reports and show that majority of the long-time survival CD8+ T cells specific for SARS S HLA-A*0201 restricted peptides in vivo are effector memory cells.
Fig. 7.
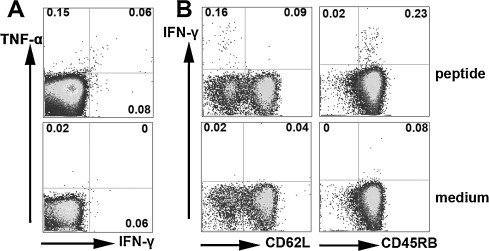
The percentage and phenotype of long-term lived HLA-A*0201 restricted peptide specific CD8+ T cells. Splenocytes were prepared 30 d after HLA-A*0201 restricted peptides plus CpG boost vaccination and were incubated with cognate peptides for 6 h. Then cells were stained with surface anti-CD8 Ab, anti-CD45RB Ab, anti-CD62L Ab, and intracellular cytokine Abs anti-IFN-γ and anti-TNF-α. CD8+ T cells were first gated. (A) The expression of IFN-γ and TNF-α by CD8+ T cells. (B) Based on IFN-γ production, the phenotype of peptide-specific CD8+ T cells. The numbers at the corner represent the mean percentages of positive cells from three independent experiments.
4. Discussion
In the previous study, we evaluated the cellular immune responses of HLA-A*0201 Tg mice which were primed intramuscularly with SARS S DNA and boosted subcutaneously with HLA-A*0201 restricted peptides. Our previous data showed that antigen specific memory CD8+ T cells were induced in the DNA prime and peptide boost strategy [13]. Considering the poor immunogenicity and stability of oligopeptide in vivo, we focused on the immune responses of peptide-specific CD8+ T cells induced by HLA-A*0201 restricted SARS-CoV S epitope vaccine. Interestingly, we found that antigen specific CD8+ T cells were elicited by HLA-A*0201 restricted SARS-CoV S epitopes which might possess stable chemical and physical properties in vivo and that with memory phenotype could exist for a long time at the absence of cognate peptides. These data provided credible evidence for the direct application of epitopes to SARS vaccine design.
Currently, the adjuvant which has been approved by FDA to be used clinically is alum. Although aluminum is able to induce a good Th2 response, it has weak capacity to stimulate strong cellular immune responses which are very important for protection against many pathogens [21]. An adjuvant which is usually used to enhance the immunogenicity of a vaccine will be required to promote not only humoral immune responses but also cellular immune responses. The molecular mechanism underlying adjuvant activity has been poorly understood for a long time, but this field has been rapidly evolving since the discovery of the TLR family of proteins and their corresponding innate ligands [22]. CpG ODN, PolyI:C and R848 are specific ligands for TLR which are the critical links between the innate and the adaptive immunity [23], [24], [25]. In our previous reports, we have studied that CpG ODN, R848 and PIKA as adjuvants could enhance HBs Ag-specific T cell responses [19], [20]. With regard to SARS, there was a new report proved that co-administration of inactivated SARS-CoV vaccine with PIKA as adjuvant could induce a much higher neutralizing activity in animal models [26]. However, there is no related report to ascertain which adjuvant will be superior in HLA-A*0201 restricted SARS S peptide vaccine. Therefore, in the present study we evaluated the efficiency of synthetic CpG ODN, PolyI:C and R848 as adjuvants in combination with CD8+ T cell epitope vaccine of SARS to immunize HLA-A*0201 Tg mice.
CpG ODN, PolyI:C and R848 as adjuvants are identified to be specific ligands for TLR which are potent activators of innate immune responses, activating dendritic cell (DC) maturation and inflammatory cytokine secretion, inducing an increase in the cross talk between the innate and adaptive immune systems, and thereby driving the expansion of CTLs that can destroy virus infected target cells. Our results showed that the CD8+ T cell responses to CpG ODN were superior to those of PolyI:C and R848, which may be due to several reasons. First, different TLRs or even different ligands for the same TLR can differ substantially in their ability to induce production of peptide-specific primary and memory CD8+ T cell responses. It has been reported that CpG ODN is a very potent inducer of IL-12, which directs differentiation of CD4+ T and CD8+ T cells into type I effectors [27]. McCluskie et al. compared the efficacy of CpG ODN and R-848 for topical immunoprophylaxis or immunotherapy of vaginal herpes simplex virus type 2 (HSV-2) infections in mice. They found that efficacy against HSV infection was observed with CpG ODN but less so with R-848, even after repeated administrations [28]. Furthermore, evidence from Rothenfusser et al. proved that two distinct types of CpG ODN have been identified that differ in their capacity to stimulate CD8+ T cells. CpG-B seems to be superior for priming CD8+ T cell responses and CpG-A selectively enhances memory CD8+ T cell responses [29]. As we know, CpG ODN as ligand for TLR9 can signal via MyD88-independent pathway to lead to the activation of NF-κB, a critical transcription factor for induction of inflammatory cytokines. In addition, TLR9 signaling can also induce type I interferon via IRF7 pathway. TLR3 recognizing PolyI:C uses TRIF- IRF3 pathway, while TLR7 recognizing R848 uses MyD88 but not TIRAP or TIRF. Therefore, it is clear that different TLR signaling profoundly affects the innate immune response which is related to the cascading activation of adaptive immune responses. Furthermore, there are differences in the responses of DC to different TLR ligands. TLR9 is expressed by both plasmacytoid and non-plasmacytoid DC, whether from spleen, thymus or grown in vitro from bone marrow progenitors, and respond to CpG [30]. Mouse spleen plasmacytoid DC also express TLR7, but especially striking is the fact that splenic CD8α+ DCs do not express TLR7 mRNA and do not respond to R848 in vitro [31]. In contrast, CD8α+ DCs display the highest expression of TLR3 message, which appears to be absent from plasmacytoid DC [30], [31]. However, the situation is somewhat different in human. Because human blood contains at least two distinct DC types, the CD11c+ DC and the plasmacytoid DC, as well as the monocyte precursors of Mo-DC [32]. If the present results are also the case in human, it is possible that use of CpG ODN with SARS epitope vaccine in human would prove superior to PolyI:C and R848. However, this would need to be further tested in clinical studies. Finally, detailed dose response analyses are also required to allow distinction between quantitative and qualitative effects of TLR ligation. According to the previous reports, the response was optimal when 25 μg of soluble CpG ODN was coinjected per animal, but it also decreased at higher doses. The same phenomenon was found in PolyI:C which optimal CTL responses were obtained at 5 μg [33]. Whether these declines occurred because of functional exhaustion of CD8+ T cells by overloading the system with antigen or not remains to be investigated.
There is evidence supported by previous findings that the presence of CpG ODN during CD8+ T cell expansion lead to increased CD56 expression which is reported to be associated with cytolytic activity of peptide-specific CD8+ T cells [29]. It suggests that CpG ODN maybe not only increase the frequency of peptide-specific CD8+ T cells but also affect their phenotype. Therefore, we further focused on the phenotype of peptide-specific memory CD8+ T cells and detected the expression of CD45RB as a surface marker for memory T cell in mice and the expression of CD62L, which is considered as a standard to distinguish central memory T cells from effector memory T cells. We detected the expression of CD45RB and CD62L on CD8+ T cells from mice immunized with peptides in the absence or presence of CpG ODN. On the basis of IFN-γ production, it showed that the percentage of peptide-specific CD8+ T cells was significantly enhanced by CpG ODN. Meanwhile, antigen-specific CD8+ T cells carried a phenotype of CD45RB+ and CD62L−. However, no significant difference was found in the expression of CD45RB and CD62L between the peptides alone and peptides plus CpG ODN group. These results indicate that CpG ODN increases the quantity of peptide-specific CD8+ T cells but does not affect the quality of the antigen specific memory CD8+ T cells.
In addition, a challenge for adjuvant usage is to develop safe adjuvants that can stimulate both antibodies and cell-mediated immunity. In the present study, no humoral immune response was observed because the peptides belong to HLA-A*0201 restricted antigen which can only stimulate CD8+ T cells. However, our previous data demonstrated that there were significantly large amounts of anti-HBs Abs in the sera obtained from the mice immunized with HBsAg in combinations with CpG ODN and R848 [19]. It suggests that they can not only augment cellular immune responses but also have remarkable effects on enhancing the humoral immune responses. The efficacy of these adjuvants inducing humoral immune responses in SARS S vaccine is also very important and undoubtedly should be paid more attention in the ongoing research plans.
As we know, the induction and survival of immune memory cells in vivo lay foundations for the development of vaccine. Excited to us, our data showed that peptide specific CD8+ T cells were long-lived memory cells in peptides combined with CpG immunized mice. Although the frequency and the percentage of antigen-specific CD8+ T cells were lightly decreased, there was considerable level of IFN-γ production (Fig. 6). Furthermore, following the cognate peptide stimulation in vitro, the long-lasting antigen-specific memory CD8+ T cells had the capacity to produce IFN-γ and TNF-α at the same time (Fig. 7). To say in other words, one of the hallmarks of successful vaccination is the induction of strong and persistent memory T cell responses [34]. Therefore, the results provided us a shining perspective to use it in clinical vaccine development.
In conclusion, CpG ODN can augment SARS S peptide specific CD8+ T cell immune responses. These antigen-specific CD8+ T cells which carry the memory T cell phenotype can exist for a long time in vivo. Vaccinations with adjuvants that mimic TLR ligands are advantageous as they are capable of eliciting positive effects across the entire spectrum of innate and adaptive immunity. Therefore, these results provide some novel information for the vaccine design and ongoing clinical trials will provide further insight into the activity of CpG ODN as immune adjuvant for epitope vaccine against SARS.
Acknowledgments
We thank Prof. Yuzhang Wu, Institute of Immunology, Third Military Medical University, Chongqing, China, for his kind present of HLA-A*0201/Kb transgenic mice. We also want to thank Dr. Richard Miller from 3M Pharmaceuticals Corps for his kindness in providing R-848. This work was supported by grants from National “863” Project (No. 2007AA02Z415).
Conflict of interest statement: The authors have no conflicts of interest.
References
- 1.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 2.Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epidemiology Consortium, CSM Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303(5664):1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 4.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 5.Orellana C. Laboratory-acquired SARS raises worries on biosafety. Lancet Infect Dis. 2004;4(2):64. doi: 10.1016/S1473-3099(04)00911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao W.C., Liu W., Zhang P.H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Fontanet A., Zhang P.H., Zhan L., Xin Z.T., Baril L. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193(6):792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y.Y., Huang Z.T., Li L., Wu M.H., Yu T., Koup R.A. Characterization of SARS-CoV-specific memory T cells from recovered individuals 4 years after infection. Arch Virol. 2009;154(7):1093–1099. doi: 10.1007/s00705-009-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herd K.A., Mahalingam S., Mackay I.M., Nissen M., Sloots T.P., Tindle R.W. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. J Virol. 2006;80(4):2034–2044. doi: 10.1128/JVI.80.4.2034-2044.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanabe S. Epitope peptides and immunotherapy. Curr Protein Pept Sci. 2007;8(1):109–118. doi: 10.2174/138920307779941569. [DOI] [PubMed] [Google Scholar]
- 11.Hanke T., Neumann V.C., Blanchard T.J., Sweeney P., Hill A.V., Smith G.L. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine. 1999;17(6):589–596. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- 12.Olsen A.W., Hansen P.R., Holm A., Andersen P. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur J Immunol. 2000;30(6):1724–1732. doi: 10.1002/1521-4141(200006)30:6<1724::AID-IMMU1724>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K., Yang B., Xu Y., Wu C. CD8(+) T cell response in HLA-A*0201 transgenic mice is elicited by epitopes from SARS-CoV S protein. Vaccine. 2010;(28):6666–6674. doi: 10.1016/j.vaccine.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv Y., Ruan Z., Wang L., Ni B., Wu Y. Identification of a novel conserved HLA-A*0201-restricted epitope from the spike protein of SARS-CoV. BMC Immunol. 2009;10:61. doi: 10.1186/1471-2172-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J., Cao Y., Du J., Bu X., Ma R., Wu C. Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses. Vaccine. 2007;25(39–40):6981–6991. doi: 10.1016/j.vaccine.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao Y.P., Lin J.Y., Jan J.T., Leng C.H., Chu C.C., Yang Y.C. HLA-A*0201 T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus nucleocapsid and spike proteins. Biochem Biophys Res Commun. 2006;344(1):63–71. doi: 10.1016/j.bbrc.2006.03.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M., Xu D., Li X., Li H., Shan M., Tang J. Screening and identification of severe acute respiratory syndrome-associated coronavirus-specific CTL epitopes. J Immunol. 2006;177(4):2138–2145. doi: 10.4049/jimmunol.177.4.2138. [DOI] [PubMed] [Google Scholar]
- 18.Wang B., Chen H., Jiang X., Zhang M., Wan T., Li N. Identification of an HLA-A*0201-restricted CD8+ T-cell epitope SSp-1 of SARS-CoV spike protein. Blood. 2004;104(1):200–206. doi: 10.1182/blood-2003-11-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma R., Du J.L., Huang J., Wu C.Y. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem Biophys Res Commun. 2007;361(2):537–542. doi: 10.1016/j.bbrc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Shen E.X., Li L., Li L.T., Feng L.Q., Lu L., Yao Z.L. PIKA as an adjuvant enhances specific humoral and cellular immune responses following the vaccination of mice with HBsAg plus PIKA. Cell Mol Immunol. 2007;4(2):113–120. [PubMed] [Google Scholar]
- 21.Valensi J.P., Carlson J.R., Van Nest G.A. Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J Immunol. 1994;153(9):4029–4039. [PubMed] [Google Scholar]
- 22.He Y., Barker S.J., MacDonald A.J., Yu Y., Cao L., Li J. Recombinant Ov-ASP-1, a Th1-biased protein adjuvant derived from the helminth Onchocerca volvulus, can directly bind and activate antigen-presenting cells. J Immunol. 2009;182(7):4005–4016. doi: 10.4049/jimmunol.0800531. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M., Seya T. Role of Toll-like receptors in innate and adaptive immune responses. Seikagaku. 2009;81(3):156–164. [PubMed] [Google Scholar]
- 24.Majewska M., Szczepanik M. The role of Toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Postepy Hig Med Dosw (Online) 2006;60:52–63. [PubMed] [Google Scholar]
- 25.Akira S., Takeda K., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 26.Gai W.W., Zhang Y., Zhou D.H., Chen Y.Q., Yang J.Y., Yan H.M. PIKA provides an adjuvant effect to induce strong mucosal and systemic humoral immunity against SARS-CoV. Virol. Sin. 2011;26(2):81–94. doi: 10.1007/s12250-011-3183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krug A., Towarowski A., Britsch S., Rothenfusser S., Hornung V., Bals R. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31(10):3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.McCluskie M.J., Cartier J.L., Patrick A.J., Sajic D., Weeratna R.D., Rosenthal K.L. Treatment of intravaginal HSV-2 infection in mice: a comparison of CpG oligodeoxynucleotides and resiquimod (R-848) Antiviral Res. 2006;69(2):77–85. doi: 10.1016/j.antiviral.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Rothenfusser S., Hornung V., Ayyoub M., Britsch S., Towarowski A., Krug A. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood. 2004;103(6):2162–2169. doi: 10.1182/blood-2003-04-1091. [DOI] [PubMed] [Google Scholar]
- 30.Reis E.S.C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16(1):27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Edwards A.D., Diebold S.S., Slack E.M., Tomizawa H., Hemmi H., Kaisho T. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33(4):827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 32.Shortman K., Liu Y.J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 33.Schlosser E., Mueller M., Fischer S., Basta S., Busch D.H., Gander B. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008;26(13):1626–1637. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Salerno-Goncalves R., Sztein M.B. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 2006;14(12):536–542. doi: 10.1016/j.tim.2006.10.004. [DOI] [PubMed] [Google Scholar]


