Abstract
To explore the best prime–boost regimen and evaluate the T-cellular response memory against HCV, we constructed two DNA vaccine candidates (pVRC-CE1E2 and pAAV-CE1E2) and two recombinant viruses (rTTV-E1E2 and rAAV-E1E2) and then assessed the immune response to different prime–boost patterns in BALB/c mice. The rTTV-E1E2 boosted the immune response to HCV DNA vaccine prime significantly, and the inverted terminal repeat sequence harboring DNA construct PAAV-CE1E2 was the best prime agent in this study. Our study provides new information for both the prime–boost regimen and long-term T-cell response for HCV vaccine development.
Abbreviations: HCV, hepatitis C virus; IFA, immunofluorescence assay; ITR, inverted terminal repeat; rAAV, recombinant adeno-associated virus; rTTV, recombinant vaccinia TianTan strain; VV, vaccinia virus; SFCs, spot-forming cells; TKR, right thymidine kinase; TKL, left thymidine kinase
Keywords: Hepatitis C virus, T-cell response, Prime–boost regimen
1. Introduction
For certain infection, T-cells are central to acquired immunity [1], [2], [3], [4]. Studies have shown that the control of acute HCV infection is associated with vigorous, broadly directed, and sustained activation of HCV-specific T-cells [2], [3], [4]. Thus, engineering an efficient adaptive immune response, especially the T-cell response, should be the goal of a HCV vaccine strategy [2], [3].
DNA immunization has been used to induce either humoral or cellular immune responses against various viral, bacterial, and parasitic pathogens [5], [6]. Immune responses induced by DNA immunization can be enhanced or modulated by the molecular adjuvant, plasmid design strategies (choice of antigen or multiple antigens, and T-cell epitope adjustment), the method and location of immunization, and vaccine regimens [5], [6], [7], [8].
Many vaccines are enhanced by combining DNA with other vaccines in “prime–boost” regimens [5], [7], [8], in which the boosting vaccine is often a recombinant viral vector or purified protein subunit. Vaccine strategies that involve primary vaccinations with a DNA vaccine followed by boosting with a recombinant virus vector encoding the same immunogen have emerged as the favored approach for generating protective CD8 T-cell responses against many diseases, including HIV, malaria, and cancer [5], [7], [8], [9].
The pVRC vector was used in DNA vaccine developments against SARS-CoV, highly pathogenic avian influenza virus (H5N1), and HIV-1 virus infections, and elicited a much stronger immune response compared to regular DNA vaccine vectors such as the pcDNA 3 series [6]. Studies have shown that the inverted terminal repeat (ITR) [cis-acting elements required for packaging, integration, and replication that flank the adeno-associated virus (AAV) genome] of AAV may strengthen the immunity of DNA vaccines [10], [11]. Meanwhile, DNA immunity can be boosted by different viral vectors or proteins, of which, the most popular vectors being derived from adenovirus and poxvirus [5], [7], [8], [9].
In this study, we constructed a pVRC-CE1E2 plasmid and a pAAV-CE1E2 plasmid, both of which encode the HCV core (C) and envelop glycoproteins (E1 and E2). We also constructed two recombinant viruses, a recombinant vaccinia TianTan strain (rTTV-E1E2) and a recombinant adeno-associated virus (rAAV-E1E2), which similarly encode HCV E1 and E2. The optimal prime–boost regimen was evaluated in BALB/c mice.
2. Materials and methods
2.1. Generation of vaccine candidates
The C/E1/E2 region of HCV (aa 1–746, NCBI accession no. L02836 [12]) was inserted into pVRC [6] and pAAV [13], [14] vectors to construct prime DNA vaccine candidates pVRC-CE1E2 and pAAV-CE1E2 (Fig. 1 ).
Fig. 1.
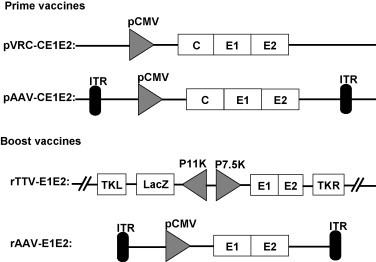
Schemes of vaccine candidates. Constructs of priming and boosting vaccines; rAAV-E1E2 was packaged with the cap and rep protein of AAV2; ITR, inverted terminal repeat; P7.5K, P7.5 later promoter; P11K, P11 later promoter; TKR, right thymidine kinase; TKL, left thymidine kinase.
The original TTV strain and dual-promoter insertion vector pJSA1175 were derived in our lab [15], [16]. The HCV E1E2 gene (aa 173–746, genotype 1b) was inserted into the SmaI site of the pJSA1175 vector. The rTTV was produced by transfection of pJSA1175 into CEF cells that were infected by TTV and was designated rTTV-E1E2 (Fig. 1).
To generate the rAAV-E1E2, the same HCV E1E2 cDNA was inserted into the pAAV2 neo vector (Fig. 1). Large-scale rAAV production and purification were described previously [13], [14], and the viral titer was determined by dot blot DNA analysis. Titers are given as vector genomes (vg) per milliliter.
2.2. Animals and immunization
Nine groups of female BALB/c mice (6–8 weeks old) were immunized as described in Table 1 . The Animal Care and Use Committees of the China CDC approved all protocols for the animal experiments.
Table 1.
Immunization strategy.
| Groups | Immunization strategy |
|
|---|---|---|
| Prime | Boost | |
| 1 | Mock (PBS) | |
| Mock (PBS) | rTTV-E1E2 | |
| Mock (PBS) | rAAV-E1E2 | |
| 2 | pVRC-CE1E2 | |
| pVRC-CE1E2 | rTTV-E1E2 | |
| pVRC-CE1E2 | rAAV-E1E2 | |
| 3 | pAAV-CE1E2 | |
| pAAV-CE1E2 | rTTV-E1E2 | |
| pAAV-CE1E2 | rAAV-E1E2 | |
Prime–boost regimens were divided into 3 groups (3 subgroups of each, 6 mice/subgroup). The plasmid DNA prime were inoculated in 0, 2, 4 weeks with dose of 50 μg, followed by the recombinant virus boost in 20 weeks with 107 pfu of rTTV-E1E2 or 1011 vector genomes (vg) of rAAV-E1E2, respectively.
2.3. Immune response analysis of vaccinated mice
A synthetic peptide representing HCV E2 (aa 384–413) of genotype 1b and a mixture of four synthetic HCV E1 peptides (1, YEVRNVSGIYHVTNDCSNSS; 2, PGCVPCVREGNSSRCWVALT; 3, REGNSSRCWVALTPTLAARNATI, and 4, PRRYETIQDCNCSIYPG) representing the E1 linear conserved B-cell epitopes among HCV genotypes were coated onto enzyme-linked immunosorbent assay (ELISA) plates to assess the antibody response [17], [18]. IgG titers were expressed as OD values measured at 405 nm.
Quantification of HCV-specific IFN-γ secreting cells was calculated via an ELIspot assay. A mixture of CTL peptides (H-2Db-restricted; New England Biolabs, Ipswich, MA) was used for stimulation: E1, GHRMAWDM (aa 315–322); E2, SGPSQKIQLV (aa 405–414); E2, PPQANWFGCTWMNSTGFTKT (aa 544–563), and E2, RLWHYPCTI (aa 614–622). A human immunodeficiency virus peptide (RIQRGPGRAFVTIGK) was used as a control [19], [20], [21]. ELIspot kits were purchased from BD PharMingen (San Diego, CA). The results are presented as the mean ELIspot positive cells/million cells ± SD for each group.
2.4. Data analysis
Significant differences between the experimental and control groups were evaluated using a two-tailed Fisher's exact test (release 12.1; SPSS Inc., Chicago, IL). Differences were considered significant at p < 0.05.
3. Results
3.1. Confirmation on the expression of HCV structural proteins
Plasmids pVRC-CE1E2 and pAAV-CE1E2 were transfected to 293 cells and their expressions were evaluated after 48 h by immunocellular staining and Western blot analysis. Similarly, rTTV-E1E2 and rAAV-E1E2 infected cells were also evaluated by the same approach. All four constructs expressed HCV proteins well (data not showed).
3.2. Humoral immune responses of all prime–boost regimens
To optimize the prime–boost regimen, a series of prime–boost regimes were performed in BALB/c mice as listed in Table 1. Sera from immunized mice were sampled and pooled at week 39 and tested via ELISA for antibodies in response to the HCV structural proteins. As shown in Fig. 2A, the strongest humoral response was induced by the regimen consisting of pAAV-CE1E2 prime and rTTV-E1E2 boost, in the third group. The titer from this regimen was 1.12 at the 1:100 dilution, and the antibody titer remained as high as 0.34 at the 1:800 dilution. The second best humoral response was induced by the regimen consisting of pVRC-CE1E2 prime and rTTV-E1E2 boost from the second group, in which the titer was 0.98 at the 1:100 dilution and 0.21 at the 1:800 dilution. The third strongest humoral response was induced by the regimen consisting of pAAV-CE1E2 prime and no boost, also from the third group; the titer was 0.53 at the 1:100 dilution and decreased to the background level at the 1:800 dilution. The remaining regimens did not raise sufficient humoral responses in this study. Our data suggest that the pAAV-CE1E2 plasmid is the best choice for prime, and that rTTV-E1E2 is the best choice for the boost; although pVRC-CE1E2 alone could not induce a high enough humoral response at the time point blood samples were taken, the rTTV-E1E2 may even boost the humoral response to a fairly high level (Fig. 2A).
Fig. 2.
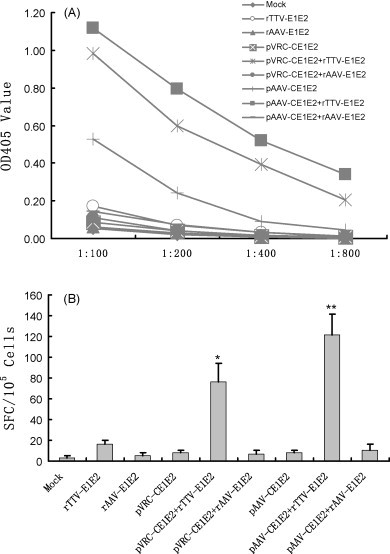
Assessment of the immune response. (A) Humoral immune response, each titration curve indicates the representative value of the total E1/E2 specific IgG in serial dilution of pooled sera of each group at OD 405 nm, therein points representing the average OD reading value of each group. (B) T cellular response, data shown represent the average response value of 6 mice each group minus the cutoff value (the mean value of spot-forming cells stimulated by HIV peptide). **p < 0.05, SFCs are the highest one; *p < 0.05, SFCs are the second highest one.
3.3. Cellular immune responses of all prime–boost regimens
To evaluate the duration of CD8+ T-cell responses induced by our prime–boost regimen, an IFN-γ ELISpot assay was performed at 39 weeks after primary injection (19 weeks after the last boost). Meanwhile, the capacity of viral vector injection to recall memory responses was explored via the same assays at 16 weeks after the last prime DNA vaccination.
The number of IFN-γ-secreting T-cells was determined by counting spot-forming cells (SFCs). The mice immunized with the pAAV-CE1E2 prime and rTTV-E1E2 boost regimen had the most IFN-γ-secreting T-cells (121 ± 20; Fig. 2B). Similarly, mice immunized with the pVRC-CE1E2 prime and rTTV-E1E2 boost regimen had the second highest number of IFN-γ-secreting T-cells (76 ± 18; Fig. 2B). Unlike the antibody response to HCV, SFCs of the mice injected with pAAV-CE1E2 were similar to that of the control group (Fig. 2B). These results suggest that the pAAV-CE1E2 prime and rTTV-E1E2 boost is the best regimen to increase a robust long-term cellular immune response. Furthermore, our study revealed that rTTV-E1E2 is a better boost method to induce both humoral and cellular immune response.
4. Discussion
To induce a strong and long-term protective T-cell response, priming of the host immune system is critical and the optimizations of vaccine candidates and prime–boost regimes are also important [7], [8], [9]. In this study, we evaluated the immune response of several vaccine candidates to explore the regimen that may induce an effective long-term T-cell response to the HCV envelope proteins in BALB/c mice. Our results demonstrated that priming with pAAV-CE1E2 followed by rTTV-E1E2 boosting induces a strong long-term T-cell response.
A previous study found that a DNA vaccine containing an ITR of AAV increased immunity to HIV [13]. To optimize our vaccination, we constructed pVRC-CE1E2 (which had no ITR sequence) and pAAV-CE1E2 (which contained an ITR sequence). Our results indicate that pAAV-CE1E2 is better than pVRC-CE1E2 as a priming candidate, suggesting that the ITR can improve and extend the T-cell response to HCV. Recombinant vaccinia viruses (rVVs) were generated in the early 1980s and shown to be capable of inducing protective T-cell responses in small animals [22], meanwhile, the rVVs have many advantages for immunization [7], [9]. We selected a highly replicating rTTV as a boosting vaccine since it might induce long-lasting immune responses via a single dose and its safety is well documented for a large population. Compared to rTTV-E1E2 boosting, rAAV-E1E2 elicited a much lower T-cell response, which was consistent with the recent reports that AAV may impair the T-cell response in certain situations [23].
The duration of T-cell responses has been assessed in several studies of DNA-MVA immunization regimes in small animals. Complete protection at 3.5 months after priming was reported with an adenovirus-vaccinia immunization regime in the Plasmodium yoelii model [24], in which better immunogenicity and protection were observed after increasing the interval between priming and boosting from 2 to 8 weeks. A study on the durability of T-cell responses to different prime–boost regimes would be valuable; our results showed that priming with pVRC-CE1E2 or pAAV-CE1E2 followed by rTTV-E1E2 boosting might elicit sustained, potent T-cell responses in mice, even when the priming-boosting interval is as long as 16 weeks.
Current HCV vaccine developments have focused mainly on strength and cross-protection, but long-term assessments of the T-cell response are limited [2], [3], [17], [18], [19], [20], [21]. Although our assessment is not sufficiently comprehensive, our data did provide an information of long-term T-cell responses to HCV in BALB/c mice at 19 weeks after the boosting.
Acknowledgments
The authors thank Dr. Gary Nabel (VRC, NIH) for providing the pVRC plasmid and Dr. A.H. Patel (MRC Virology Unit, UK) for the anti-E2 mAb AP33. This study was supported by the 863 Hi-Tech Research and Development Program of China (2007AA02Z157 and 2007AA02Z455) and the National Natural Science Foundation of China (30571673 and 30671961).
Contributor Information
Wenjie Tan, Email: tanwj28@yahoo.cn.
Yue Wang, Email: euy-tokyo@umin.ac.jp.
References
- 1.Liao G., Wang Y., Chang J., Bian T., Tan W., Sun M. Hepatitis B virus precore protein augments genetic immunizations of the truncated hepatitis C virus core in BALB/c mice. Hepatology. 2008;47:25–34. doi: 10.1002/hep.21992. [DOI] [PubMed] [Google Scholar]
- 2.Dustin L.B., Rice C.M. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 3.Rehermann B., Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [review] [DOI] [PubMed] [Google Scholar]
- 4.Cooper S., Erickson A.L., Adams E.J., Kansopon J., Weiner A.J., Chien D.Y. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 5.Lu S., Wang S., Grimes-Serrano J.M. Current progress of DNA vaccine studies in humans. Expert Rev Vaccines. 2008;7:175–191. doi: 10.1586/14760584.7.2.175. [DOI] [PubMed] [Google Scholar]
- 6.Barouch D.H., Yang Z.-Y., Kong W.-P., Korioth-Schmitz B., Sumida S.M., Truitt D.M. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79:8828–8834. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McConkey S.J., Reece W.H., Moorthy V.S., Webster D., Dunachie S., Butcher G. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 8.Woodberry T., Gardner J., Elliott S.L., Leyrer S., Purdie D.M., Chaplin P. Prime boost vaccination strategies: CD8 T cell numbers, protection, and Th1 bias. J Immunol. 2003;170:2599–2604. doi: 10.4049/jimmunol.170.5.2599. [DOI] [PubMed] [Google Scholar]
- 9.Schoenly K.A., Weiner D.B. Human immunodeficiency virus type 1 vaccine development: recent advances in the cytotoxic T-lymphocyte platform “spotty business”. J Virol. 2008;82:3166–3180. doi: 10.1128/JVI.01634-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikhlikar P., Barros de Arruda L., Agrawal S., Byrne B., Guggino W., August J.T. Inverted terminal repeat sequences of adeno-associated virus enhance the antibody and CD8(+) responses to a HIV-1 p55Gag/LAMP DNA vaccine chimera. Virology. 2004;323:220–232. doi: 10.1016/j.virol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Xin K.Q., Ooki T., Jounai N., Mizukami H., Hamajima K., Kojima Y. A DNA vaccine containing inverted terminal repeats from adeno-associated virus increases immunity to HIV. J Gene Med. 2003;5:438–445. doi: 10.1002/jgm.356. [DOI] [PubMed] [Google Scholar]
- 12.Bi S.L., Bai X.H., Cong M.E., Liu C.B. Sequence analysis and variation of hepatitis C virus isolate from Hebei Province, China. Chin J Virol. 1993;9:114–127. [Google Scholar]
- 13.Wu X.B., Dong X.Y., Wu Z.J., Cao H., Niu D.B., Qu J.G. A novel method for purification of recombinant adeno-associated virus vectors on a large scale. Chin Sci Bull. 2004;46:485–489. [Google Scholar]
- 14.Hauswirth W.W., Lewin A.S., Zolotukhin S., Muzyczka N. Production and purification of recombinant adeno-associated virus. Methods Enzymol. 2000;316:743–761. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- 15.Tsao H., Liu G.Q., Ruan L., Chu C.M. Construction and application of plasmids containing bidirectional promoters of vaccinia virus. J Virol. 1988;62:4832–4834. doi: 10.1128/jvi.62.12.4832-4834.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan L., Zheng H.Q., Xu S.C., Wang S.P., Tsao X., Xie Y. Expression of both glycoprotein F and G of respiratory syncytial virus (RSV) in a single recombinant vaccinia virus. Chin J Virol. 1992;8:101–109. [Google Scholar]
- 17.Youn J.W., Park S.H., Lavillette D., Cosset F.L., Yang S.H., Lee C.G. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology. 2005;42:1429–1436. doi: 10.1002/hep.20934. [DOI] [PubMed] [Google Scholar]
- 18.Encke J., Radunz W., Eisenbach C., Geib J., Gehrke S., Pfaff E. Development of a heterologous, multigenotype vaccine against hepatitis C virus infection. Eur J Clin Invest. 2007;37:396–406. doi: 10.1111/j.1365-2362.2007.01802.x. [DOI] [PubMed] [Google Scholar]
- 19.Majid A.M., Ezelle H., Shah S., Barber G.N. Evaluating replication-defective vesicular stomatitis virus as a vaccine vehicle. J Virol. 2006;80:6993–7008. doi: 10.1128/JVI.00365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S.H., Yang S.H., Lee C.G., Youn J.W., Chang J., Sung Y.C. Efficient induction of T helper 1 CD4+ T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boost. Vaccine. 2003;21:4555–4564. doi: 10.1016/s0264-410x(03)00499-7. [DOI] [PubMed] [Google Scholar]
- 21.Murata K., Lechmann M., Qiao M., Gunji T., Alter H.J., Liang T.J. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc Natl Acad Sci USA. 2003;100:6753–6758. doi: 10.1073/pnas.1131929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci USA. 1982;79:4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S.W., Hensley S.E., Tatsis N., Lasaro M.O., Ertl H.C. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruña-Romero O., González-Aseguinolaza G., Hafalla J.C., Tsuji M., Nussenzweig R.S. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc Natl Acad Sci USA. 2001;98:11491–11496. doi: 10.1073/pnas.191380898. [DOI] [PMC free article] [PubMed] [Google Scholar]


