Abstract
In the face of an almost unprecedented threat of a global pandemic of influenza it is imperative that stockpiling of appropriate drugs and devices begin now. One vital device is an appropriate syringe for delivering vaccine. With the potential for millions to be infected and the vaccine supply severely stretched it is imperative that the syringe used to vaccinate waste as little vaccine as possible and thus allow for a maximum number of persons to be vaccinated. Our study tested seven leading candidate vaccine syringes for dosing accuracy, dose-capacity per vial, medication wastage and a battery of ergonomic features. One device, the Flu+™ syringe, proved superior to the others in all important categories, possibly due to its low dead-space volume and its dosing accuracy. The data suggest that switching to this device from any of the others tested would provide between 2 and 19% additional vaccine doses per vial if the current 10-dose vials are used. Extrapolations from this data suggest that many thousands to millions of additional persons could be vaccinated in mass campaigns. Use of a syringe of this type, and the vaccine savings that would accrue, would likely be important in reducing morbidity and mortality in the event of a pandemic of influenza.
Keywords: Influenza, Pandemic, Avian flu, H5N1, Vaccine, Syringe
1. Introduction
Hardly a day passes without a new alarm raised regarding H5N1 avian influenza. Its steady march westward from Asia, its striking case:mortality rate and the expectation that it will soon ‘humanize’ (become easily transmissible from person to person) are causing tremors throughout the world [1]. At the back of everyone's mind is the question, ‘could this become another 1918?’ The influenza pandemic of 1918 may well have been the worst catastrophe in human history. The most recent estimates, which now include deaths from India and China, are that as many as a 100 million people worldwide lost their lives [2].
Unlike yearly epidemics, pandemic flu viruses have a predilection for young, healthy persons, whom they attack with astonishing virulence. It was not uncommon in 1918 for a young person to go to bed feeling fine, wake up achy and feverish, be bedridden by noontime and be dead before sundown [3].
Influenza experts have long predicted a 1918-like influenza pandemic from southeastern Asia, the region from which the SARS coronavirus emerged [4], [5]. All three influenza pandemics in the last century have come from this region. The 1918 pandemic took 3 weeks to circle the globe in an age without commercial air travel. A pandemic today might take as little as 2 days [6], [7], [8]. A recurrence of 1918 would mean 100 million cases of flu in North America alone and an equal number in Europe [4], [7]. These would come in two giant waves (if previous pandemics are a guide), the first beginning in late fall and the second in spring. Upwards of 2 million deaths could result on each continent [9], [10]. The possible toll worldwide is almost unfathomable.
The health-care response during the first days into a pandemic would focus on vigorous, proactive public health measures such as isolation of those infected, closures of schools, the obligatory wearing of masks, gloves and other protective gear and frequent hand-washing [8], [11], [12], [13]. Anti-influenza drugs, which are currently in short supply, would play a critical role, assuming resistance to them did not develop quickly. The newer Neuraminidase Inhibitors, oseltamivir and zanamivir, would be made available during the first wave to key population groups in countries which have been wise (and wealthy) enough to stockpile [14].
It is axiomatic that a vaccine will not be immediately available, but will take several months to develop with current technology [15], [16]. Worldwide there are only a handful of manufacturers [17] who produce the vast majority of influenza vaccines. It would require between 3 and 8 months to produce and release a new influenza subtype vaccine, meaning that no or limited doses of vaccine would be available in the first half year of the pandemic.
Pandemic preparedness involves stockpiling of the key medical supplies known to be needed in the first months of a pandemic (e.g. anti-virals) as well as those that are likely to be in short supply when the vaccine is ready, most critically syringes. The choice of syringe by stockpilers is not merely of academic or financial interest; it will be a major determinant of life or death in a pandemic. Given the scale of need for vaccine and the limited capacity of manufacturers to supply it, shortages will be inevitable. Such will be the outcry for vaccine that officials expect major social and political upheavals [18]. It is therefore of paramount importance to stock a syringe which will reduce vaccine wastage to a minimum and thereby allow the greatest number of persons to be vaccinated. In our experience, discussions on pandemic preparedness often overlook the critical role of the ‘lowly’ syringe. We continue to do this at our own peril.
This article reports on a study which will inform officials and planners of the public health stakes in their choice of a pandemic syringe for vaccination. It is intended to help them estimate the degree of vaccine wastage/savings they should expect with each potentially stockable device. We provide information for estimating the additional number of individuals who might benefit from vaccination as a function of the device.
The study compared the accuracy and reproducibility of a 0.5 mL dose administered with each of seven device which are being considered as stocking options in pandemic preparedness (Table 1 ). We compared the total number of 0.5 mL doses obtained from a 10-dose vial (5.0 mL) with the optimal number of ‘expected draws’. From this we estimated the number of persons who could be vaccinated with each device per million vials purchased. Finally, we compared the ease and speed of use of each device as determined by users.
Table 1.
Devices tested in current study
| Device number | Trade name/description | Dose range (mL) | Needle fixation | Needle gauge (G) | Needle length (in.) | Manufacturer |
|---|---|---|---|---|---|---|
| 1 | Flu+ | 0.25–1.0 | Attached | 25 | 1 | BD |
| 2 | SoloShot IX | 0.5 (fixed) | Attached | 25 | 1 | BD |
| 3 | Plastipak Luer slip | 0.0–1.0 | Detached | 25 | 1 | BD |
| 4 | Plastipak Luer slip | 0.0–2.0 | Detached | 25 | 1 | BD |
| 5 | Injekt Luer slip | 0.0–2.0 | Detached | 25 | 1 | B Braun |
| 6 | Monoject Luer slip | 0.0–1.0 | Detached | 25 | 1 | Tyco |
| 7 | Terumo Luer slip | 0.0–1.0 | Detached | 25 | 1 | Terumo |
2. Materials and methods
2.1. Number of syringes and users
Seventy study syringes per clinician were assessed by five clinicians per site in nine European sites (3150 devices in total). Forty-five clinicians (nurses and physicians) were enrolled at three sites in Poland, three in Sweden and three in the United Kingdom (England, Scotland, Wales) (Table 2 ). Participating clinicians were required to have at least 6 months of injecting experience and to perform at least five percutaneous injections (intramuscular, intradermal or subcutaneous) per week. Sites varied from academic medical centres to private clinics to regional medical centres and users were felt to constitute a representative sample of injecting health-care professionals.
Table 2.
Participating centres
| Poland | Sweden | UK |
|---|---|---|
| Szpital Bielanski, Warsaw | University Hospital Malmo, Malmo | East Oxford Health Center, Oxford, England |
| NZOXZ Sana S.C. Policlinic, Warsaw | Helsingborg Hospital, Helsingborg | Prince Charles Hospital, North Glamorgan NHS Trust, Merthyr Tidfil, Wales |
| Miedzyleski District Hospital, Warsaw | University of Lund Hospital, Lund | Fife NHS Primary Care Trust, Kirckaldy, Scotland |
2.2. Products trialed
At enrollment, each clinician received seven 5 mL vials of sterile saline (Abbott Laboratories, North Chicago, IL, USA); 10 BD Flu+™ 0.25–1 mL variable dose syringes with attached 25 G 1 in. (25 mm) needles (BD, Franklin Lakes, NJ, USA); 10 BD 0.5 mL SoloShot™ IX Auto-Disable Syringes and attached 25 G 1 in. needles; ten 1 mL BD Plastipak™ Luer slip syringes with detached 10 BD Microlance™ 25 G 1 in. needles; 10 BD 2 mL Plastipak™ Luer slip syringes with 10 BD Microlance™ 25 G 1 in. needles; ten 2 mL B Braun Injekt™ Luer slip syringes with 10 detached B Braun Sterican™ 25 G 1 in. needles (B Braun, Melsungen, Germany); ten 1 mL Tyco Monoject™ Luer slip syringes with 10 detached Tyco Monoject™ 25 G 1 in. needles (Tyco, Mansfield, MA, USA); ten 1 mL Terumo™ Luer slip syringes with 10 detached Terumo Neolus™ 25 G 1 in. needles (Terumo, Leuven, Belgium). These devices were chosen based on contacts with pandemic preparedness planners in Europe who indicated that they were considering stocking these or similar syringes. Saline was chosen as a substitute for influenza vaccine based on similarity of physical properties as well as saline's overall safety profile for participants.
2.3. Study process
The order of use of each syringe was randomized. Study staff neither intentionally identified nor concealed the identity of the device being used. All devices were CE-marked and branded, thus it was impossible to blind users to the manufacturer. Staff weighed a 5 mL vial containing saline in a highly-sensitive electronic balance (Excellence SX™ Analytical Balance, Mettler-Toledo, OH, USA) and gave it to the participant with instructions to draw up consecutive doses of 0.5 mL and inject them into a plastic tub on the balance using the drawing-up and injection techniques they customarily used in practice; no training on technique was given beyond a simple verbal explanation of the function of each syringe. Opportunity was given to the users to practice with the device beforehand, as desired.
The goal for the user was to draw up the maximum number of full doses from the vial. Each dose was weighted (Vx) and a running sum of weights was kept (sum Vx). When the sum Vx approached the expected maximum the vial contained, the vial was again weighed. If there was adequate remaining volume, the drawing up of another full dose was attempted. Afterwards the vial with its remaining volume (less than a full dose) was weighed (d = weight of residual liquid and vial). This allowed the calculation of the wasted volume (e = weight of only the residual liquid in a vial calculated by d − a = e). Following the study, staff weighed each empty, dry vial (a = weight of empty, dry vial) in order to compare with weights of full vials (b = weight of full vial) and assess the weight of liquid in the vial (c = weight of only the liquid in a full vial [b − a = c]). The participant and staff repeated all the above steps until all seven devices were tested.
2.4. Calibration
In order to ensure that no error was introduced due to variability in the electronic balance, a calibration was performed using FACT (Fully Automatic Calibration Technology™, Mettler-Toledo, OH, USA). This internal, automatic calibrating function was used to ensure standardization between each move (from centre to centre and from site to site within a centre) and before any study samples were weighed. FACT was also run between each participant.
2.5. Reproducibility
Standard deviations (S.D.) were used to assess the reproducibility of results for the different variables (within device, within vial, within person, within centre, within country).
2.6. Accuracy
The target value for each injection was 0.500 g. Measurement of the mean and S.D. and a comparison of its proximity to 0.500 g were used to assess the accuracy and reproducibility of the various devices.
2.7. Dose capacity
The goal was to draw 10 or more doses per vial. Measurement of the mean and S.D. of total doses obtained and a comparison of its proximity to 10 doses per vial were used to assess the performance of the various devices to make maximum use of vial capacity as well as an indirect assessment of accuracy and residual/waste. From the distribution of volumes from 5 mL vials we were able to estimate how many draws (under optimal conditions; i.e. where there is zero loss) might be achieved. Knowing that there was usually a 10% overfill, we estimated, before study commencement, that ideally the percentage of 9-draw vials would be approximately 2%, 10-draw vials approximately 15%, 11-draw vials approximately 80% and 12-draw vials approximately 3%. Comparison of the performance of each device to these optimal draw values was made.
2.8. Residual/waste
Using this procedure we were able to calculate e, the residual volume. Sum e and mean e were assessed for each device. The comparison within the same vial enabled us to negate the variation due to differences in non-liquid vial components (glass, plastic, stopper, label, aluminium wrapper, etc.).
2.9. Assumptions
Several assumptions were made regarding the possible vaccine configuration in a pandemic. The first was that vials used would be the same as those currently marketed, i.e. 5 mL; secondly, that the dose of flu vaccine would be the same as currently used, i.e. 0.5 mL; finally, that the variable amount of over-fill per vial (often allowing more than 10 doses to be drawn from each) would continue to be provided by manufacturers throughout a pandemic.
2.10. Possible sources of error
One possible source of error was the difference in the empty weight of one vial (i.e. capacity before filling) compared to another. Another was the difference in the full weight of one vial (i.e. capacity after filling and before drawing) compared to another. These possible sources of error were evaluated by comparing weights of all empty and filled vials to determine if there were differences within device categories. A third potential confounding factor might be differences in the dead-space volume of one device category compared to another. The dead-space in a syringe is the volume of fluid that is drawn up but not injected; this volume is trapped inside the syringe as a function of the design of the device. This parameter was evaluated in a separate bench experiment in which 40 syringes from each of the 7 device categories were weighed empty and then filled with 0.5 mL of saline; the saline was expelled by fully depressing the plunger and the device was immediately weighed. The difference between the final and the first weights was considered the dead-space volume.
2.11. Performance: ease of use, safety, preference
A standard questionnaire was administered to each participant after completing all injections within one device category to obtain their assessment of following parameters:
-
•
Ease of use: ease of learning (intuitiveness), ease of drawing up, legibility of calibrations/markings, air bubble formation and expulsion, ease of displacement of piston, ease of disposal, time expenditure of the procedure.
-
•
Safety: prevention of reuse, potential for needlestick injuries, disposal issues.
Additionally, study monitors observed and recorded user performance on the following parameters: speed of learning on each of the devices, correct use of each of the seven devices and drawing-up techniques.
2.12. Statistics
2.12.1. Predicate studies
A previous 2003 study in South Africa ([19]; see also [20]) showed that the average dose per injection was 0.575 mL (=0.575 g) with BD 0.5 mL SoloShot™ syringes and 0.637 mL (=0.637 g) with conventional disposable syringes. This difference (0.062 mL on average) was statistically highly significant (p = 0.0025). In that trial, the standard deviation the BD 0.5 mL SoloShot™was 0.037 and 0.060 with the conventional syringes.
2.12.2. Sample size determination
One hundred and eighty injections of each type in each centre were required to detect a mean difference between doses delivered with the test devices of 0.015 mL (mg) at a 0.05 level of significance with a power of 90% assuming a standard deviation of 0.030 mL. A target of 200 injections per centre was set based on the above estimate. Because of the potential for large centre-to-centre variation (due to air shots with conventional syringes wasting up to 0.2 ml per injection) the study was powered to be analyzed centre by centre.
2.12.3. Statistical tests
Two-sample t-test was used to compare mean doses for accuracy and reproducibility. Multi-variant ANOVA was used to determine relative influence of each of the following factors: device, subject, centre and country. Fisher exact test compared percentages. Contingency tables using chi-squared and McNamar tests were used to assess the difference in dose/vial as a function of device.
2.13. Regulatory
The study was conducted in compliance with Good Clinical Practices and applicable local and European Union regulatory requirements and directives. Ethics Committee approval was sought for the trial at each centre. In several cases this process was waived by the committee due to the low risk entailed by the study; in these cases a letter exempting the sponsor from this requirement was obtained from the chairperson of the Ethics Committee.
3. Results
3.1. Accuracy
There was a statistically significant difference in the accuracy of the 7 devices (p = 0.001) with devices 1 and 6 being the most accurate (closest means to 0.500 g) and devices 4 and 5, the least accurate (Fig. 1 ).
Fig. 1.
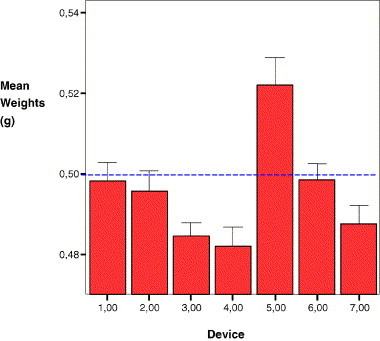
A comparison of the mean weights in g of 540 doses delivered by each of the 7 devices (3780 total doses). Devices that delivers less than 0.500 g are considered to underdose (e.g. devices 3, 4 and 7), those that deliver more than 0.500 g to overdose (device 5) and those that deliver a mean closest to 0.500 g are the most accurate (e.g. devices 1 and 6). Error bars represent the 95% CI.
3.2. Doses obtained from each vial
We observed a highly statistically significant difference (p = 0.001) amongst devices in the number of doses that could be drawn per vial (Table 3A ). We extrapolated this data to 1 million vials used per device (Table 3B ) and calculated how many persons could be vaccinated from these 1 million vials as a function of device (Table 3C ). It is clear from our data that device 1 allows for the maximum number of doses to be drawn from each vial. Table 4 shows the additional number of persons who could be vaccinated by switching from any of the other devices to device 1. Conversion from any other device to device 1 results in between 222,222 and 1,577,777 additional persons vaccinated per million vials purchased. These estimates must be taken as indicative of a trend and not as absolute figures, as any error in our study of 315 vials will be multiplied 3000-fold when an extrapolation to a million vials is made. However, despite this error the data show that switching to this device from any of the others tested would provide between 2 and 19% additional vaccine doses per vial if the current 10-dose vials are used.
Table 3A.
Actual study data (numbers in the columns under doses drawn represent the number of vials which yielded this number of doses)
| Devices | Doses drawn per vial |
Total | |||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 0 | 0 | 16 | 19 | 9 | 1 | 45 |
| 2 | 0 | 1 | 20 | 18 | 5 | 1 | 45 |
| 3 | 0 | 4 | 40 | 1 | 0 | 0 | 45 |
| 4 | 0 | 6 | 27 | 9 | 2 | 0 | 44 |
| 5 | 2 | 28 | 14 | 1 | 0 | 0 | 45 |
| 6 | 0 | 2 | 39 | 5 | 0 | 0 | 46 |
| 7 | 0 | 5 | 39 | 1 | 0 | 0 | 45 |
| Total | 2 | 46 | 195 | 54 | 16 | 2 | 315 |
Table 3B.
Study data extrapolation to 1 million vials/device
| Device | Number of vials delivering the following doses, assuming 1 million vials used for each device |
Total | |||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 0 | 0 | 355555 | 422222 | 200000 | 22222 | 1000000 |
| 2 | 0 | 22222 | 444444 | 400000 | 111111 | 22222 | 1000000 |
| 3 | 0 | 88888 | 888888 | 22222 | 0 | 0 | 1000000 |
| 4 | 0 | 136363 | 613636 | 204545 | 45454 | 0 | 1000000 |
| 5 | 44444 | 622222 | 311111 | 22222 | 0 | 0 | 1000000 |
| 6 | 0 | 43478 | 847826 | 108695 | 0 | 0 | 1000000 |
| 7 | 0 | 111111 | 866666 | 22222 | 0 | 0 | 1000000 |
Table 3C.
Number of persons who could be vaccinated from these 1 million vials as a function of device
| Device | Persons vaccinated per vial type |
Total | |||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 0 | 0 | 3200000 | 4222222 | 2200000 | 266666 | 9888888 |
| 2 | 0 | 177777 | 4000000 | 4000000 | 1222222 | 266666 | 9666666 |
| 3 | 0 | 711111 | 8000000 | 222222 | 0 | 0 | 8933333 |
| 4 | 0 | 1090909 | 5522727 | 2045454 | 500000 | 0 | 9159090 |
| 5 | 311111 | 4977777 | 2800000 | 222222 | 0 | 0 | 8311111 |
| 6 | 0 | 347826 | 7630434 | 1086956 | 0 | 0 | 9065217 |
| 7 | 0 | 888888 | 7800000 | 222222 | 0 | 0 | 8911111 |
Table 4.
Additional number of persons who could be vaccinated by switching from any of the other devices to device 1
| Device | Total persons vaccinated | By switching from | Additional persons vaccinated per 1 million vials |
|---|---|---|---|
| 1 | 9888888 | NA | |
| 2 | 9666666 | Device 2–1 | 222222 |
| 3 | 8933333 | Device 3–1 | 955555 |
| 4 | 9159090 | Device 4–1 | 729798 |
| 5 | 8311111 | Device 5–1 | 1577777 |
| 6 | 9065217 | Device 6–1 | 823671 |
| 7 | 8911111 | Device 7–1 | 977777 |
3.3. Residual volume (waste)
The volume of liquid left in the vials after all possible doses were drawn up was measured indirectly by weighing the vial once user felt all possible doses had been drawn and subtracting the weight of the dry, empty vial. The means of these weights are shown in Fig. 2 . There is a highly statistically significant difference (p = 0.001) in the residual volumes in vials as a function of device used. Device 1 leaves the least amount of residual volume (less than half that of devices 3–7) and thus is the least wasteful.
Fig. 2.
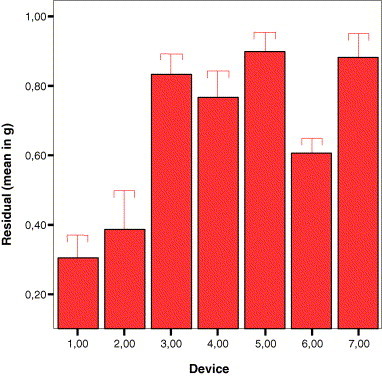
Means of residual volumes (waste) left in vials per device category. The least wasteful are devices 1 and 2 while the others are approximately twice as wasteful. Error bars indicate the 95% CI.
3.4. Reproducibility
Reproducibility is a measure of how equally and consistently the doses are drawn up from one injection to another. There was no statistically significant difference in reproducibility as measured by the standard deviation of the mean of dose weights, with values varying from 0.03395 g for device 3 to 0.06631 g for device 5.
3.5. Dead-space volumes
In an attempt to explain the differences observed in accuracy and dose-sparing characteristics of the devices, we tested the dead-space by weighing 40 syringes from each device category, then, after filling them with 0.5 mL of saline and expelling it, we weighed the device again. The difference between the latter weight and the former was considered the dead-space volume. Fig. 3 shows that devices 1 and 2 had statistically significant lower dead-space volumes than the other devices. Device 1 had the lowest dead-space volume.
Fig. 3.
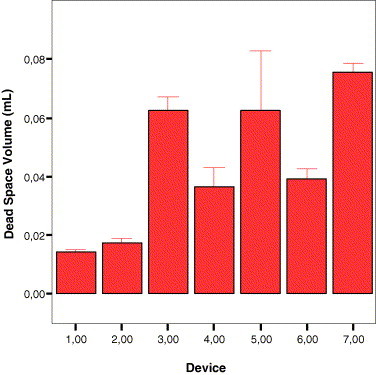
The means of dead-space volumes (n-40 per device) for the seven devices tested. Devices 1 and 2 had statistically significantly lower dead-space volumes than the other devices.
3.6. Possible sources of error
One possible source of error is the difference in the empty weight of one vial (i.e. capacity before filling) compared to another. We found no statistical difference (p = 0.751) in the dry weight of vials used in this study as a function of their use in different devices. Another possible source of error is the difference in the full weight of one vial (i.e. capacity after filling and before drawing) compared to another. We found no statistical difference (p = 0.829) in the filled weight of vials used in this study as a function of their use in different devices. Variability in either the dry or full weight of vials cannot, therefore, be used to explain the difference in number of doses obtained from vials with different devices. Multivariate analysis showed that the factor responsible for the differences found in parameters tested was the device and not the factors of country, site, clinician or vial.
3.7. Monitoring results
One study staff nurse (EF) observed each injection for a number of parameters. Virtually no users requested to see the instructions for use or a demonstration of the product or practiced with the device beforehand. However, almost all were given verbal explanations regarding the function of each device. In this regard the exposure to the devices mimics that of a pandemic, where detailed training for all users might be impossible. For all devices except number 2 almost no users required any additional training during the injection sequence. Almost a third of those using device 2 did require such training. Device 2 was the only device with a fixed dose feature (0.5 mL) and an automatic locking mechanism to prevent reuse (i.e. auto-disable syringe). It was the only device that appeared to be different from what the clinicians would normally use. For all devices except number 2 the clinicians used them correctly virtually all the time (>97.8%). For device 2 only 93.3% of users used it correctly, a difference that was statistically significant (p = 0.01).
3.8. Clinician feedback results
There was no statistical difference amongst devices regarding ease of use. There was no statistical difference amongst devices in the adequacy of training given; almost all clinicians felt it was adequate. There was a statistical difference amongst devices in ability to draw up using one's usual technique; device 2 accounted for this difference, as it required significant numbers of clinicians to change technique. There was a statistical difference amongst devices in terms of amount of time to draw up and administer an injection; devices 1 and 2 took less time to operate, while devices 3 and 7 took more.
4. Discussion
With ‘avian flu’ literally staring us in the face and the catastrophic experience of previous influenza pandemics as a backdrop, it is imperative that wise planning, backed by political will and resources, take place now. In addition to stocking anti-viral drugs and preparing vaccine manufacturers for the challenge ahead, national governments have a duty to stock the means of delivery for the vaccine. Officials and medical decision makers must be asking the question, what would constitute an optimal injecting device for influenza vaccine in a pandemic? We would like to offer the following suggested answers as a prelude to discussing the results of our study.
4.1. One should stock a syringe that reduces vaccine wastage and increases dosages per vial
It will be important to reduce vaccine wastage (given the inevitability of a shortage of vaccine); therefore, devices with a low dead-space feature will provide important advantages. Experience shows that by using a low dead-space device and by appropriate drawing-up technique (expelling excess vaccine back into the vial rather than into the air when getting rid of bubbles or aligning meniscus to dose line) one may increase the number of doses drawn from a 10-dose vial from an average of 8 to an average of 10. In some instances, involving over-filled vials (a common practice by vaccine suppliers), one may even get 11 or 12 doses from a vial. There are enormous financial benefits that will accrue if, from each vial, 20% more vaccine is saved; this may be expected to translate into 20% lower vaccine costs, 20% less workload for vaccinators and 20% greater numbers of persons vaccinated.
4.2. One should stock a syringe that is safe and simple to use
In the event of a pandemic, where tens of millions of persons must be vaccinated in a short period of time, the engagement of many non-professional persons as vaccinators is inevitable. In this case, insisting on previously used injecting conventions (e.g. two-needle per injection) is distracting and irrelevant as these people will not have had any previous experience. Techniques, in this case, must be the simplest possible, easy to teach and learn as well as free of any additional risk. A one-needle per injection technique is the easiest to learn, safer for the injector, simpler logistically and produces less sharps waste overall.
4.3. One should stock a syringe that has an integrated needle
A device with an integrated needle requires the injector to use the same needle to inject as was used for drawing up. This technique is not currently used in many health-care settings (e.g. the UK), but is the most common practice in many settings worldwide (including parts of the USA and Europe). Integrated needles save doses (due to dead-space engineering), save time (spent changing the drawing-up needle), are simpler to stock and deliver logistically (only one stocking item). They are generally considered to be safer because there is only one-needle requiring disposal.
4.4. One should stock a syringe which manufacturers can supply in the volumes needed
A three-piece syringe consists of a barrel, a plunger and a stopper on the end of the plunger. Newer manufacturing techniques have incorporated the stopper into the plunger, making these devices two-piece rather than three-piece. Two-piece syringes used for influenza vaccination are ideal in that they are less expensive, perform this function virtually indistinguishably from three-piece and are available in volumes that manufacturers have capacity to deliver.
4.5. One should stock a needle whose length permits injectors to reach muscle without hitting nerves, vessels or bone
The official recommendations [21] for influenza vaccinations by the Centers for Disease Control are as follows: adults and older children should be vaccinated in the deltoid muscle. A needle length ≥1 in. can be considered for these age groups because needles ≤1 in. might be of insufficient length to penetrate muscle tissue in certain adults and older children. Infants and young children should be vaccinated in the anterolateral aspect of the thigh. ACIP recommends a needle length of 7/8–1 in. for children aged ≤12 months for intramuscular vaccination into the anterolateral thigh. When injecting into the deltoid muscle among children with adequate deltoid muscle mass, a needle length of 7/8–1.25 in. is recommended.
Table 5 presents a summary of the key findings in our study of seven candidate devices. It would appear that there are compelling reasons to recommend device 1 for stocking in the event of an influenza pandemic. Device 1 yielded more doses per vial, probably because it has significantly lower dead-space volumes (Fig. 4 ). The additional persons vaccinated per million vials with this device is striking, and its use could probably lead to significant decreases in morbidity and mortality. In addition to sparing doses and ‘stretching’ the vaccine supply, the use of device 1 would likely mean considerable financial savings to health-care systems due to less vaccine wastage and more efficient (speedier) injections. How much this savings would be is difficult to calculate. We do not know what the cost of the pandemic vaccine will be or, more importantly, the total cost of caring for victims who could have been spared had they been vaccinated. However, if the current cost of flu vaccine is used as a reference, it is clear that the financial savings which would accrue from vaccine savings on the scale reported here would more than cover the additional costs of stockpiling the device.
Table 5.
A summary of key findings on each device
| Device number | Name/description | Accuracy | Dose-sparing | Minimal residual volume | Speed in use |
|---|---|---|---|---|---|
| 1 | Flu+ | ++++ | ++++ | ++++ | ++++ |
| 2 | SoloShot IX | +++ | +++ | +++ | ++++ |
| 3 | Plastipak Luer slip | + | ++ | + | + |
| 4 | Plastipak Luer slip | + | ++ | + | +++ |
| 5 | Injekt Luer slip | + | + | + | ++ |
| 6 | Monoject Luer slip | ++++ | ++ | ++ | +++ |
| 7 | Terumo Luer slip | ++ | ++ | + | + |
Fig. 4.
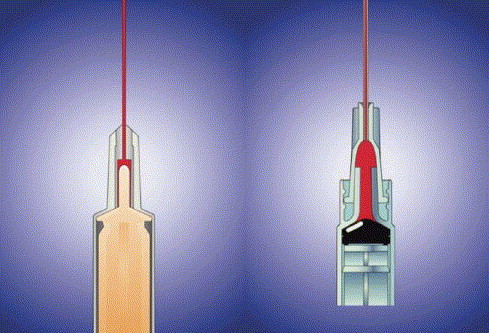
The syringe on the left has a plunger tip that fits into the cone-shaped end of the barrel, decreasing the amount of dead (waste) space. Compare to the conventional device on the right, where a considerable amount of dead-space (red) remains when the plunger is pushed in.
Device 1 is a two-piece (plastic plunger + barrel) syringe with an integral (non removable) needle. Unlike many conventional syringes, the plunger tip is pointed (Fig. 4) to minimize vaccine residue upon completion of each dose. Pharmaceutical manufacturers are advised by the US Pharmacopeia Formulary [22] to over-fill drug vials by 20–25% to compensate for wastage, from, among other factors, the dead-space volumes of standard syringes. There is, however, no guarantee that vaccine manufacturers, pressed by the inevitable vaccine shortage, would continue to offer this (or any) degree of overfill during a pandemic.
Inevitably some vaccine is discarded with the syringe following use. Injection devices with less dead-space, also known as ‘dose-sparing’ devices, can increase the amount of useable vaccine in a multi-dose vial allowing for greater distribution of the vaccine. Clinical testing and customer-documented experience have shown that the available vaccine can be extended by 7–10% through the use of these devices. Therefore the use of injection devices with dose-sparing capabilities is a relatively low cost solution to maximizing flu vaccination yields.
In summary, our study attempts to bring to light a neglected aspect of pandemic planning, the choice of a syringe which will waste as little vaccine as possible while being accurate, reliable and available in quantities sufficient to supply worldwide demand. One syringe, device 1 in our study, seems to rise above the others in this regard and its use could prove to be life-saving in the event of a pandemic of influenza.
Acknowledgements
The authors wish to thank the study staff, Eta Tierney and Elizabeth Foster, as well as the following Principal Investigators: Warsaw, Poland: Barbara Kisielinska-Holka, Nurse, Miedzyleski District Hospital. Krystyna Gorecka, Nurse, NZOXZ Sana S.C. Policlinic. Marzena Krupa, Nurse, Szpital Bielanski. United Kingdom: Pippa Clarke, Nurse, East Oxford Health Centre. Harry Stevens, Head of Resuscitation, Prince Charles Hospital, Merthyr Tidfil, Wales. Julie Peacock, Clinical Practice Development Officer, Fife NHS Primary Care Trust, Kirckaldy, Scotland. Sweden: Magdalena Annersten, Nurse, Oresund Diabetes Team, Lund. We also thank the 45 doctors and nurses who participated in this study.
Conflict of interest: One of our authors (KS) is medical director for BD, a manufacturer of injecting syringes. Another author (AF) has a consulting agreement with BD.
References
- 1.The Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5 Avian influenza A (H5N1) infection in humans. NEJM. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 2.Johnson N.P., Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 3.Langford C. The age pattern of mortality in the 1918–19 influenza pandemic: an attempted explanation based on data for England and Wales. Med Hist. 2002;46(1):1–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Trampuz A., Prabhu R.M., Smith T.F., Baddour L.M. Avian influenza: a new pandemic threat? Mayo Clin Proc. 2004;79:523–530. doi: 10.4065/79.4.523. [Erratum, Mayo Clin Proc 2004;79:833] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO inter-country consultation: influenza A/H5N1 in humans in Asia, Manila, May 6–7 2005 (accessed October 12, 2005, at http://www.who.int/csr/disease/avianinfluenza/H5N1IntercountryAssessment.pdf).
- 6.Mills C.E., Robins J.M., Lipsitch M. Transmissibility of 1918 pandemic influenza. Nature. 2004;432:904–906. doi: 10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balicer R.D., Huerta M., Grotto I. Tackling the next influenza pandemic. BMJ. 2004;328:1391–1392. doi: 10.1136/bmj.328.7453.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO consultation on priority public health interventions before and during an influenza pandemic. April 2004 (accessed October 12, 2005, at http://www.who.int/csr/disease/avian_influenza/consultation/en/).
- 9.European Influenza Surveillance Scheme, site http://www.eiss.org/index.cgi.
- 10.Centers for Disease Control and Prevention. Update on influenza A (H5N1) and SARS: interim recommendations for enhanced U.S. surveillance, testing, and infection controls. February 3, 2004 (accessed October 12, 2005, at http://www.cdc.gov/flu/avian/professional/han020302.htm).
- 11.Cox N., Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 12.Monto A.S., Gravenstein S., Elliott M., Colopy M., Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 13.Seto W.H., Tsang D., Yung R.W.H., Ching T.Y., Ng T.K., Ho M. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moscona A. Neuraminidase inhibitors for influenza. NEJM. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 15.Hayden F.G. Pandemic influenza: is an antiviral response realistic? Pediatr Infect Dis J. 2004;23(Suppl):S262–S269. doi: 10.1097/01.inf.0000144680.39895.ce. [DOI] [PubMed] [Google Scholar]
- 16.Harper SA, Fukuda K, Uyeki TM, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004;53:1–40. [PubMed]
- 17.WHO site: http://www.who.int/csr/disease/influenza/pandemic/en/.
- 18.Hayden F.G. Perspectives on antiviral use during pandemic influenza. Philos Trans R Soc Lond B Biol Sci. 2001;356:1877–1884. doi: 10.1098/rstb.2001.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aspinall S. Comparison of BD SoloShot™ IX auto-disable syringes with conventional disposable syringes in the South African EPI programme (on file and available from BD on request).
- 20.Nelson CM. Comparison of SoloShot™ IX auto destruct syringe to a disposable syringe in a national immunization campaign in Indonesia. WHO/EPI/TECHNET.98/BK.3.
- 21.CDC site: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5102a1.htm.
- 22.US Pharmacopeia Formulary 2004, chapter 1151, can be viewed at http://www.uspnf.com/uspnf/pub/index?usp=28&nf=23&s=2.


