Abstract
Despite new agent development and short-term benefits in patients with colorectal cancer (CRC), metastatic CRC cure rates have not improved due to high rates of 5-fluorouracil (5-FU)/leucovorin/oxaliplatin (FOLFOX)-resistance and a clinical therapeutic plateau. At the same time, this treatment regime leads to significant toxicity, cost, and patient inconvenience. Drug-resistance is linked to CRC stem cells, which are associated with the epidermal-to-mesenchymal transition (EMT) pathway. Thus, to optimally treat CRC, a therapy that can target the cell survival and EMT pathways in both CRC bulk and stem cell populations is critical. We recently identified a novel small molecule NSC30049 (7a) that is effective alone, and in combination potentiates 5-FU-mediated growth inhibition of CRC bulk, FOLFOX-resistant, and CRC stem cells both in vitro and in vivo models. In the present study, we report the synthesis and anti-CRC evaluation of several stable and effective 7a analogs. ASR352 (7b) was identified as one of the equipotent 7a analogs that inhibited the growth of CRC bulk cells, sensitized FOLFOX-resistant cells, and reduced the sphere formation capacity of CRC stem cells. It appears that the complex mechanism of cytotoxicity for 7b includes abrogation of 5-FU-induced the S phase, reduction of the phosphorylation of Chk1 at S317P, S345P and S296P, increased γH2AX staining, activation of caspase 3/PARP1 cleavage, and enhancement of Bax/Bcl2 ratio. Further 7b-mediated reduced phosphorylation of Chk1 was an indirect effect, since it did not inhibit Chk1 activity in an in vitro kinase assay. Our findings suggest that 7b as a single agent, or in combination with 5-FU can be developed as a therapeutic agent in CRC bulk, FOLFOX-resistant, and CRC stem cell populations for unmanageable metastatic CRC conditions.
Keywords: Colorectal cancer, Stem cells, Tetraazaadamantane, Apoptosis, Checkpoint kinase 1
Graphical abstract
Highlights
-
•
Novel analogs of orphan molecule NSC30049 were synthesized.
-
•
ASR352 (7b) was identified as most cytotoxic to colorectal cancer (CRC) cells.
-
•
7b inhibited the growth of CRC bulk and sensitized FOLFOX-resistant cells.
-
•
7b reduced sphere formation capacity of CRC stem cells.
-
•
7b abrogated 5-FU-induced the S phase and inhibited the phosphorylation of Chk1.
1. Introduction
Despite the attempts to improve patient outcomes by incorporating new active systemic agents into clinical practice, there has been little improvement in the metastatic CRC patient cure rate. In spite of 5-fluorouracil (5-FU) use, with or without additional therapy, to eradicate metastatic disease after “curative” surgery for CRC, most cancers relapse within the first few years following treatment completion. This suggests that there is a relatively rapid repopulation of neoplastic progeny, i.e., CRC stem cells [1,2]. A more aggressive treatment with higher or prolonged doses of multiple drug combinations, such as 5-FU and oxaliplatin, leads to serious side-effects [3,4]. Despite the frequent use of 5-FU in cancer, a thorough understanding of its mechanisms of action and interventions to overcome resistance are still relatively limited. One of mechanisms of resistance to 5-FU occurs through the induction of the EMT pathway [5,6]. The down-regulation of epithelial markers (APC, β-catenin and E-cadherin) is characteristic to the EMT pathway EMT, which is involved in CRC stem cell invasion, metastasis and drug-resistance [[7], [8], [9], [10], [11], [12]].
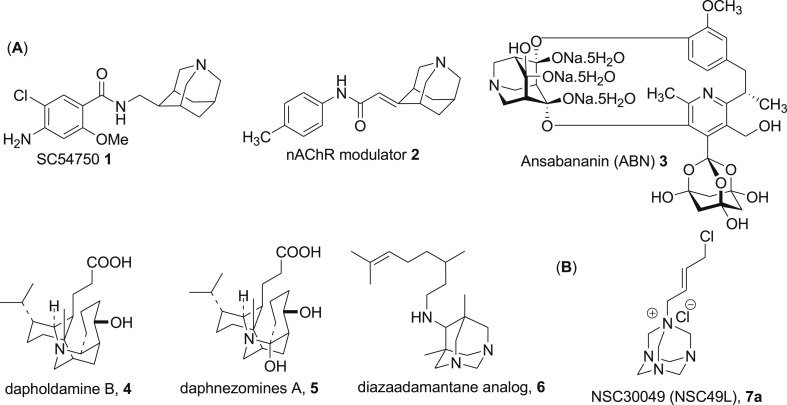
In an effort to develop a more effective drug that has a minimal side effect profile on normal epithelial cells in CRC treatment, we recently identified a novel tetraazaadamantane containing small molecule NSC30049 (1-(4-Chloro-2-butenyl)-1λ∼5∼,3,5,7-tetraazatricyclo[3.3.1.1–3,7∼]decane) that either alone or in combination potentiates 5-FU-mediated growth inhibition of CRC heterogeneous bulk, FOLFOX-resistant and CRC stem cells while maintaining the integrity of the normal colonic epithelial cells [13]. Nitrogen containing adamantane analogs [14,15], e.g., 1-azaadamantanes, 1,3-diazaadamantanes, 1,3,5-triazaadamantanes and 1,3,5,7-tetraazaadamantanes are of great interest as conformationally constrained motifs for the design of various pharmacological tools [16]. Structural uniqueness of these azaadamantanes is related to both physical and chemical properties, most notably in the reduction of lipophilicity thus inducing higher aqueous solubility of these compounds compared to that of adamantanes consisting solely of carbon and hydrogen atoms [14,15]. However, studies of the biological activity of azaadamantanes and their derivatives are still relatively less explored despite having good pharmacological properties. A few representative examples of 1-azaadamantane motif (Fig. 1 ) include 5-HT4 receptor agonist SC-54750 (1) [17], a modulator of neuronal nicotinic acetylcholine receptors (nAChRs) 2 [[18], [19], [20]], inhibitors of σ1 (CNS related diseases) and σ2 (cancer), inhibitors of human T-cell leukemia virus-1 (HTLV-1), and anti-viral and analgesic agents [[21], [22], [23]]. Furthermore, the natural product, ansabananin (ABN) (3) that inhibits SARS Coronavirus (SCV) [24], and other natural alkaloids dapholdamine B (4) [25], daphnezomines A (5) [26] possess 1-azaadamantane moiety (Fig. 1). Recently, a diazaadamantane derivative 6 has been reported as an inhibitor of the rimantadine-resistant strain of the influenza A/Puerto Rico/8/34 (H1N1) virus [27]. It is noteworthy that although methenamine is clinically used to prevent urinary tract infections [28], the tetrazaadementane skeleton is barely utilized to design small drug-like molecules except in synthesizing the crucial reaction intermediate phenacyl amines [[29], [30], [31]]. NSC30049 (7a) is a unique tetraazaadamentane (methenamine) analog identified recently by our group that showed activity against 5-FU/oxaliplatin resistant and stem cells [13].
Fig. 1.
Panel A shows the structure of pharmacologically important natural and synthetic azaadamantane compounds. Panel B is the structure of the lead tetraazaadamantane Chk1 inhibitor 7a.
The expected mechanism of action of 7a in CRC is through the inhibition of checkpoint kinase 1 (Chk1) pathway through an indirect mechanism(s) [13]. Since Chk1 plays a critical role in cell cycle regulation in response to DNA damage, several Chk1 inhibitors have been developed and tested as chemo-sensitizing agents [[32], [33], [34], [35], [36], [37], [38], [39]]. Compound 7a has been shown to inhibit Chk1 phosphorylation both in vitro and in vivo CRC models [13]; however, the pharmacokinetic analysis showed a short plasma half-life similar to 5-FU [40]. The short plasma half-life of 7a is likely due to the presence of a reactive alkyl chloride group. To overcome this problem, we designed and synthesized several novel tetraazaadamantane 7a analogs, and tested their cytotoxic efficacy against CRC bulk, FOLFOX-resistant as well as CRC stem cells.
2. Results and discussion
2.1. Design
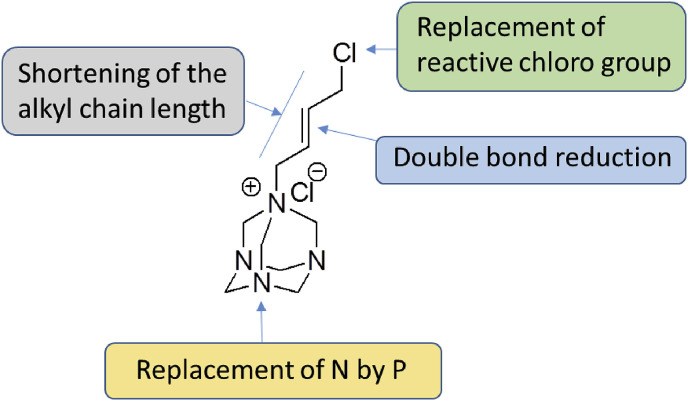
The structural optimization of 7a was focused mainly on replacing the reactive alkyl chloride group with more stable alkyl/alkenyl/aryl moieties. The rationale is that reactive alkyl chloride can potentially react with protein thiols and amines to compromise its plasma half-life and thus the biological activity. The functionalities that replaced cholo (Cl) group were chosen to enhance the overall stability of the molecule while retaining or possibly enhancing the potency (Fig. 2 ). In addition, the approaches of modification also included saturation of the olefinic group, shortening of the alkyl chain length, and replacement of nitrogen (N-7) of tetraazaadmantane ring with phosphorus having more labile valence shell electrons (Fig. 2).
Fig. 2.
Optimization strategy for 7a.
2.2. Chemistry
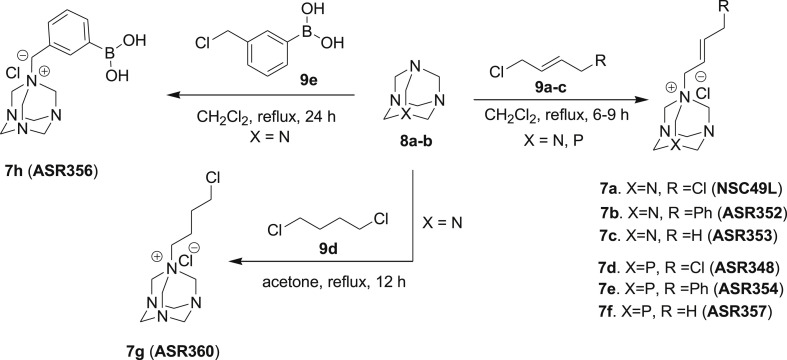
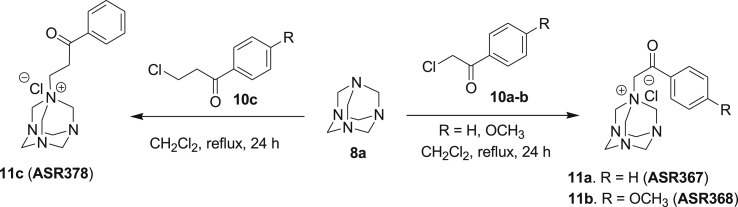
Novel 1,3,5,7-tetraazaadamantane (7a-c, g, f & 11a-c) and 1,3,5-triaza-7-phosphaadamantane (7d-f) analogs of NSC30049 (7a) were prepared as depicted in Scheme 1, Scheme 2 . Compounds 7a-c were synthesized by the reaction of readily available tetraazaadamantane 8a with various alkenyl halides 9a-c in CH2Cl2 under reflux conditions in quantitative yields (Scheme 1) [41]. To evaluate the difference in activity between the nitrogen and corresponding phosphorus analogs, we also synthesized isosteric 7-phosphorus analogs (7d-f) of lead compound 7a. 1,3,5-Triaza-7-phophaadamanatne 8b was reacted under reflux conditions in CH2Cl2 with different alkenyl halides 9a-c to furnish the corresponding phosphorus analogs 7d-f in excellent yields (Scheme 1). Butyl chloride analog 7g and the boronic acid analog 7h were also synthesized using similar reaction conditions by refluxing for 12 h and 24 h, respectively.
Scheme 1.
Synthesis of 1,3,5,7-tetraaza- and 1,3,5-triaza-7-phospha-adamentane derivatives (7a-h).
Scheme 2.
Synthesis of 1,3,5,7-tetrazaadamentane phenacyl derivatives (11a-c).
To further diversify the structure activity relationship study on azaadamantane 7a derivatives, we synthesized azaadamantane analogs 11a-c as depicted in Scheme 2. Compounds 11a-c were synthesized by reacting 8a with readily available phenacyl chlorides 10a-c in CH2Cl2 under reflux conditions in good yields (Scheme 2) [41]. The structures of all the novel NSC30049 derivatives were confirmed by 1H NMR, 13C NMR and HRMS analysis. The compounds purity (≥98%) was analyzed by analytical high-performance liquid chromatography (HPLC) before proceeding for in vitro biological assays.
2.3. Biology
2.3.1. Cytotoxicity evaluation of novel azaadamantane: ASR352 (7b) induces cytotoxicity and reduces the effective concentration of 5-FU in CRC cells
We determined the IC50 of the novel azaadamantane (7a-c, g, h and 11a-c) and aza-phosphaadamantane (7d-f) analogs of NSC30049 (7a) in HCT116 cells by MTT-cell survival assay. Results showed a variable range of IC50 of these analogs. Based on the results of this cell viability assay, some structure-activity relationship (SAR) can be inferred: First, reducing the olefinic double bond by retaining chlorine atom (7g) reduced the potency of the compounds on cancer cell viability. Second, replacing the chlorine atom of 7a by a phenyl (ASR352, 7b) retained the activity of the molecule while the removal of the chlorine atom (7c) led to reduced potency (Table 1 ). Third, isosteric phosphorous analogs of 7a (i.e. 7d) and 7b (i.e. 7e) displayed only moderate cell viability when compared to its parent nitrogen analogs, while both 7c and its isosteric phosphorus analog 7h exhibited poor activity (Table 1). Compound 7h having a 3-phenyl boronic acid functionality also led to a reduced potency. Fourth, among the analogs designed by replacing chlorine atom of 7a with phenacyl groups of different alkyl chain lengths (11a-c), analogs having phenyl (ASR367, 11a, IC50 18.53 ± 1.41 μM) and 4-methoxy phenyl (11b, IC50 13.10 ± 0.16 μM) displayed moderate inhibition of cell viability, while 11c designed by extending the alkyl chain length of 11a lost the activity (Table 1). Overall, except replacement of Cl− group of 7a by a phenyl group, all other modifications in structure including chain length, functional group variation, or isosterically replacing nitrogen by phosphorous, caused a dramatic reduction in or the total loss of cytotoxicity against HCT116 cells (Table 1). Compounds 7d, 11a and 11b showed IC50 in the range of 13–18 μM; other analogs, such as 7c, 7e, 7f, 7h, 7g and 11c did not show any or very poor inhibitory activity against HCT116 cells (Table 1).
Table 1.
Screening of NSC30049 (7a) analogs for their toxicity with HCT116 cells.
| Compounds | IC50 (μM)a |
|---|---|
| 7a | 2.46 ± 0.14 |
| 7b | 5.75 ± 0.09 |
| 7c | ND |
| 7d | 18.39 ± 2.79 |
| 7e | ND |
| 7f | ND |
| 7h | ND |
| 7g | ND |
| 11a | 18.53 ± 1.41 |
| 11b | 13.10 ± 0.16 |
| 11c | ND |
The IC50 of different analogs was determined by MTT-cell survival assay. Data is the mean ± SE of four estimations. ND, not detectable.
Among all these analogs, 7b exhibited a low micromole range IC50 (5.75 ± 0.09 μM) that was close to the IC50 of 7a (2.46 ± 0.14 μM) (Table 1). Thus, 7b was identified as the lead novel compound having potency comparable to 7a. This result suggests that the Cl− of the 7a is not an active group responsible for the cytotoxic activity of this compound and 7b appears pharmacologically more stable and favorable for further development.
Next, we determined whether 7b can enhance the potency of 5-FU in CRC cells. Results showed that the 7b treatment reduced the IC50 of 5-FU with HCT116 and HT29 cell lines in a dose-dependent manner that was similar to 7a (Table 2 ). These results indicate that 7a and its analog 7b can reduce the effective dose of 5-FU and inhibit the growth of all CRC cells, thus potentiating the effect of current therapy.
Table 2.
IC50 of 5-FU in the presence of 7a and 7b with HCT116 and HT29 cell lines.
| IC50 (μM)a | ||
|---|---|---|
| Treatments | HCT116 cells | HT29 cells |
| 5-FU | 15.16 ± 4.20 | 16.45 ± 2.627 |
| 5FU + 0.5 μM 7a | 7.05 ± 0.79 | 8.12 ± 0.14* |
| 5FU + 1 μM 7a | 6.27 ± 0.6* | 7.38 ± 0.29* |
| 5FU + 0.5 μM 7b | 8.19 ± 0.05* | 8.52 ± 0.49* |
| 5FU + 1 μM 7b | 7.56 ± 0.05* | 6.57 ± 0.36* |
The IC50 of 7a and 7b was determined by MTT-cell survival assay. Data is the mean ± SE of four estimations. * = Significantly different than 5-FU alone.
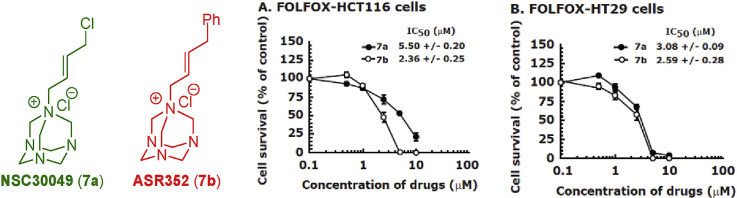
2.3.2. Compound 7b inhibits the growth of FOLFOX-HCT116 and FOLFOX-HT29 cells with similar efficiency as 7a
To determine whether 7b can inhibit the survival of FOLFOX-resistant HCT116 and HT29 cell lines [42], we performed MTT-cell survival assay and compared the results with 7a. Indeed, treatment with 7b effectively sensitized both FOLFOX-HCT116 and FOLFOX-HT29 cell lines with an IC50 of 2.38 ± 0.25 μM and 2.59 ± 0.28 μM, respectively, that was similar to or slightly better than the IC50 of 7a; IC50 of 7a was 5.50 ± 0.20 μM and 3.08 ± 0.09 μM, respectively, for FOLFOX-HCT116 and FOLFOX-HT29 cell lines (Table 3 ). Since the growth of FOLFOX-resistant HCT116 and HT29 cell lines are maintained in the continuous presence of 50 μM 5-FU and 1.25 μM oxaliplatin, the effect on reduced IC50 of 7b and 7a with these cell lines is independent of 5-FU and oxaliplatin treatments.
Table 3.
IC50 of 7a and 7b with FOLFOX-HCT116, FOLFOX-HT29 and CA2 cell lines.
| IC50 (μM)a | |||
|---|---|---|---|
| Compounds | FOLFOX-HCT116 cells | FOLFOX-HT29 cells | CA2 cells |
| 7a | 5.50 ± 0.20 | 3.08 ± 0.09 | 7.96 ± 1.03 |
| 7b | 2.38 ± 0.25 | 2.59 ± 0.28 | 9.06 ± 0.71 |
The IC50 of 7a and 7b was determined by MTT-cell survival assay with FOLFOX-HCT116 and FOLFOX-HT-29 cell lines and by spheroid formation assay with CA2 cells. Data is the mean ± SE of three estimations.
2.3.3. Compound 7b inhibits the spheroid formation capacity of CA2 cells
In recent studies we have shown that 7a inhibits the spheroid formation activity of CRC stem cells [13]. Here, we determined whether 7b has similar activity against CRC stem cells. For these experiments, we used a well-characterized CRC stem cell line, CA2 [43,44] and performed sphere formation assays involving three-dimensional (3D) culture system in serum-free conditions. Results showed that 7b inhibited the growth of spheres similar to 7a (Table 3). The IC50 of 7b in CA2 cells were greater than the IC50 of heterogeneous population of CRC bulk cell lines HCT116 and HT29, suggesting that CA2 cells are more resistant to these drugs than CRC bulk cells.
2.3.4. Compound 7b abrogates S phase arrest of HCT116 cells
In recent years many anticancer drugs have been discovered that target cell cycle checkpoints, arrest cell proliferation, and at the same time, provides opportunity for the repair of the damaged DNA [45,46]. The consequence of this leads to drug resistance and cell survival [47,48]. Therefore, current efforts are to induce death of the arrested cells by using inhibitors of the checkpoint kinases [[49], [50], [51]]. It was known that the therapeutic activity of 5-FU is linked to the replication stress as one of the mechanisms [52,53]. Several other inducers of the replication stress pathways have been implicated as therapeutic agents [51,[53], [54], [55], [56]]. By enhancing replicative stress through perturbing the S-G2/M checkpoints in cancer cells, a mitotic catastrophe can be induced with accumulated single-stranded DNA (ssDNA), and double-stranded DNA (dsDNA) breaks, that exceeds the repair capacity of the cell; and leads to cell death [[57], [58], [59]].
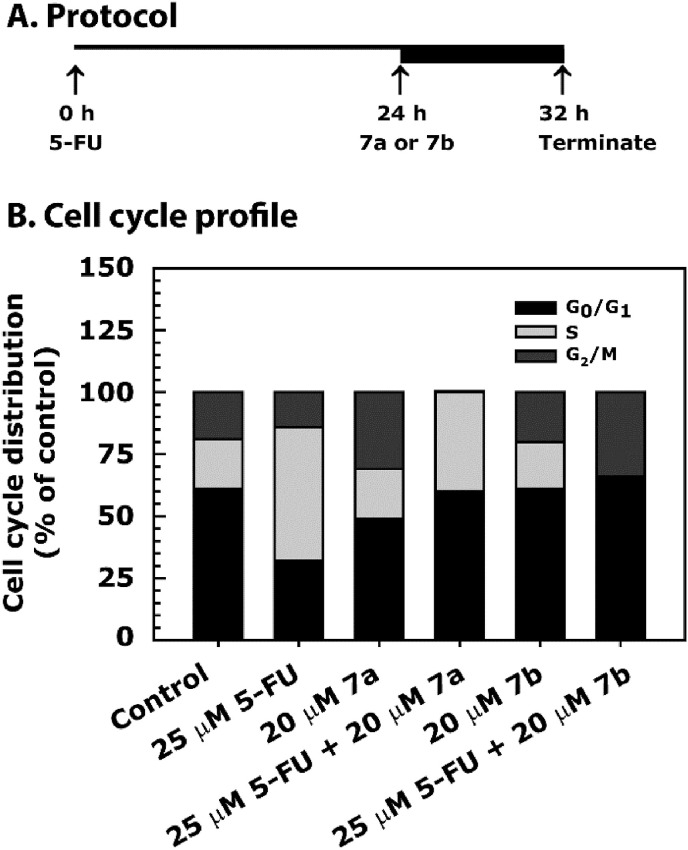
In recent studies, we have shown that 7a treatment further increases S phase arrest of the cell cycle over hydroxyurea (HU) treatment [13], which is a pure inducer of replication stress-dependent S phase arrest [60,61]. In the present study, HCT116 cells were treated with 25 μM of 5-FU for 24 h, then with 20 μM of 7a or 7b for additional 8 h (Fig. 3 A). The cell cycle analysis results showed 5-FU-induced S phase arrest of HCT116 cells (Fig. 3B). However, after combination treatment with 7a, while S phase arrest continued the G2/M phase arrest was abrogated. Interestingly, in combination with 5-FU, 7b abrogated the S phase and increased the G2/M phase arrest (Fig. 4 B). The structural differences in 7a and 7b seem to be important for the differential activity of these two compounds on cell cycle profiling.
Fig. 3.
Cell cycle profile of HCT116 cell line-treated with 7a and 7b either alone or in combination with 5-FU. Panel A describes the assay protocol. Panel B shows the data as a stacked bar graph. Cells were grown in 0.5% FBS for 20 h and then treated with 25 μM of 5-FU for 24 h, followed by treatment with 20 μM of 7a or 7b for an additional 8 h. After treatment, cells were processed for cell cycle analysis by FACS analysis. The ranges for G0/G1, S and G2/M-phase arrested cells were established on the basis of the corresponding DNA content of the histograms. Data are mean percent distribution of DNA content of two different experiments.
Fig. 4.
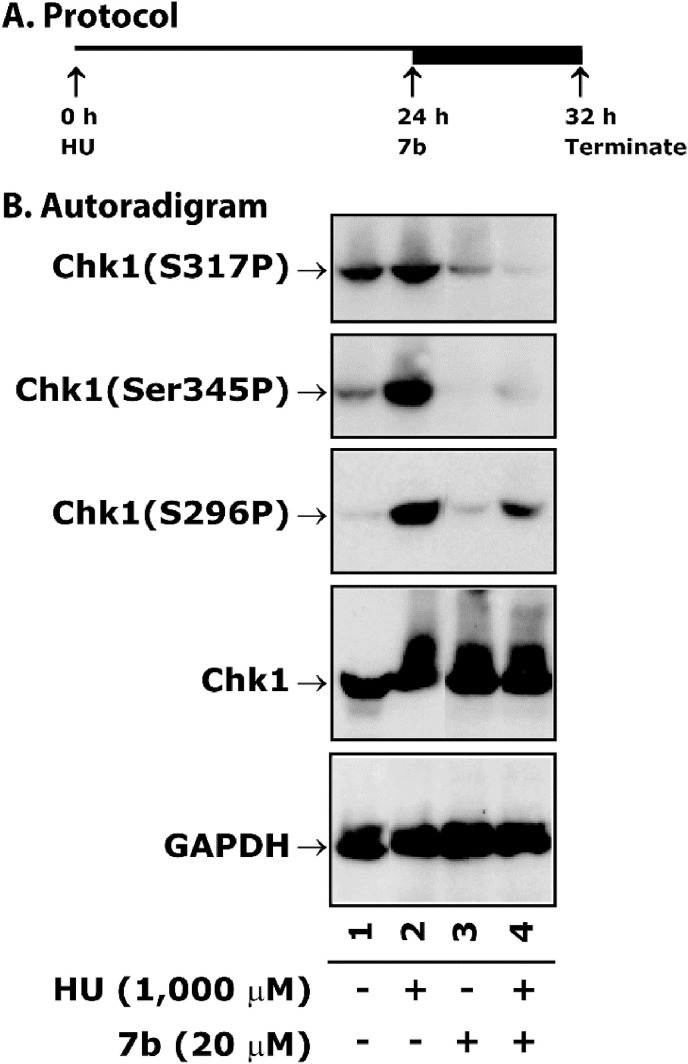
Treatment of 7b inhibits HU-induced phosphorylation of Chk1 in HCT116 cells. Panel A depicts the protocol of the assay. Cells were treated with 1 mM of HU for 24 h followed by 20 μM of 7b for an additional 8 h. Panel B shows representative autoradiograms of Chk1 western blots analyzed with whole cell extract. Normalization of protein loading was assessed by GAPDH.
2.3.5. Compound 7b blocks HU-induced phosphorylation of Chk1 in HCT116 cells
Chk1 after phosphorylation at S317 subsequently phosphorylates itself at S345 for efficient progression of DNA replication forks and prevention of fork stalling. Therefore, to impair DNA repair or cellular division, Chk1 (S317P) inhibitors as single agent or in combination with chemotherapeutic agents may be important for further research. It is well-established that ATR-mediated Chk1 phosphorylation at S317P and S345P plays a critical role in DNA replication, DNA repair, S-G2/M phase checkpoint control [62], and has been the target for cancer therapy [63], It has been also shown that the inhibition of Chk1(S317P) and Chk1(S296P) abrogates S-phase progression [64,65]. Since 7b abrogates S phase arrest, we determined the phosphorylation level of Chk1 after treatment with 7b either alone or in combination with HU, a pure inducer of S phase arrest [60,61]. The experimental protocol is described in Fig. 4A. Results showed a high level phosphorylation of Chk1(S317P), Chk1(S345P) and Chk1(S296P) after the treatment with HU (Fig. 5 compare lane 1 with 2)’ which was reduced after combination treatment with 7b (Fig. 4, compare lane 2 with 4). However, treatment with 7b alone did not affect the phosphorylation of Chk1 (Fig. 4, compare lane 1 with 3). These results suggest that 7b blocks HU-induced Chk1 phosphorylation and thus is involved in the abrogation of S phase as seen in Fig. 4.
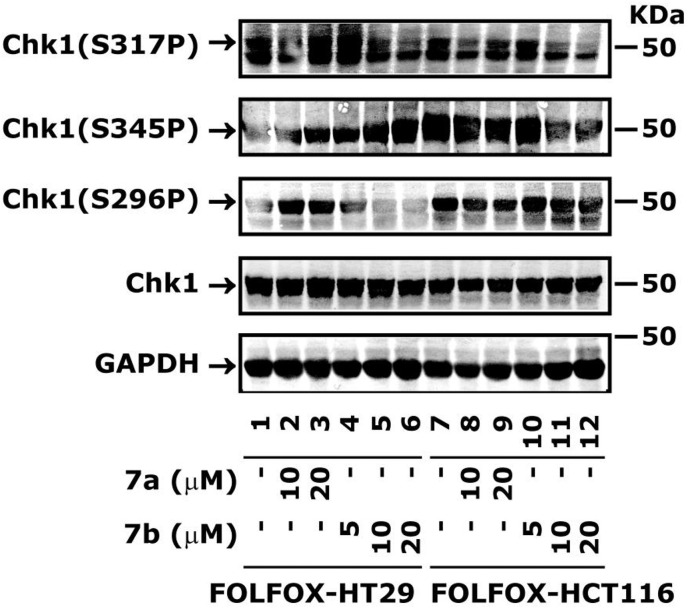
Fig. 5.
Treatment of 7a and 7b inhibit Chk1 in FOLFOX-HT29 and FOLFOX-HCT116 cells. Cells were treated with different concentrations of 7a and 7b for 24 h. The whole cell extract was processed for the determination of Chk1 levels by western blot analysis. Normalization of protein loading was assessed by GAPDH.
2.3.6. Compound 7b blocks Chk1 phosphorylation in FOLFOX-HCT116 and FOLFOX-HT29 cell lines
We determined whether 7a and 7b can also block Chk1 phosphorylation, as observed with bulk HCT116 cells. The FOLFOX-HCT116 and FOLFOX-HT29 cell lines were treated with different concentrations of 7a and 7b for 24 h. The results showed inhibition of Chk1(S317P) and Chk1(S296P) in a dose-dependent manner (Fig. 5), as seen with bulk cells (Fig. 4B). There was an increase in Chk1(S345P) phosphorylation by both 7a and 7b with FOLFOX-HT29 cells; however, with FOLFOX-HCT116 cells it was reduced in a dose-dependent manner (Fig. 5). This differential effect of 7a and 7b on these two cell lines may be due to their p53 status, where FOLFOX-HT29 cells express mutant p53 and FOLFOX-HCT116 cells express wild-type p53. However, the mechanism by which p53 might be regulating Chk1 phosphorylation in these two cells lines is currently unknown.
2.3.7. Compound 7b induces double-stranded break of DNA in HCT116 cells, both as a single agent and in combination with 5-FU
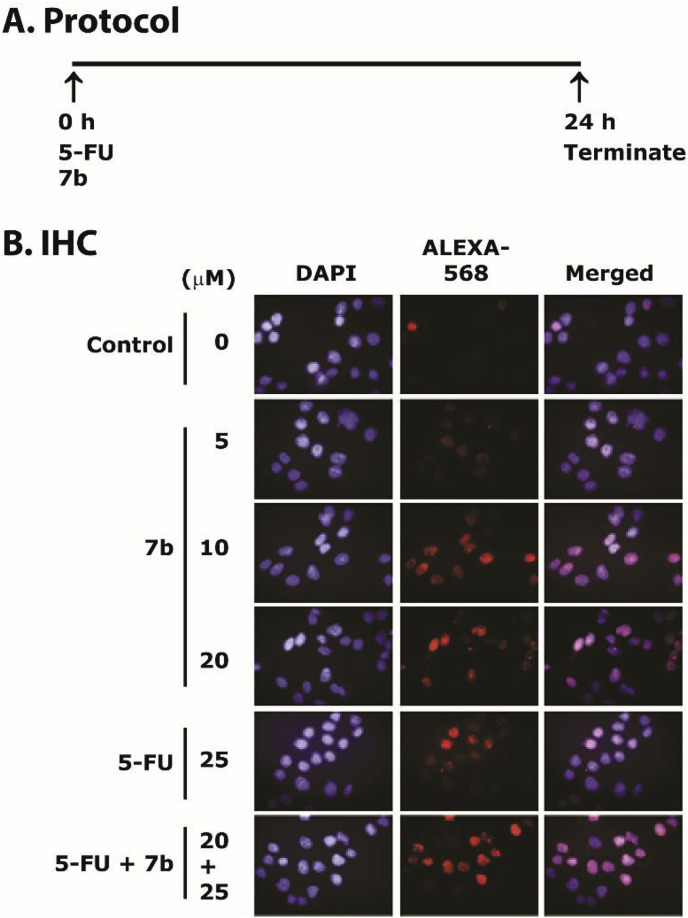
Blockage of replication from UV, hydroxyurea, or 5-FU treatment activates the ATR-Chk1 pathway, stalls replication fork, and leads to double-stranded DNA breaks (DSBs), cell cycle arrest and apoptosis [66,67]. ATR phosphorylates histone H2A variant H2AX at Ser139 to form γH2AX that is considered as a marker of DSBs [68,69]. Previously, it has been shown that the inhibition of Chk1 by gemcitabine and 5-FU treatments increases γH2AX levels through ATR-mediated replicative stress [66,68]. In the present study, we examined whether 7b-induced toxicity either alone or in combination with 5-FU, was related to DSBs and phosphorylation of γH2AX. We examined the levels of γH2AX by IHC staining. The cells treatment protocol is given in Fig. 6 A. We observed an increased γH2AX-foci after treatment with 7b alone in HCT-116 cells that was further increased in combination with 5-FU (Fig. 7 B). Thus, these results show that 7b either alone or in combination with 5-FU induces replication stress-mediated DNA damage in HCT-116 cells.
Fig. 6.
Compound 7b and 5-FU treatments increase γH2AX-foci in HCT116 cells. Panel A describes the protocol of the assay. Panel B shows the IHC staining of γH2AX-foci formation (red) in nuclei of HCT-116 cells treated with 7b (0, 5, 10 and 20 μM), 5-FU (25 μM) and combination of 5-FU (25 μM) and 7b (20 μM) for 24 h. Magnification = 40×. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 7.
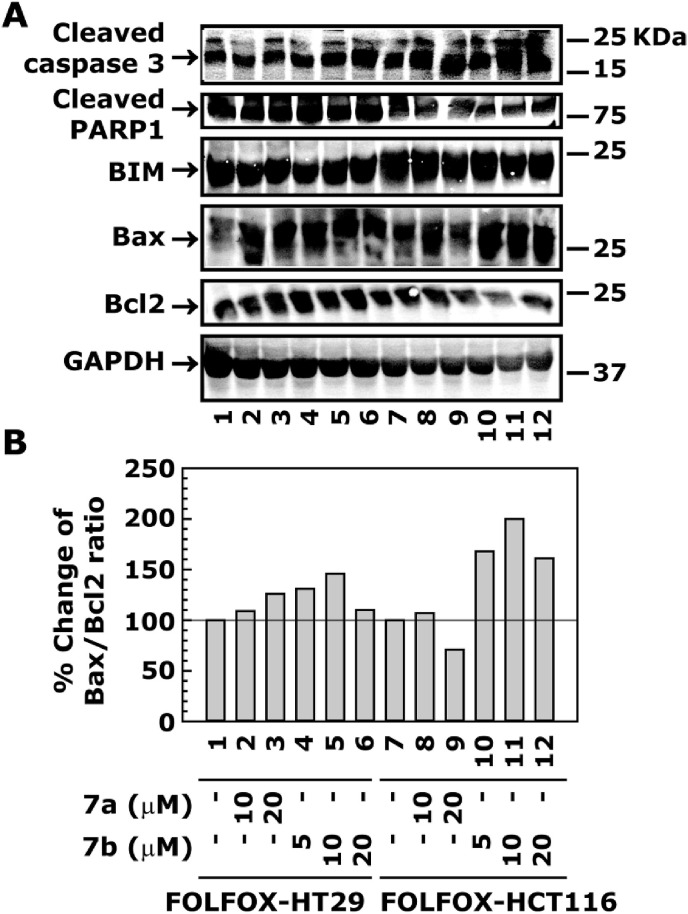
Effect of 7a and 7b treatments on the expression of apoptotic marker proteins in FOLFOX-HT29 and FOLFOX-HCT116 cell lines. Cells were treated with different concentrations of 7a and 7b for 24 h. The whole cell extract was processed for the determination of protein levels by western blot analysis. Panel A shows the protein levels of cleaved caspase 3, cleaved PARP 1, BIM, Bax and Bcl2. Normalization of protein loading was assessed by GAPDH. Panel B shows the Bax/Bcl2 ration.
2.3.8. Compounds 7a and 7b do not inhibit Chk1 activity in a reconstituted purified system.
While from our cell-based studies it was clear that 7a and 7b inhibit Chk1 phosphorylation in FOLFOX-HCT116 and FOLFOX-HT29 cells, we do not know whether the inhibition of Chk1 phosphorylation by 7a and 7b was through direct or indirect mechanisms. For direct mechanism, we performed in vitro Chk1 kinase assay by using purified Chk1 protein. Results showed no inhibition of the Chk1 activity by any of these compounds (Table 4 ). Next, we tested whether any other kinases may be a target of these compounds. We profiled the activity of 366 kinases in an in vitro assay system but did not find a significant inhibition by 7a and 7b (see Table S1, Supplementary Material). From these results it became clear that the inhibition of Chk1 phosphorylation is not directly caused by 7a and 7b, but possibly due to some unknown mechanism(s) which remain to be elucidated.
Table 4.
Effect of 7a and 7b on the Chk1 activity in an in vitro assay system.
| NSC30049 (7a) and ASR352 (7b) Concentration (M) |
Percent activity |
Saturosporine Concentration (M) |
||
|---|---|---|---|---|
| NSC30049 (7a) | ASR352 (7b) | Staurosporine | ||
| 1.00E-04 | 91.54 | 90.47 | 0.31 | 2.00E-05 |
| 3.33E-05 | 105.37 | 95.76 | −1.28 | 5.00E-06 |
| 1.11E-05 | 94.37 | 94.16 | 1.47 | 1.25E-06 |
| 3.70E-06 | 106.53 | 96.66 | 2.55 | 3.13E-07 |
| 1.23E-06 | 113.75 | 95.86 | −0.27 | 7.81E-08 |
| 4.12E-07 | 99.00 | 105.75 | 6.54 | 1.95E-08 |
| 1.37E-07 | 100.06 | 106.27 | 9.81 | 4.88E-09 |
| 4.57E-08 | 92.52 | 106.18 | 13.67 | 1.22E-09 |
| 1.52E-08 | 92.23 | 90.13 | 41.24 | 3.05E-10 |
| 5.08E-09 | 112.15 | 104.82 | 55.99 | 7.63E-11 |
| DMSO | 103.06 | 100.00 | 96.94 | DMSO |
Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 100 μM. Control compound, Staurosporine, was tested in 10-dose IC50 mode with 4-fold serial dilution starting at 20 μM. Reactions were carried out at 10 μM ATP. Data is presented as % enzyme activity (relative to DMSO controls).
ATR is one of the key activators of Chk1 phosphorylation at Ser345 and Ser317 [70], which promotes autophosphorylation of the same kinase at Ser296 that plays an important role in cell cycle control and DNA replication [71]. In our studies, the inhibition of Chk1 phosphorylation by 7a and 7b is caused only at Ser317 not at Ser345, indicating that ATR may not be a target of 7a and 7b. However, this possibility cannot be completely ruled out as the ATR was not included in our kinase profiling assays. It is also possible that Chk1 is indirectly inhibited by proteins, such as Claspin, that serves as a scaffold protein in ATR-mediated Chk1 phosphorylation [72,73]. The down-regulation of Claspin expression have been shown to inhibit Chk1 activation in response to replication stress [74]. Studies are awaited to examine whether 7a- and 7b-mediated inhibition of Chk1 phosphorylation is due to down-regulation of Claspin. Furthermore, 7a and 7b might down-regulate mammalian TOR complex 1 (mTORC1) activity, that in turn, may down-regulate Chk1 phosphorylation and induce γH2AX phosphorylation [75]. However, we have not yet experimentally verified the role of mTORC1 in 7a- and 7b-mediated inhibition of Chk1 phosphorylation.
2.3.9. Treatment of 7a and 7b increases the levels of apoptosis marker proteins in FOLFOX-HT29 and FOLFOX-HCT116 cell lines
In previous studies, increased expression of Bax, an important member of the Bcl2 family, has pro-apoptotic effect [76], and has been implicated in the better survival of colorectal cancer patients [[77], [78], [79]]. On the other hand, increased expression of Bcl2 prevents apoptosis by inhibiting the activity of Bax [80]. Thus, the increased Bax/Bcl2 ratio plays a critical role in determining the susceptibility to apoptosis in cancer cells. We determined the effect of 7a and 7b treatments on the expression of apoptotic marker proteins e.g. cleaved caspase 3 and PARAP 1 and then determined their correlation with Bax/Bcl2 ratio in FOLFOX-HT29 and FOLFOX-HCT116 cells. Results showed an increased levels of cleaved caspase 3 and PARP1 in FOLFOX-HT29 and FOLFOX-HCT116 cells after treatment with 7a and 7b, suggesting an increased level of apoptosis in these cell lines (Fig. 7A). The apoptotic effect of 7a and 7b was further supported by the increased Bax/Bcl2 ratio (Fig. 7A and B).
2.3.10. Drug-like properties of 7a and 7b
With an aim to check to the drug-likeness of our compounds, we explored the Lipinski's parameters [81] for our two potent compounds (7a and 7b) using the “Filter by Lipinski and Veber Rules” embedded in the Discovery Studio (DS) 4.0 client (Accelrys, San Diego, CA, USA), and are summarized in Table 5 . According to Lipinski's rule, any compound with the molecular weight (MW) greater than 500 Da, hydrogen bond acceptors (HBAs) more than 10, hydrogen bond donors (HBDs) more than five, and an octanol-water partition coefficient (logP) value more than 5, is likely to have poor absorption or membrane permeation under physiological conditions. Similarly, Veber et al., based on their studies in rats, suggested that any compound with rotatable bonds ≤10 would have good oral availability [82]. A closer inspection of Table 5 shows that both 7a and 7b showed compliance with the Lipinski's as well as Veber indicators. The topological polar surface area (TPSA), which is an indicator for hydrogen bond forming capability of molecules, was also computationally predicted using the same tool in DS. More than 90% drugs targeting human proteins are reported to have PSA <140 Å2, hence considered to be an important indicator to predict the bioavailability of drug candidates after oral absorption. Both 7a and 7b with TPSA around 10 Å2 exhibited compliance with TPSA indicator (Table 5), again suggesting their potential to be developed as drug molecules.
Table 5.
Predicted Lipinski's and Verber's parameters for 7a and 7b.
| Compound | logP | MWt | HBA | HBD | TPSA | Rotatable bonds |
|---|---|---|---|---|---|---|
| 7a | −0.13 | 229.7 | 4 | 0 | 10.07 | 3 |
| 7b | 1.2 | 271.3 | 4 | 0 | 10.07 | 4 |
3. Conclusion
In conclusion, SAR based on novel 7a analogs led to the identification of 7b which showed comparable potency in inhibiting the growth of CRC bulk, sensitized FOLFOX-resistant, and reduced sphere formation capacity of CRC stem cells. Experiments to evaluate underlying mechanisms of action revealed that 7a and 7b abrogated 5-FU-induced S phase, reduced the phosphorylation of Chk1 at S315P, S345P and S296P, increased γH2AX staining, and increased the expression of apoptotic marker proteins. Thus, these compounds have the potential to be developed further as a therapeutic agent that is effective both as a single agent and in combination with 5-FU, with solid mechanistic basis by targeting the Chk1 pathway in 5-FU-resistant CRC heterogeneous bulk and CRC stem cell populations.
4. Experimental
4.1. Chemistry
4.1.1. General
Melting points were recorded on a Fischer-Johns melting point apparatus and are uncorrected. 1H NMR spectra were recorded on a Bruker Advance 500 instrument in CDCl3, operating at 500 MHz. Chemical shifts are reported in δ values (ppm) and J values are reported in hertz (Hz). The signals are quoted as s (singlet), d (doublet), t (triplet), m (multiplet), and dd (doublet of doublet). High-resolution MS were recorded on AB Sciex 5600 TripleTOF instrument at Pennsylvania State University, University Park. Experiments were performed under a nitrogen atmosphere in oven-dried glassware. Reagents, starting materials, and anhydrous solvents were purchased from commercial suppliers and were used as received. Reaction courses were monitored by thin-layer chromatography (TLC) on pre-coated silica gel 60 F254 aluminum sheets (Merck, Darmstadt, Germany), and the spots were visualized under UV light. The crude reaction products were purified by recrystallization in ethanol-methylene chloride mixture. Tetraazaadamantane (8a) triaza-phophaadamanatne (8b), various alkyl/alkenyl/aryl halides 9 and phenacyl derivatives 10a-c were purchased from commercial sources. The purity of the final compounds (≥97%) was quantified by analytical high performance liquid chromatography analysis by comparing the peak areas of the product relative to any impurities. Synthesis of 7a was accomplished in our laboratory following the procedure of Warmus et al. [83], as described in our recent studies [13].
4.1.2. General procedures for the synthesis of novel azaadamantane NSC30049 analogs (7a-h)
A mixture of 1.0 mmol of 1,3,5,7-tetraazaadamantane (8a) or 1,3,5-triaza-phosphaadamantane 8b and alkenyl halides 9a-c (1.0 mmol) in CH2Cl2 (10 ml) was refluxed 6 h (for reaction with 8a) and 9 h (for reaction with 8b). After completion of the reaction as indicated by TLC, reaction mass was cooled to room temperature, filtered the residue, and washed with excess methylene dichloride. The crude solid thus obtained was recrystallized in an EtOH/CH2Cl2 mixture and was dried under vacuum (1 mm) to afford the corresponding pure analogs 7a-h.
4.1.3. 1-(4-Chloro-but-2-enyl)-3,5,7-triaza-1-azonia-tricyclo[3.3.1.13,7]decane; chloride (7a)
Yield 92% (0.243 g); white solid; mp 199–201 °C; 1H NMR (500 MHz, D2O) δ [ppm]: 6.31–6.27 (m, 1H), 5.97–5.93 (m, 1H), 5.08 (s, 6H), 4.71 (s, 3H), 4.54 (d, J = 11.0 Hz, 3H), 4.20 (d, J = 5.0 Hz, 2H), 3.57 (d, J = 6.0 Hz, 2H); 13C NMR (125 MHz, DMSO‑d 6) δ [ppm]: 140.6, 117.1, 78.1, 70.13, 57.8, 43.0; HRMS (ESI, M+) calcd. for C10H18ClN4 229.1219, found 229.1218.
4.1.4. 1-(3-Phenyl-allyl)-3,5,7-triaza-1-azonia-tricyclo[3.3.1.13,7]decane; chloride (7b)
Yield 90% (0.262 g); white solid; mp 207–209 °C; 1H NMR (600 MHz, DMSO‑d 6) δ [ppm]: 7.59 (d, J = 7.5 Hz, 2H), 7.41 (t, J = 7.5 Hz, 2H), 7.36 (d, J = 6.0 Hz, 1H), 6.84 (d, J = 16.0 Hz, 1H), 6.47–6.44 (m, 1H), 5.17 (s, 6H), 4.61 (d, J = 12.5 Hz, 3H), 4.50 (d, J = 12.5 Hz, 3H), 3.67 (d, J = 7.5 Hz, 2H); 13C NMR (150 MHz, DMSO‑d 6) δ [ppm]: 140.7, 135.8, 129.3, 129.1, 129.1, 127.7, 115.2, 78.1, 71.8, 70.3, 58.0; HRMS (ESI, M+) calcd. for C15H21N4 257.1766, found 257.1744.
4.1.5. 1-But-2-enyl-3,5,7-triaza-1-azonia-tricyclo[3.3.1.13,7]decane; chloride (7c)
Yield 82% (0.188 g, as mixture of E:Z = 7.4:2.6); white solid; mp 227–229 °C; 1H NMR-E isomer (600 MHz, DMSO‑d 6) δ [ppm]: 6.01–5.96 (m, 1H), 5.63–5.60 (m, 1H), 5.07 (s, 6H), 4.59 (d, J = 12.5 Hz, 3H), 4.49–4.45 (m, 3H), 3.45 (d, J = 7.5 Hz, 2H), 1.75 (d, J = 6.0 Hz, 3H); 13C NMR (150 MHz, DMSO‑d 6) δ [ppm]: 139.2, 116.7, 79.6, 70.3, 57.8, 18.4; HRMS (ESI, M+) calcd. for C10H19N4 195.1609, found 195.1592.
4.1.6. 7-(4-Chloro-but-2-enyl)-1,3,5-triaza-7-phosphonia-tricyclo[3.3.1.13,7]decane; chloride (7d)
Yield 92% (0.259 g); pale yellow solid; mp 219–221 °C (dec.); 1H NMR (500 MHz, D2O) δ [ppm]: 6.33–6.28 (m, 1H), 6.02–5.96 (m, 1H), 4.95 (d, J = 11.5 Hz, 2H), 4.85 (d, J = 12.0 Hz, 2H), 4.61 (d, J = 13.5 Hz, 1H), 4.45 (d, J = 14.0 Hz, 1H), 4.43–4.21 (m, 4H), 4.00–3.94 (m, 2H), 3.86–3.83 (m, 2H), 3.61 (d, J = 7.5 Hz, 2H); 13C NMR (125 MHz, D2O) δ [ppm]: 80.4, 80.3, 67.91, 67.8, 58.3, 57.8, 51.0, 50.6, 48.6, 24.5.
4.1.7. 7-(3-Phenyl-allyl)-1,3,5-triaza-7-phosphonia-tricyclo[3.3.1.13,7]decane; chloride (7e)
Yield 88% (0.271 g); pale yellow solid; mp 203–205 °C; 1H NMR (500 MHz, DMSO‑d 6) δ [ppm]: 7.59 (d, J = 6.5 Hz, 2H), 7.59 (t, J = 6.0 Hz, 2H), 7.34 (t, J = 6.0 Hz, 1H), 6.85 (d, J = 13.0 Hz, 1H), 6.47–6.43 (m, 1H), 5.01 (d, J = 9.5 Hz, 2H), 4.95 (d, J = 9.5 Hz, 2H), 4.52 (d, J = 11.0 Hz, 1H), 4.39–4.36 (m, 3H), 3.92–3.90 (m, 2H), 3.86–3.85 (m, 2H), 3.69 (d, J = 6.5 Hz, 2H); 13C NMR (125 MHz, DMSO‑d 6) δ [ppm]: 141.2, 135.8, 129.3, 129.1, 127.7, 115.3, 78.8, 69.7, 63.8, 52.5, 52.3, 45.9, 45.8; HRMS (ESI, M+) calcd. for C15H21N3P 274.1473, found 274.1448.
4.1.8. 7-But-2-enyl-1,3,5-triaza-7-phosphonia-tricyclo[3.3.1.13,7]decane; chloride (7f)
Yield 78% (0.192 g, as mixture of E:Z = 7.6:2.4); pale yellow solid; mp 249–251 °C; 1H NMR-E isomer (500 MHz, DMSO‑d 6) δ [ppm]: 6.04–5.99 (m, 1H), 5.66–5.62 (m, 1H), 4.93–4.88 (m, 4H), 4.52 (d, J = 15.0 Hz, 1H), 4.39–4.33 (m, 2H), 4.30 (d, J = 5.0 Hz, 1H), 3.92 (t, J = 14.0 Hz, 2H), 3.84–3.80 (m, 2H), 3.49 (d, J = 7.5 Hz, 2H), 1.79 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, DMSO‑d 6) δ [ppm]: 139.8, 116.8, 78.5, 69.7, 63.6, 52.2, 46.0, 18.6; HRMS (ESI, M+) calcd. for C10H19N3P 212.1316, found: 212.1295.
4.1.9. 1-(4-Chloro-butyl)-3,5,7-triaza-1-azonia-tricyclo[3.3.1.1 3,7]decane; chloride (7g)
The mixture of 1,3,5,7-tetraazaadamantane 8a (1.40 g, 10 mmol) and 1,4-dichlorobutane (1.27 g, 10 mmol) in acetone (10 mL) was refluxed for 24 h. After completion of the reaction as indicated by TLC, reaction mixture was cooled to room temperature, filtered the residue, and washed with excess acetone. The crude solid thus obtained was recrystallized in an EtOH/CH2Cl2 mixture and was dried under vacuum (1 mm) to afford the corresponding pure analog 7g. Yield 87% (0.232 g); white solid; mp 229–231 °C; 1H NMR (500 MHz, D2O) δ [ppm]: 5.09 (s, 6H), 4.71–4.69 (m, 3H), 4.56–4.53 (m, 3H), 3.63 (t, J = 6.5 Hz, 2H), 2.94–2.91 (m, 2H), 1.88–1.77 (m, 4H); 13C NMR (150 MHz, D2O) δ [ppm]: 81.8, 79.9, 78.4, 78.3, 71.4, 70.1, 69.8, 56.3, 44.2, 43.0, 28.7, 17.0; HRMS (ESI, M+) calcd. for C10H20ClN4 231.1376, found: 231.1358.
4.1.10. 1-(3-boronobenzyl)-3,5,7-triaza-1-azonia-tricyclo[3.3.1.13,7]decane; chloride (7h)
The mixture of 1,3,5,7-tetraazaadamantane 8a (0.140 g, 1.0 mmol) and [3-(Chloromethyl)phenyl]boronic acid (0.170 g, 1.0 mmol) in CH2Cl2 (10 mL) was refluxed for 24 h. After completion of the reaction as indicated by TLC, reaction mass was cooled to room temperature, filtered the residue, and washed with excess CH2Cl2. The crude solid thus obtained was recrystallized in an EtOH/CH2Cl2 mixture and was dried under vacuum (1 mm) to afford the corresponding pure analog 7h.
Yield 75% (0.232 g); white solid; mp 235–237 °C; 1H NMR (600 MHz, DMSO‑d 6) δ [ppm]: 8.30–8.20 (brs, 2xOH, 2H), 7.92 (d, J = 6.0 Hz, 1H), 7.81 (s, 1H), 7.51–7.46 (m, 2H), 5.05 (s, 6H), 4.58 (d, J = 10.5 Hz, 3H), 4.43 (d, J = 10.0 Hz, 3H), 4.04 (s, 2H); 13C NMR (150 MHz, DMSO‑d 6) δ [ppm]: 138.4, 136.2, 134.4, 128.6, 125.1, 78.1, 73.5, 73.3, 70.3, 70.2, 60.0, 55.4; HRMS (ESI, M+) calcd. for C13H20BN4O2 275.1679, found: 275.1659.
4.1.11. General procedures for the synthesis of novel azaadamantane NSC30049 (7a) analogs 11a-c
A mixture of azaadamantane 8a (1 mmol) and phenacyl chlorides 10a-c (1 mmol) in CH2Cl2 (10 mL) was refluxed 24 h. After completion of the reaction as indicated by TLC, reaction mass was cooled to 25 °C, the residue was filtered and washed with excess methylene dichloride. The crude solid thus obtained was recrystallized from a mixture of EtOH and CH2Cl2 and was further dried under vacuum (1 mm) at 25 °C to afford the corresponding pure 11a-c.
4.1.12. 1-(2-Oxo-2-phenyl-ethyl)-3,5,7-triaza-1-azonia-tricyclo[3.3.1.13,7]decane; chloride (11a)
Yield 87% (0.255 g); white solid; mp 167–169 °C; 1H NMR (600 MHz, DMSO‑d 6) δ [ppm]: 7.99 (d, J = 6.0 Hz, 2H), 7.75 (t, J = 6.0 Hz, 1H), 7.61 (t, J = 7.0 Hz, 2H), 5.42 (s, 6H), 4.95 (s, 2H), 4.67 (d, J = 10.5 Hz, 3H), 4.58 (d, J = 10.5 Hz, 3H); 13C NMR (150 MHz, DMSO‑d 6) δ [ppm]: 191.6, 135.2, 134.9, 129.5, 129.2, 129.2, 128.6, 121.4, 79.5, 72.6, 71.3, 70.7, 59.3, 57.7; HRMS (ESI) calcd. for C14H19N4O 259.1558, found: 259.1542.
4.1.13. 1-[2-(4-Methoxy-phenyl)-2-oxo-ethyl]-3,5,7-triaza-1-azonia-tricyclo[3.3.1.13,7]decane; chloride (11b)
Yield 82% (0.265 g); white solid; mp 182–184 °C; 1H NMR (600 MHz, DMSO‑d 6) δ [ppm]: 7.92 (d, J = 7.0 Hz, 2H), 7.07 (d, J = 7.5 Hz, 2H), 5.43 (s, 6H), 4.80–4.75 (m, 6H), 4.64 (s, 2H), 3.90 (s, 3H); 13C NMR (150 MHz, DMSO‑d 6) δ [ppm]: 189.7, 164.8, 131.4, 130.8, 127.0, 114.5, 81.8, 79.4, 71.8, 70.3, 55.8; HRMS (ESI, M+) calcd. for C15H21N4O2 289.1664, found: 289.1640.
4.1.14. 1-(3-Oxo-3-phenyl-propyl)-3,5,7-triaza-1-azonia-tricyclo[3.3.1.13,7]decane; chloride (11c)
Yield 77% (0.237 g); white solid; mp 204–206 °C; 1H NMR (600 MHz, DMSO‑d 6) δ [ppm]: 8.08 (dd, J = 7.0, 1.0 Hz, 2H), 7.71 (t, J = 6.5 Hz, 1H), 7.59 (t, J = 6.5 Hz, 2H), 5.21 (s, 6H), 4.63 (d, J = 10.5 Hz, 3H), 4.52 (d, J = 10.5 Hz, 3H), 3.71 (t, J = 6.5 Hz, 2H), 3.18 (t, J = 6.5 Hz, 2H); 13C NMR (150 MHz, DMSO‑d 6) δ [ppm]: 196.9, 136.4, 134.3, 129.2, 128.6, 78.6, 71.5, 70.3, 51.4, 30.0; HRMS (ESI, M+) calcd. for C15H21N4O 273.1715, found: 273.1704.
4.2. Biological evaluations
4.2.1. Ethics statement
All procedures performed in this study have been in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
4.2.2. Cell lines and cell cultures
Human colon cancer cell lines HCT-116 and HT-29 were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were maintained as recommended by ATCC either in McCoy's 5a or Dulbecco's modified Eagle medium (DMEM; 4.5 g/L d-glucose) supplemented with 10% FBS and 1% antibiotic/antimycotic in tissue culture flasks in a humidified incubator at 37 °C in an atmosphere of 95% air and 5% carbon dioxide. The medium was changed two times a week and cells were passaged using 0.05% trypsin/EDTA.
The FOLFOX-resistant HCT-116 and HT29 cell lines were obtained from Dr. Adhip P. Majumdar (John D. Dingell VA Medical Centre, Detroit, MI) [42]. These cells were generated by exposure to clinically relevant doses and schedules. The exposing schedule was for 12 cycles; each cycle lasted for one week. Briefly, the cells were first exposed to FOLFOX (25 μM 5-FU and 0.625 μM oxaliplatin) for 72 h. The surviving cells were then cultured in normal medium without the drugs for 4–5 days. This cycle was repeated 12 times. The surviving cells were then split and exposed to higher doses of FOLFOX (50 μM 5-FU + 1.25 μM oxaliplatin or 100 μM 5-FU + 2.5 μM oxaliplatin) for 2–3 days per week for approximately 4 weeks. Finally, the resistant cells were maintained in normal culture medium containing a low dose of FOLFOX (5 μM 5-FU + 0.125 μM oxaliplatin).
CRC stem cell line, CA2, was obtained from Dr. Emina Huang (Lerner Research Institute, Cleveland, OH), as described in our recent publication [13]. These cells were characterized for the expression of ESAhigh, ALDH1+, CD44+and CD133+ stem cell markers [43,44]. These cells were maintained in DMEM/F12 (50/50 1X) medium containing, 6 g/ml d-glucose, 1 mg/ml NaHCO3, 4 mg/ml bovine serum albumin, 2 mM glutamate, 25 mg/ml ITS (insulin, transferrin, and selenium), 20 nM progesterone, 9.6 μg/ml putrescine, and 1% antibiotic/antimycotic solution in ultra-low attachment 6-well plates. Cultures were maintained in a humidified incubator at 37 °C in an atmosphere of 95% air and 5% carbon dioxide.
4.2.3. Cell survival assay
The survival of cells was determined by MTT (3-(4,5-Dimethylthiazol-2yl-)-2,5-diphenyltetrazolium bromide) assay (ATCC, Manassas, VA). In principle, the viable cell number is directly proportional to the purple formazan color of the reduced MTT dye, which can be quantitatively measured by spectrophotometry. Briefly, 1500 cells were plated in quadruplets in 96-well flat-bottom tissue culture plates. After treatment with compounds for certain periods as described in respective figure legends, 10 μl of MTT reagent was added to each well and incubated at 37 °C for 4 h to allow the formation of purple color crystals of formazan. In total, 100 μl of detergent solution was added to each well, and the reaction mixture was incubated in dark for 2–4 h at room temperature. The developed color density was then measured spectrophotometrically at 570 nm using the POLARstar Omega micro-plate reader (BMG Labtech, Inc., Cary, NC).
4.2.4. Spheroid formation assay
Live CA2 cells (100) were seeded in 384-well ultra-low attachment plates. Next, day cells were treated with different concentrations of 7a and 5-FU either alone or in combination. Cells were incubated for 7 days and the number of spheroids were counted in control and treated groups under an Olympus inverted microscope with a 10 × magnification.
4.2.5. Fluorescence-activated cell sorting (FACS) analysis
For determining the cell cycle profile, cells were plated in 60 mm tissue culture dishes and grown until 60% confluence. Cells were treated with 25 μM 5-FU for 24 h followed by treatment with 20 μM 7a for an additional 8 h, as described previously [84]. After treatment, cells were harvested at different time intervals, washed with ice-cold PBS and processed for FACS analysis [85,86]. The ranges for G0/G1, S, and G2/M phase arrested and sub-G1 apoptotic cells were established on the basis of the corresponding DNA content of the histograms. At minimum, 10,000 cells were counted from each sample.
4.2.6. Western blot analysis
The levels of various proteins were determined by Western blot analysis with our previously described procedure [87,88]. The antibodies for phospho-Chk1(Ser317), phospho-Chk1(Ser317), phospho-Chk1(Ser319), phospho-Chk1(Ser345), Chk1, and GAPDH were purchased from Cell Signaling Technology (Danvers, MA).
4.2.7. Kinase activity profiling
To determine whether NSC30049 and ASR352 are Chk1 kinase inhibitors, an in vitro Chk1 kinase activity was determined by Eurofins Pharma Discovery Services, Wolverhampton, UK. To further determine whether 7a and 7b can inhibit any kinases, a profiling of 366 kinases was tested by Reaction Biology Corp., Malvern, PA.
4.2.8. Statistical analysis
All experiments were repeated at least three times and results were expressed as mean ± SE. One-way analysis of variance (ANOVA) was calculated with Sigma-Plot 9. A one-tailed t-test was used to compare any significant differences between control and treated groups. The criterion for statistical significance was p < 0.05. For western blotting data, band intensities were measured using ImageJ and normalized with GAPDH.
Acknowledgement
This work was supported partially by the Team Science Project #00110481, University of Florida Shands Cancer Center, Gainesville, FL to SN. AKS thanks the Department of Pharmacology, Penn State College of Medicine, and Penn State Cancer Institute (PSCI) for financial support. The authors thank Dr. Jyh-Ming Lin, Solution Phase NMR Facility at Core Research Facilities of the Penn State College of Medicine for recording NMR spectra, and Organic Synthesis Shared Resource of PSCI. The authors also thank the Centre for High Computing Performance (CHPC) based in Cape Town (South Africa) for access and use of computational resources.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmech.2018.10.052.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Copies of the 1H NMR and 13C NMR spectra for new compounds 7a-h and 11a-c, and the Kinase Profiling Report.
References
- 1.Desch C.E., Benson A.B., 3rd, Somerfield M.R., Flynn P.J., Krause C., Loprinzi C.L., Minsky B.D., Pfister D.G., Virgo K.S., Petrelli N.J., American Society of Clinical O. Colorectal cancer surveillance: 2005 update of an American Society of clinical Oncology practice guideline. J. Clin. Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 2.Kosmider S., Lipton L. Adjuvant therapies for colorectal cancer. World J. Gastroenterol. 2007;13:3799–3805. doi: 10.3748/wjg.v13.i28.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy J., Misset J.L. Oxaliplatin-related side effects: characteristics and management. Semin. Oncol. 2002;29:11–20. doi: 10.1053/sonc.2002.35524. [DOI] [PubMed] [Google Scholar]
- 4.Sanoff H.K., Carpenter W.R., Freburger J., Li L., Chen K., Zullig L.L., Goldberg R.M., Schymura M.J., Schrag D. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118:4309–4320. doi: 10.1002/cncr.27422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Feng M., Zheng G., Chen Y., Wang X., Pen B., Yin J., Yu Y., He Z. Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cells. Biochem. Biophys. Res. Commun. 2012;417:679–685. doi: 10.1016/j.bbrc.2011.11.142. [DOI] [PubMed] [Google Scholar]
- 6.Izumiya M., Kabashima A., Higuchi H., Igarashi T., Sakai G., Iizuka H., Nakamura S., Adachi M., Hamamoto Y., Funakoshi S., Takaishi H., Hibi T. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2012;32:3847–3853. [PubMed] [Google Scholar]
- 7.Brabletz T., Jung A., Spaderna S., Hlubek F., Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat. Rev. Canc. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 8.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., Campbell L.L., Polyak K., Brisken C., Yang J., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han X.Y., Wei B., Fang J.F., Zhang S., Zhang F.C., Zhang H.B., Lan T.Y., Lu H.Q., Wei H.B. Epithelial-mesenchymal transition associates with maintenance of stemness in spheroid-derived stem-like colon cancer cells. PloS One. 2013;8 doi: 10.1371/journal.pone.0073341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Tillo E., de Barrios O., Siles L., Cuatrecasas M., Castells A., Postigo A. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc. Natl. Acad. Sci. U.S.A. 2011;108:19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmalhofer O., Brabletz S., Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis. Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 12.Onder T.T., Gupta P.B., Mani S.A., Yang J., Lander E.S., Weinberg R.A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 13.Narayan S., Jaiswal A.S., Sharma R., Nawab A., Duckworth L.V., Law B.K., Zajac-Kaye M., George T.J., Sharma J., Sharma A.K., Hromas R.A. NSC30049 inhibits Chk1 pathway in 5-FU-resistant CRC bulk and stem cell populations. Oncotarget. 2017;8:57246–57264. doi: 10.18632/oncotarget.19778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuznetsov A.I., Zefirov N.S. Azaadamantanes with nitrogen atoms in the bridgehead positions. Russ. Chem. Bull. 1989;58:1033–1047. [Google Scholar]
- 15.Jimenez-Cruz F., Rios-Olivares H., Gutierrez J.L.G. Molecular structure of 1-azaadamantanes and 1,3-diazaadamantanes. In: Iriepa I., editor. Structural Analysis of Cyclic Systems. Research Signpost; Trivandrum: 2005. pp. 101–125. [Google Scholar]
- 16.Izumi H., Yamagami S., Futamura S. 1-Azaadamantanes: pharmacological applications and synthetic approaches. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2003;1:99–111. doi: 10.2174/1568016033477478. [DOI] [PubMed] [Google Scholar]
- 17.Becker D.P., Flynn D.L., Shone R.L., Gullikson G. Azaadamantane benzamide 5-HT4 agonists: gastrointestinal prokinetic SC-54750. Bioorg. Med. Chem. Lett. 2004;14:5509–5512. doi: 10.1016/j.bmcl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Shi L., Scanio M.J.C., Bunnelle W.H. Amimomethyl azaadamantane derivatives and use thereof as selective modulators of the alpha7- neuronal nicotinic acetylcholine receptor (nnrs) PCT Int. Appl. WO 2008118747A1; Chem. Abstr. 2008;149:425803. [Google Scholar]
- 19.Bunnelle W.H. 4-substituted azaadamantane derivatives and methods of use thereof. PCT Int. Appl. WO 2008118745A1; Chem. Abstr. 2008;149:425802. [Google Scholar]
- 20.Schrimpf M.R., Nersesian D.L., Sippy K.B., Ji J., Li T., Shi L. Azaadamantane ester and carbamate derivatives and methods of use thereof. PCT Int. Appl. WO 2008118742A1; Chem. Abstr. 2008;149:425805. [Google Scholar]
- 21.Banister S.D., Yoo D.T., Chua S.W., Cui J., Mach R.H., Kassiou M. N-Arylalkyl-2-azaadamantanes as cage-expanded polycarbocyclic sigma (sigma) receptor ligands. Bioorg. Med. Chem. Lett. 2011;21:5289–5292. doi: 10.1016/j.bmcl.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Demir A., Oguariri R.M., Magis A., Ostrov D.A., Imamichi T., Dunn B.M. Kinetic characterization of newly discovered inhibitors of various constructs of human T-cell leukemia virus-1 (HTLV-1) protease and their effect on HTLV-1-infected cells. Antivir. Ther. 2012;17:883–892. doi: 10.3851/IMP2090. [DOI] [PubMed] [Google Scholar]
- 23.Kesel A.J. Synthesis of novel test compounds for antiviral chemotherapy of severe acute respiratory syndrome (SARS) Curr. Med. Chem. 2005;12:2095–2162. doi: 10.2174/0929867054637644. [DOI] [PubMed] [Google Scholar]
- 24.Tanner J.A., Zheng B.J., Zhou J., Watt R.M., Jiang J.Q., Wong K.L., Lin Y.P., Lu L.Y., He M.L., Kung H.F., Kesel A.J., Huang J.D. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem. Biol. 2005;12:303–311. doi: 10.1016/j.chembiol.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., Di Y.T., Mu S.Z., Li C.S., Zhang Q., Tan C.J., Zhang Z., Fang X., Hao X.J. Dapholdhamines A-D, alkaloids from Daphniphyllum oldhami. J. Nat. Prod. 2009;72:1325–1327. doi: 10.1021/np900112d. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C.R., Liu H.B., Feng T., Zhu J.Y., Geng M.Y., Yue J.M. Alkaloids from the leaves of Daphniphyllum subverticillatum. J. Nat. Prod. 2009;72:1669–1672. doi: 10.1021/np9003799. [DOI] [PubMed] [Google Scholar]
- 27.Suslov E., Zarubaev V.V., Slita A.V., Ponomarev K., Korchagina D., Ayine-Tora D.M., Reynisson J., Volcho K., Salakhutdinov N. Anti-influenza activity of diazaadamantanes combined with monoterpene moieties. Bioorg. Med. Chem. Lett. 2017;27:4531–4535. doi: 10.1016/j.bmcl.2017.08.062. [DOI] [PubMed] [Google Scholar]
- 28.Lo T.S., Hammer K.D., Zegarra M., Cho W.C. Methenamine: a forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era. Expert Rev. Anti-infe. 2014;12:549–554. doi: 10.1586/14787210.2014.904202. [DOI] [PubMed] [Google Scholar]
- 29.Li X.Q., Xu Q., Luo J., Wang L.J., Jiang B., Zhang R.S., Shi D.Y. Design, synthesis and biological evaluation of uncharged catechol derivatives as selective inhibitors of PTP1B. Eur. J. Med. Chem. 2017;136:348–359. doi: 10.1016/j.ejmech.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P.Y., Wong I.L., Yan C.S., Zhang X.Y., Jiang T., Chow L.M., Wan S.B. Design and syntheses of permethyl ningalin B analogues: potent multidrug resistance (MDR) reversal agents of cancer cells. J. Med. Chem. 2010;53:5108–5120. doi: 10.1021/jm100035c. [DOI] [PubMed] [Google Scholar]
- 31.Saha S., Reddy Ch V., Xu S., Sankar S., Neamati N., Patro B. Synthesis and SAR studies of marine natural products ma'edamines A, B and their analogues. Bioorg. Med. Chem. Lett. 2013;23:5135–5139. doi: 10.1016/j.bmcl.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Morgan M.A., Parsels L.A., Zhao L., Parsels J.D., Davis M.A., Hassan M.C., Arumugarajah S., Hylander-Gans L., Morosini D., Simeone D.M., Canman C.E., Normolle D.P., Zabludoff S.D., Maybaum J., Lawrence T.S. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 2010;70:4972–4981. doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez-Gonzalez A., Belda-Iniesta C., Bargiela-Iparraguirre J., Dominguez G., Garcia Alfonso P., Perona R., Sanchez-Perez I. Targeting Chk2 improves gastric cancer chemotherapy by impairing DNA damage repair. Apoptosis Int. J. Program. Cell Death. 2013;18:347–360. doi: 10.1007/s10495-012-0794-2. [DOI] [PubMed] [Google Scholar]
- 34.Zabludoff S.D., Deng C., Grondine M.R., Sheehy A.M., Ashwell S., Caleb B.L., Green S., Haye H.R., Horn C.L., Janetka J.W., Liu D., Mouchet E., Ready S., Rosenthal J.L., Queva C., Schwartz G.K., Taylor K.J., Tse A.N., Walker G.E., White A.M. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol. Canc. Therapeut. 2008;7:2955–2966. doi: 10.1158/1535-7163.MCT-08-0492. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Z., Xue J., Sowin T.J., Rosenberg S.H., Zhang H. A novel mechanism of checkpoint abrogation conferred by Chk1 downregulation. Oncogene. 2005;24:1403–1411. doi: 10.1038/sj.onc.1208309. [DOI] [PubMed] [Google Scholar]
- 36.Morgan M.A., Parsels L.A., Parsels J.D., Mesiwala A.K., Maybaum J., Lawrence T.S. Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res. 2005;65:6835–6842. doi: 10.1158/0008-5472.CAN-04-2246. [DOI] [PubMed] [Google Scholar]
- 37.Blackwood E., Epler J., Yen I., Flagella M., O'Brien T., Evangelista M., Schmidt S., Xiao Y., Choi J., Kowanetz K., Ramiscal J., Wong K., Jakubiak D., Yee S., Cain G., Gazzard L., Williams K., Halladay J., Jackson P.K., Malek S. Combination drug scheduling defines a "window of opportunity" for chemopotentiation of gemcitabine by an orally bioavailable, selective ChK1 inhibitor, GNE-900. Mol. Canc. Therapeut. 2013;12:1968–1980. doi: 10.1158/1535-7163.MCT-12-1218. [DOI] [PubMed] [Google Scholar]
- 38.Lapenna S., Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 39.Toledo L.I., Murga M., Zur R., Soria R., Rodriguez A., Martinez S., Oyarzabal J., Pastor J., Bischoff J.R., Fernandez-Capetillo O. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat. Struct. Mol. Biol. 2011;18:721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diasio R.B., Harris B.E. Clinical pharmacology of 5-fluorouracil. Clin. Pharmacokinet. 1989;16:215–237. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 41.Rajesh T., Azeez S.A., Naresh E., Madhusudhan G., Mukkanti K. Practical one-pot and large-scale synthesis of N-(tert-Butyloxycarbonyl)-3-pyrroline. Org. Process Res. Dev. 2009;13:638–640. [Google Scholar]
- 42.Yu Y., Kanwar S.S., Patel B.B., Nautiyal J., Sarkar F.H., Majumdar A.P. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl. oncol. 2009;2:321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang E.H., Hynes M.J., Zhang T., Ginestier C., Dontu G., Appelman H., Fields J.Z., Wicha M.S., Boman B.M. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpentino J.E., Hynes M.J., Appelman H.D., Zheng T., Steindler D.A., Scott E.W., Huang E.H. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storchova Z., Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 46.Sherr C.J., Bartek J. Cell cycle-targeted cancer therapies. Annu. Rev. Cell Biol. 2017;1:47–57. [Google Scholar]
- 47.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Canc. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 48.Longley D.B., Johnston P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 49.Manic G., Obrist F., Sistigu A., Vitale I., Trial Watch Targeting ATM-CHK2 and ATR-CHK1 pathways for anticancer therapy. Mol. Cell Oncol. 2015;2 doi: 10.1080/23723556.2015.1012976. e1012976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherr C.J., Roberts J.M. CDK inhibitors: positive and negative regulators of G1-phase progression. Gene Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 51.Mills C.C., Kolb E.A., Sampson V.B. Development of chemotherapy with cell-cycle inhibitors for adult and pediatric cancer therapy. Cancer Res. 2018;78:320–325. doi: 10.1158/0008-5472.CAN-17-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martino-Echarri E., Henderson B.R., Brocardo M.G. Targeting the DNA replication checkpoint by pharmacologic inhibition of Chk1 kinase: a strategy to sensitize APC mutant colon cancer cells to 5-fluorouracil chemotherapy. Oncotarget. 2014;5:9889–9900. doi: 10.18632/oncotarget.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Dai Q., Park D., Deng X. Targeting DNA replication stress for cancer therapy. Genes. 2016;7:51. doi: 10.3390/genes7080051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobbelstein M., Sorensen C.S. Exploiting replicative stress to treat cancer. Nat. Rev. Drug Discov. 2015;14:405–423. doi: 10.1038/nrd4553. [DOI] [PubMed] [Google Scholar]
- 55.Zhou B.B., Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat. Rev. Canc. 2004;4:216–225. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- 56.Kitao H., Iimori M., Kataoka Y., Wakasa T., Tokunaga E., Saeki H., Oki E., Maehara Y. DNA replication stress and cancer chemotherapy. Cancer Sci. 2018;109:264–271. doi: 10.1111/cas.13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berti M., Vindigni A. Replication stress: getting back on track. Nat. Struct. Mol. Biol. 2016;23:103–109. doi: 10.1038/nsmb.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaillard H., Garcia-Muse T., Aguilera A. Replication stress and cancer. Nat. Rev. Canc. 2015;15:276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 59.Zeman M.K., Cimprich K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014;16:2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vesela E., Chroma K., Turi Z., Mistrik M. Common chemical inductors of replication stress: focus on cell-based studies. Biomolecules. 2017;7:19. doi: 10.3390/biom7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skog S., Tribukait B., Wallstrom B., Eriksson S. Hydroxyurea-induced cell death as related to cell cycle in mouse and human T-lymphoma cells. Cancer Res. 1987;47:6490–6493. [PubMed] [Google Scholar]
- 62.Walworth N.C., Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- 63.Rundle S., Bradbury A., Drew Y., Curtin N.J. Targeting the ATR-CHK1 Axis in cancer therapy. Cancers. 2017;9:41. doi: 10.3390/cancers9050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang W.H., Poh A., Fanous A.A., Eastman A. DNA damage-induced S phase arrest in human breast cancer depends on Chk1, but G2 arrest can occur independently of Chk1, Chk2 or MAPKAPK2. Cell Cycle. 2008;7:1668–1677. doi: 10.4161/cc.7.11.5982. [DOI] [PubMed] [Google Scholar]
- 65.Blasina A., Hallin J., Chen E., Arango M.E., Kraynov E., Register J., Grant S., Ninkovic S., Chen P., Nichols T., O'Connor P., Anderes K. Breaching the DNA damage checkpoint via PF-00477736, a novel small-molecule inhibitor of checkpoint kinase 1. Mol. Canc. Therapeut. 2008;7:2394–2404. doi: 10.1158/1535-7163.MCT-07-2391. [DOI] [PubMed] [Google Scholar]
- 66.McNeely S., Conti C., Sheikh T., Patel H., Zabludoff S., Pommier Y., Schwartz G., Tse A. Chk1 inhibition after replicative stress activates a double strand break response mediated by ATM and DNA-dependent protein kinase. Cell Cycle. 2010;9:995–1004. doi: 10.4161/cc.9.5.10935. [DOI] [PubMed] [Google Scholar]
- 67.Srivastava M., Raghavan S.C. DNA double-strand break repair inhibitors as cancer therapeutics. Chem. Biol. 2015;22:17–29. doi: 10.1016/j.chembiol.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Ward I.M., Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 69.Downey M., Durocher D. gammaH2AX as a checkpoint maintenance signal. Cell Cycle. 2006;5:1376–1381. doi: 10.4161/cc.5.13.2899. [DOI] [PubMed] [Google Scholar]
- 70.Cimprich K.A., Cortez D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nam E.A., Cortez D. ATR signalling: more than meeting at the fork. Biochem. J. 2011;436:527–536. doi: 10.1042/BJ20102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumagai A., Dunphy W.G. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell. 2000;6:839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- 73.Jeong S.Y., Kumagai A., Lee J., Dunphy W.G. Phosphorylated claspin interacts with a phosphate-binding site in the kinase domain of Chk1 during ATR-mediated activation. J. Biol. Chem. 2003;278:46782–46788. doi: 10.1074/jbc.M304551200. [DOI] [PubMed] [Google Scholar]
- 74.Chini C.C., Chen J. Human claspin is required for replication checkpoint control. J. Biol. Chem. 2003;278:30057–30062. doi: 10.1074/jbc.M301136200. [DOI] [PubMed] [Google Scholar]
- 75.Zhou X., Liu W., Hu X., Dorrance A., Garzon R., Houghton P.J., Shen C. Regulation of CHK1 by mTOR contributes to the evasion of DNA damage barrier of cancer cells. Sci. Rep. 2017;7:1535. doi: 10.1038/s41598-017-01729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oltvai Z.N., Milliman C.L., Korsmeyer S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L., Yu J., Park B.H., Kinzler K.W., Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 78.Nehls O., Okech T., Hsieh C.J., Enzinger T., Sarbia M., Borchard F., Gruenagel H.H., Gaco V., Hass H.G., Arkenau H.T., Hartmann J.T., Porschen R., Gregor M., Klump B. Studies on p53, BAX and Bcl-2 protein expression and microsatellite instability in stage III (UICC) colon cancer treated by adjuvant chemotherapy: major prognostic impact of proapoptotic BAX. Br. J. Canc. 2007;96:1409–1418. doi: 10.1038/sj.bjc.6603728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sturm I., Kohne C.H., Wolff G., Petrowsky H., Hillebrand T., Hauptmann S., Lorenz M., Dorken B., Daniel P.T. Analysis of the p53/BAX pathway in colorectal cancer: low BAX is a negative prognostic factor in patients with resected liver metastases. J. Clin. Oncol. 1999;17:1364–1374. doi: 10.1200/JCO.1999.17.5.1364. [DOI] [PubMed] [Google Scholar]
- 80.Hector S., Prehn J.H. Apoptosis signaling proteins as prognostic biomarkers in colorectal cancer: a review. Biochim. Biophys. Acta. 2009;1795:117–129. doi: 10.1016/j.bbcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:2–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 82.Veber D.F., Johnson S.R., Cheng H.-Y., Smith B.R., Ward K.W., Kopple K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 83.Warmus J.S., Dilley G.J., Meyers A.I. A modified procedure for the preparation of 2,5-dihydropyrrole (3-pyrroline) J. Org. Chem. 1993;58:270–271. [Google Scholar]
- 84.Montano R., Chung I., Garner K.M., Parry D., Eastman A. Preclinical development of the novel Chk1 inhibitor SCH900776 in combination with DNA-damaging agents and antimetabolites. Mol. Canc. Therapeut. 2012;11:427–438. doi: 10.1158/1535-7163.MCT-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaiswal A.S., Marlow B.P., Gupta N., Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 86.Jaiswal A.S., Aneja R., Connors S.K., Joshi H.C., Multani A.S., Pathak S., Narayan S. 9-bromonoscapine-induced mitotic arrest of cigarette smoke condensate-transformed breast epithelial cells. J. Cell. Biochem. 2009;106:1146–1156. doi: 10.1002/jcb.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Narayan S., Jaiswal A.S. Activation of adenomatous polyposis coli (APC) gene expression by the DNA-alkylating agent N-methyl-N'-nitro-N-nitrosoguanidine requires p53. J. Biol. Chem. 1997;272:30619–30622. doi: 10.1074/jbc.272.49.30619. [DOI] [PubMed] [Google Scholar]
- 88.Narayan S., Jaiswal A.S., Kang D., Srivastava P., Das G.M., Gairola C.G. Cigarette smoke condensate-induced transformation of normal human breast epithelial cells in vitro. Oncogene. 2004;23:5880–5889. doi: 10.1038/sj.onc.1207792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.