Summary
Dendritic cell–specific intracellular adhesion molecule-3–grabbing nonintegrin (DC-SIGN) and DC-SIGNR are C-type lectins that serve both as cell adhesion and pathogen recognition receptors. Because of the essential role of the these molecules in the immune response, the implication of their alleles in human disease states, and the possible genetic variation at these loci among ethnically diverse populations, we undertook a study to analyze the full extent of DC-SIGN and DC-SIGNR polymorphisms in Caucasian Canadian and indigenous African populations. We report several novel nucleotide variants within regulatory 5′- and 3′-untranslated regions of the genes that could affect their transcription and translation. There were significant differences in the distribution of DC-SIGN and DC-SIGNR alleles among African and non-African populations. Finally, our study clearly demonstrates that Africans show greater genetic diversity at these two closely-related immune loci than observed in other major population groups. The differences may reflect evolutionary pressures generated by environmental factors, such as prevalent pathogens in these geographically distinct regions. Further studies will be needed to determine the net impact of DC-SIGN and DC-SIGNR genetic variants on the expression, translation, and function of the proteins and to understand how these functional polymorphisms may affect immune responses or immune escape.
Keywords: African, antigen presentation, DC-SIGN, DC-SIGNR, polymorphism
Abbreviations
- CDR
C-terminal carbohydrate recognition domain
- DC
dendritic cell
- DC-SIGN
dendritic cell–specific intracellular adhesion molecule–3–grabbing nonintegrin
- DC-SIGNR
dendritic cell–specific intracellular adhesion molecule–3–grabbing nonintegrin related protein
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IPCR
polymerase chain reaction
- SNP
single nucleotide polymorphism
- UTR
untranslated region
Introduction
Dendritic cell–specific intracellular adhesion molecule–3–grabbing nonintegrin (DC-SIGN/CD209) and DC-SIGN related (DC-SIGNR/L-SIGN/CD209L) are C-type lectins involved in both innate and adaptive immunity. DC-SIGN is expressed on subsets of dendritic cells (DC) and macrophages [1], [2], whereas expression of DC-SINGR is restricted to endothelial cells of the liver, lymph nodes and placenta [1], [3]. Despite their different expression profiles, DC-SIGN and DC-SIGNR have similar amino acid composition (77% homology) and share an identical intron-exon organisation. A C-terminal carbohydrate recognition domain (CRD) present in both receptors binds pathogens in a Ca2+ dependent manner. A neck region, made up of highly conserved 23 amino acid repeats, plays a crucial role in tetramerization and support of CRDs, thus influencing the pathogen-binding properties of these receptors. Finally, a short cytoplasmic tail at the N-terminus with LL and YKSL motifs, and a triacidic cluster (EEE) for DC-SIGN is responsible for internalisation and signal transduction [4] leading to cellular maturation, adhesion, migration and differentiation [5]. Both lectins are known to bind multiple pathogens of great public health concern such as Mycobacterium tuberculosis, Helicobacter pylori, Klebsiella pneumoniae, Dengue virus, Ebola virus, hepatitis C virus (HCV), human cytomegalovirus, human herpesvirus 8, human immunodeficiency virus (HIV)–1, HIV-2, measles virus, coronavirus severe acute respiratory syndrome, Leishmania pifanoi, Shistosoma mansoni, and Candida albicans [6], [7], [8], [9], [10], [11], [12], [13], [14], [15].
DC-SIGN and DC-SIGNR genes are located on chromosome 19 p13, in close physical proximity (∼15 Kb) in head-to-head orientation, resulting from duplication of an ancestral gene [16]. Strikingly, these two genes behave as independent entities and are not in linkage disequilibrium [17]. These receptors appear to have undergone different evolutionary processes, leading to considerable diversity in their recognition patterns. Characterization of their genetic polymorphism may provide insight into the variability of pathogen-recognition patterns among individuals of different ethnic origins, and therefore on the susceptibility or resistance to significant pathogens. In recent years, DC-SIGN and DC-SIGNR polymorphisms have been associated with several infectious diseases. DC-SIGN promoter variant at position −336G was associated with an increased risk for parenteral transmission of HIV-1 in Caucasian Americans [18] and for dengue hemorrhagic fever in the Thai population [19]. In contrast, the wild-type variant at this position (−336A), was associated with a protective effect against tuberculosis in the coloured South-African population [20] but not in Colombian individuals [21]. The neck regions of DC-SIGN and DC-SIGNR are formed by variable numbers of 69-bp tandem repeats in exon 4 that encode repeating units of 23 amino acids [16]. Heterozygosity for the number of DC-SIGN and DC-SIGNR tandem repeats has been associated with a reduced risk of sexual transmission of HIV-1 in Caucasian Americans [22], [23], but not in Caucasian Europeans [24]. Nattermann et al. [25] found that Caucasian European individuals with 5-, 6-, and 7-repeat alleles of DC-SIGNR had higher HCV-RNA levels when compared with carriers of 4- and 9-repeat alleles. Finally, Chinese individuals homozygous for the DC-SIGNR tandem repeat were less susceptible to coronavirus severe acute respiratory syndrome infection [26].
To date, little is known about DC-SIGN and DC-SIGNR polymorphisms among African populations in which the burden of health-threatening pathogens such as those mentioned above is the greatest. The allelic distribution of DC-SIGN and DC-SIGNR genes in people living in developing countries may differ from those living in industrialized countries because of overdominant selection pressure exerted on specific alleles by prevalent pathogens in these geographic areas [27]. Because of the essential role of the DC-SIGN and DC-SIGNR molecules in the immune response, the implication of their alleles in human disease states, and the possible genetic variation at these loci among ethnically diverse populations, we undertook a study to analyze the full extent of DC-SIGN and DC-SIGNR polymorphisms in Caucasian Canadians and an indigenous African population. We now report the nucleotide sequence diversity of DC-SIGN and DC-SIGNR genes in Caucasian Canadians and in the Shona people of Zimbabwe, selected to represent a homogenous ancestral group.
Subjects and methods
Sample composition
Our samples consisted of stored DNA extracts from 100 unrelated Zimbabweans of the Shona ethnic group recruited in the ZVITAMBO project in Harare (Zimbabwe, Africa) and 100 unrelated Caucasian individuals from Québec, Canada. The use of these samples for the present study was approved by the research ethics committees.
DNA extraction and DNA sequence analysis
DNA was extracted from whole peripheral blood using standard phenol-chloroform extraction procedure. The nucleotide sequence variation of the entire promoter (5′-UTR), coding regions and part of 3′-UTR of DC-SIGN and DC-SIGNR genes were determined by polymerase chain reaction (PCR)–amplified direct DNA sequencing method in 50 randomly selected samples from each population. The DNA sequencing procedures were done using the PCR conditions and specific primers as described previously [19]. The reaction products were run in an automated DNA sequencing ABI PRISM 3100 capillary sequencer (Applied Biosystems, Foster City, CA). All PCR products were sequenced in both directions. Sequences were analyzed using Lasergene software (DNA Stars, Madison, WI) and polymorphisms were identified as compared with the reference sequence of human chromosome 19 contig (GenBank accession no NT_077812.2) for DC-SIGN and DC-SIGNR. DNA sequences of the promoter region were analyzed with the TESS interface (http://www.cbil.upenn.edu/tess) for putative transcription factor binding sites using the TRANSFAC database.
Specific polymorphisms with the potential to affect the protein function and/or known to be associated with susceptibility/resistance to pathogens were typed in all study samples (Table 1, Table 2). DC-SIGN mutations in the promoter and in exon 4 were determined by direct DNA sequencing as described above. The number of DC-SIGN and DC-SIGNR exon 4 repeats was determined as described previously [22], [23].
Table 1.
Allelic distribution of selected DC-SIGN polymorphisms in study populations.
| Selected SNPs |
Population allelic frequencies (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymorphism name DCSIGN | Position on cDNA | Nucleotide change | Amino acid change | Rare allele | Zimbabwean (n = 200) | Sub-Saharan African (n = 82) | South African coloured (n = 1422) | Caucasian Canadian (n = 200) | Caucasian European (n = 86) | Thai (n= 80) | Asian (n = 86) | pa |
| p-939 | Promoter | G/A | A | 26.5 | 45.1 | 29.8 | 51.5 | 54.7 | 26.6 | 29.1 | <0.001 | |
| p-871 | Promoter | A/G | G | 5.0 | – | 11.6 | 42.0 | 38.4 | 21.2 | 20.9 | <0.001 | |
| p-745 | Promoter | G/T | T | 5.5 | – | – | 0.0 | – | 0.0 | – | <0.001 | |
| p-336 | Promoter | A/G | G | 45.0 | 37.8 | 42.8 | 18.0 | 20.9 | 8.0 | 5.8 | <0.001 | |
| p-201 | Promoter | G/T | T | 11.5 | – | – | 0.0 | – | 0.0 | – | <0.001 | |
| p-139 | Promoter | A/G | G | 74.0 | 87.8 | 72.1 | 69.5 | 75.6 | 26.7 | 33.7 | ns | |
| Ex4RPT | Exon 4 | 7,5/6,5 | del Y124-I146 | 6.5 | 1.5 | – | – | 2.0 | – | 0.6 | – | ns |
| Ex4+415 | Exon 4 | C/T | R198Q | T | 11.5 | ∼15.0 | – | 0.0 | – | 0.0 | – | <0.001 |
| Ex4+465 | Exon 4 | C/G | E214D | G | 16.0 | ∼15.0 | – | 0.0 | – | 0.0 | – | <0.001 |
| Ex4+485 | Exon 4 | C/T | R221Q | T | 16.0 | ∼15.0 | – | 0.0 | – | 0.0 | – | <0.001 |
| Ex4+547 | Exon 4 | G/C | L242V | C | 7.5 | 6.0 | – | 0.0 | – | 0.0 | – | <0.001 |
| Reference citation | [17] | [20] | [17] | [19] | [17] | |||||||
Amino residues are indicated by single-letter codes at each codon position defined by three-digit numbers.
Abbreviations: DC-SIGN = dendritic cell–specific intracellular adhesion molecule–3–grabbing nonintegrin; n = number of alleles; ns= not significant; SNPs =single nucleotide polymorphisms; – = not determined.
Difference between Zimbabweans and Canadians determined by Chi-square test.
Table 2.
Distribution of DC-SIGNR repeat-region genotypes among different populations.
| Genotypes | Populations (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zimbabwean | Caucasian Canadian | Sub-Saharan African | Middle-Easterner | Caucasian European | South Asian | East Asian | Chinese | Japanese | Oceanian | Native American | pa | |
| 4/4 | … | … | … | … | 0.6 | … | … | … | … | … | … | |
| 5/4 | … | 1.0 | … | 0.7 | 3.7 | … | … | … | … | … | … | ns |
| 5/5 | … | 8.0 | 0.8 | 6.1 | 13.0 | 7.5 | 2.0 | 2.6 | 4.3 | 2.6 | 29.6 | 0.01 |
| 6/4 | … | 3.0 | … | … | 3.7 | … | … | … | … | … | … | ns |
| 6/5 | 4.0 | 8.0 | 0.8 | 8.1 | 10.0 | 2.5 | 2.4 | 1.0 | … | 10.3 | … | ns |
| 6/6 | 11.0 | 3.0 | 11.0 | 2.0 | 2.5 | 0.5 | … | 0.5 | … | 5.3 | … | ns |
| 7/6 | 34.0 | 22.0 | 44.1 | 21.0 | 10.6 | 5.0 | 2.4 | 6.6 | 3.5 | 10.3 | … | ns |
| 7/4 | … | 3.0 | … | … | 4.4 | … | … | … | … | … | … | ns |
| 7/5 | 4.0 | 29.0 | 4.7 | 28.4 | 25.5 | 36.5 | 21.5 | 16.6 | 22.6 | 10.3 | 33.3 | <0.001 |
| 7/7 | 42.0 | 22.0 | 37.8 | 31.1 | 22.4 | 39.5 | 49.4 | 50.3 | 58.3 | 10.3 | 22.2 | 0.01 |
| 8/5 | … | … | … | … | … | … | … | … | 0.9 | … | … | |
| 8/6 | 2.0 | … | 0.8 | 0.7 | … | … | … | … | … | … | … | ns |
| 8/7 | 3.0 | 1.0 | … | … | … | 1.0 | 0.4 | … | 0.9 | … | … | ns |
| 8/8 | … | … | … | … | … | 0.5 | … | … | … | … | … | |
| 9/4 | … | … | … | 0.7 | … | … | … | … | … | … | … | |
| 9/5 | … | … | … | 0.7 | 1.9 | 1.5 | 3.2 | 3.9 | 1.7 | 7.7 | 1.9 | |
| 9/6 | … | … | … | … | 0.6 | … | 0.5 | 1.0 | … | 12.8 | … | |
| 9/7 | … | … | … | 0.7 | 1.3 | 5.5 | 16.8 | 15.8 | 7.8 | 12.8 | 10.2 | |
| 9/9 | … | … | … | … | … | … | 1.6 | 1.6 | … | 10.3 | 2.8 | |
| 10/7 | … | … | … | … | … | … | … | … | … | 7.7 | … | |
| homozygotes | 53.0 | 33.0 | 49.6 | 39.2 | 38.5 | 48.0 | 53.0 | 55.0 | 62.6 | 28.2 | 54.6 | 0.01 |
| heterozygotes | 47.0 | 67.0 | 50.4 | 60.8 | 61.5 | 52.0 | 47.0 | 45.0 | 37.4 | 71.8 | 45.4 | |
| Reference citation | [30] | [30] | [30] | [30] | [30] | [26] | [31] | [30] | [30] | |||
Abbreviations: DC-SIGNR = dendritic cell–specific intracellular adhesion molecule–3–grabbing nonintegrin related protein; ns = not significant; … = 0.0%.
Difference between Zimbabweans and Canadians determined by Chi-square test.
Statistical analysis
Allelic frequencies in our populations were compared using the Chi-square test. Genotypic frequencies were compared with Hardy-Weinberg expectations using the Chi-square test. Haplotype reconstruction was performed by use of the Bayasian statistical method implemented in PHASE, version 2.1.1 [28], [29], using single nucleotide polymorphism (SNP) with a minimum allele frequency of 2%. We applied the algorithm five times, using different randomly generated seeds, and consistent results were obtained across runs.
Results
DC-SIGN polymorphism
We identified 40 SNPs with allelic frequencies >1% in the Canadian and Zimbabwean populations after direct sequencing of the entire DC-SIGN gene (Figure 1A). Among these SNPs, 19 (47.5%) were unique to the Zimbabweans when compared with the Canadians, whereas four (10%) were found only in the Canadian samples. Five novel DC-SIGN mutations were identified in the promoter region and in intron 6. In the Zimbabwean population, two novel SNPs were found in the 5′-UTR at positions −819 (A/G) and −332 (G/A) at allelic frequencies of 2%. In the Canadian population, one novel mutation in the promoter region at position −859 (A/G) was found in 2% of the individuals. One novel SNP in intron 6 +148 (G/A) was observed exclusively in 3% of Zimbabweans, whereas another single novel mutation in intron 6–39 (insertion of G) shared by both populations was found in 2% of individuals in each population.
Figure 1.
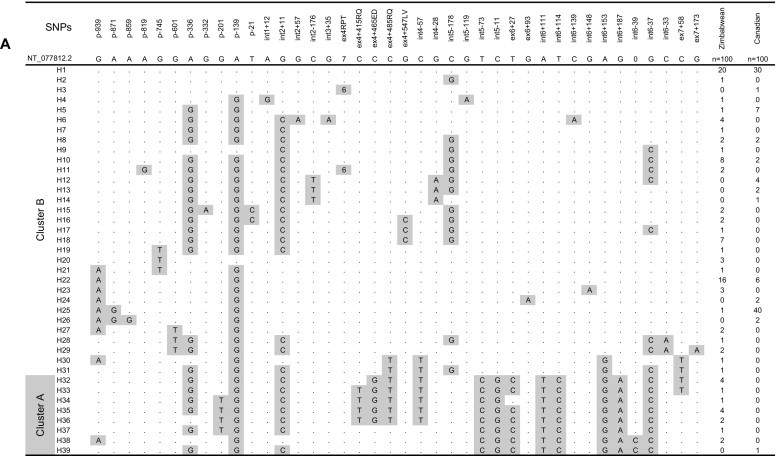

Inferred haplotypes of dendritic cell–specific intracellular adhesion molecule–3–grabbing nonintegrin (DC-SIGN) (A) and DC-SIGN–related (DC-SIGNR) (B). Dark boxes correspond to the mutations compared with the reference sequence of chromosome 19 (NCBI no NT_077812.2). n = number of alleles; SNPs = single nucleotide polymorphisms.
The allelic distribution of selected DC-SIGN polymorphisms with the potential to affect the protein function and/or known to be associated with susceptibility/resistance to pathogens were typed in all study samples and compared with those of other ethnic populations (Table 1). The genotypic distribution of DC-SIGN SNPs at each position was in Hardy-Weinberg equilibrium (p > 0.5) for both populations analyzed in the present study. There were significant differences in the distribution of DC-SIGN alleles between Zimbabweans and Canadians for most of the SNPs analyzed. The mutations at position −745 (G/T) and −201 (G/T) in the promoter region were observed exclusively in the Zimbabwean population. Promoter variants at position −336 (A/G) was found more frequently in Zimbabweans (45%) than in Canadians (18%) whereas the −871 (A/G) variant was observed less frequently in Zimbabweans (5%) than in Canadians (42%). A cluster of 8 mutations in introns 5 and 6 in high LD, identified as cluster A (H32-H39) by Barreiro et al. [17], was found in 15% of the Zimbabwean population, whereas it was almost absent in the Canadian population (1%) (Figure 1A). This cluster of mutations was found in association with the promoter variant at position −201 (G/T) and three exon 4 protein-modifying mutations coding for the neck region of the protein (H33-H36, Figure 1A): codons 198 (positive charged Arg→neutral Gln), 214 (negative charged Glu→negative charged Asp), and 221 (positive charged Arg→neutral Gln). A fourth nonsynonymous substitution in exon 4 at codon 242 (neutral Leu→neutral Val) was associated with the cluster B (H16–18) (Figure 1A). The Cluster A and exon 4 nonsynonymous mutations are observed almost exclusively in African populations (Figure 1A and Table 1), whereas the cluster B mutations (Figure 1A) are found in both Zimbabwean and Canadian populations as well as in all other major ethnic groups [17], [19], [20].
The allelic composition of DC-SIGN haplotypes and their frequency distribution in Zimbabweans and Canadians is illustrated in Figure 1A. In total, we identified 39 different haplotypes. Two major haplotypes (H1, H25) accounted for 70% of the Canadian variability, whereas the two major haplotypes (H1, H22) in the Zimbabwean population represented ∼35% of the variability. There were 32 different haplotypes in Zimbabweans and 13 different haplotypes among Canadians. Of those, 26 were found exclusively among Zimbabweans, whereas seven were unique to the Canadian population. Thus, the Zimbabwean population showed a significant greater DC-SIGN genetic diversity (82%) than the Canadian population (33%) (p < 0.03).
DC-SIGNR polymorphism
We observed the presence of 29 DC-SIGNR SNPs with allelic frequencies >1% in the Canadian and Zimbabwean populations (Figure 1B). Of these, eight were novel DC-SIGNR mutations. Two novel mutations in the 5′-UTR at positions −414 (G/A) and −133 (T/C) were detected exclusively in Canadians at frequencies of 9% and 3%, respectively. In the Zimbabwean population, there were two novel SNPs in exons 2 and 5 found in 3% and 5% of individuals, respectively. The mutation in exon 2 (C/G) is predicted to modify the amino acid composition of the protein at codon 28 (neutral Pro→neutral Leu) while the exon 5 (G/T) at codon 290 is a silent mutation (His→His). There were four novel mutations in the 3′-UTR of the Zimbabwean DC-SIGNR gene. The exon 7 + 223 (deletion of TCT) and exon 7 + 271 (C/G) were linked and found in 15% of individuals. The exon 7 + 333 (T/C) was linked with exon 5 (G/T) mutation and observed in 5% of individuals. Finally, the exon 7 + 518 (C/T) was found at an allelic frequency of 4% in the Zimbabwean population. The genotypic distribution of DC-SIGNR SNPs at each positions was in Hardy-Weinberg equilibrium (p ≥ 0.5).
Haplotype reconstruction of DC-SIGNR showed a wider haplotype diversity (52 different haplotypes) than that found for DC-SIGN (Figure 1B). Interestingly, the haplotype corresponding to the wild-type sequence (H1) was found only in 5% of Canadians and in none of the Zimbabweans. The major haplotype (H15) in Zimbabweans (22%) was also relatively frequent in the Canadian population (19%). Haplotypes (H22, H43, H46) accounted for 27% of Zimbabweans variability and were absent in Canadians. In contrast, haplotypes (H13, H33, H36, H41) accounted for 53% of Canadian variability, whereas they were at very low frequency (H33 at 1%) or absent (H13, H36, H41) in Zimbabweans. Once again, the Zimbabweans showed a greater DC-SIGNR genetic diversity with 34/52 haplotypes (65%) than Canadians with 23/52 haplotypes (44%) although the difference was not statistically significant (p > 0.05). In contrast, Zimbabweans were less likely to present polymorphic DC-SIGNR repeat-regions (Table 2). Genotypic distribution of repeat alleles in Zimbabweans was mostly represented by the wild-type 7/7-repeat form (42%). Moreover, the Zimbabweans were more likely to be homozygous (53%) for the repeat region than the Canadians (33%, p < 0.01) and other Caucasian populations (Table 2).
Discussion
Our exhaustive investigation of the nucleotide sequence of DC-SIGN and DC-SIGNR genes in Zimbabwean and Canadian populations revealed the presence of novel variants, mostly in the regulatory regions of these genes. There were two novel promoter (5′-UTR) variants that might influence gene transcription as suggested by a search in the TRANSFAC database. The DC-SIGN −332A variant found in Zimbabweans and the DC-SIGNR −414A variant observed in Caucasian Canadians create potential binding sites for TCF-1α and E1A-F transcriptional factors, respectively. Four novel mutations in DC-SIGNR 3′-UTR were observed at frequencies ranging from 4% to 15% in the Zimbabwean population exclusively. There are now a number of studies suggesting that genetic variants in 3′-UTR affecting RNA expression can be implicated in human disease (reviewed in [32]). Using computer-based prediction tools (Lasergene software, DNA Stars, Madison, WI), we observed that these novel mutations are likely to modify the secondary structure of the DC-SIGNR 3′-UTR, which in turn could affect mRNA stability or production. Further in vitro studies will be needed to assess the possible effects of these novel 5′-UTR and 3′-UTR mutations on DC-SIGN and DC-SIGNR transcriptional and translational activities.
They were significant differences in the distribution of DC-SIGN and DC-SIGNR alleles among African and non-African populations. Of interest, the DC-SIGN promoter variant −336G associated with susceptibility to HIV-1 infection [18] and dengue hemorrhagic fever [19] is found significantly more frequently in African than in Caucasian or Asian populations (p < 0.001) (Table 1). This observation is very interesting, considering the high prevalence of at least the former infection in Africa. The −336G variant, located 214 bp upstream of the major transcription site, affects a Sp1 binding site and modulates in vitro DC-SIGN transcriptional activity by decreasing its expression [19]. The reduced DC and macrophage cell surface expression of DC-SIGN in individuals carrying the −336G variant might have deleterious effects on clearance of pathogens by diminishing the antigen-presenting capability of these cells. However, recent in vitro studies have shown that HIV-1 and other important pathogens target DC-SIGN to escape immune surveillance and to promote their survival [33], [34]. Other relatively frequent promoter mutations, found exclusively in Africans, might also affect cell surface expression of DC-SIGN. Indeed, the TRANSFAC database search identified potential TBP and RSRFC4 binding sites in the presence of promoter variant −745A and a c-Myc binding site in presence of the variant −201A. At this point, it is not known whether or not these putative binding sites are biologically significant in the context of DC-SIGN transcription. More effort will be needed to determine the net impact of DC-SIGN promoter variants on its expression and to understand how the altered expression of DC-SIGN may affect immune response capacity or immune escape.
The neck region of DC-SIGN and DC-SIGNR region plays a crucial role in tetramerization of the receptor and support of CRDs, thus influencing the pathogen-binding properties of these receptors. The neck region of DC-SIGN consists of seven amino acid repeats with rare variations, whereas the number of DC-SIGNR repeats is highly variable and differs among different ethnic populations (Table 2). The Zimbabweans are more likely to be homozygous (53%) for the DC-SIGNR repeat region than the Canadians (33%, p < 0.01) and other Caucasian populations (Table 2). Genotypic distribution of repeat alleles in Zimbabweans was mostly represented by the wild-type homozygous 7/7-repeat form (42%). A recent study reported that the DC-SIGNR homozygous 7/7 wild-type genotype is associated with an increased risk of HIV-1 infection, whereas the heterozygous 7/5 genotype correlated with resistance to infection [23]. It has been suggested that heterozygous expression of polymorphic neck variants of DC-SIGNR may result in reduced ligand-binding affinity [16], [35], [36]. In fact, homozygous CHO cells transfected with DC-SIGNR containing seven tandem exon 4 repeats (7/7) had higher binding capacity than heterozygous 7/5 transfectants [26]. Whether homozygosity contributes to enhanced infection, immune escape or triggers effective immune responses remains to be established. Guo et al. [37] found that individual with homozygous or heterozygous 5-, 6-, or 7-repeat alleles would express functional DC-SIGNR, whereas shorter alleles would not oligomerize. Despite the fact that the heterozygous genotype did not affect tetramerization of the receptor, it is not excluded that shortening the neck region would significantly change position of the CRDs relative to the cell surface, thus affecting interaction with pathogens. Accordingly, the high rate of homozygosity at the wild-type repeat allele in Zimbabweans may represent a better pathogen recognition capacity for DC-SIGNR or a better chance for pathogens to subvert DC-SIGNR for immune escape.
We and others [17] have identified four protein-modifying mutations in the neck domain of DC-SIGN that are relatively frequent in Africans but absent in other major population groups (Table 1). These variants are nonconservative substitutions with respect to amino acid composition (codons 198, 214, 221, 242) and polarity (codons 198 and 221) and might induce conformational changes in the protein neck domain. This in turn could affect the oligomerization of the receptor, resulting in altered binding capacity and specificity of the DC-SIGN protein. Haplotype reconstruction of the DC-SIGN gene (Figure 1A) in the Zimbabwean population has shown that haplotypes can contained either three (H33-H36), two (H32), or one (H16,H17,H18,H30,H31) of these amino acid changes, thus affecting the conformation of the protein in different manners and contributing to the variability of DC-SIGN at the protein level in this population. Variations in the neck domain of DC-SIGN gene, leading to different forms of the DC-SIGN receptor in Africans, may be the result of selective pressure exerted by prevalent pathogens in these geographically distinct regions.
In summary, we have conducted a thorough analysis of the nucleotide sequence of the DC-SIGN and DC-SIGNR genes in samples collected from Caucasian Canadians and indigenous Zimbabweans. Several novel nucleotide variants within the regulatory 5′- and 3′-untranslated regions of the genes that are important for transcription and translation were identified. The data from this study and others [17], [19], [20], [26], [31] show that the allelic distribution of DC-SIGN and DC-SIGNR genes differs widely in populations from industrialized and developing countries, presumably because of geographically determined selection pressures. In fact, the prevalence of most of the polymorphisms that could potentially affect either the transcription, translation or function of these proteins was higher in African than in non-African populations. Moreover, haplotype reconstruction clearly demonstrates that Africans show greater genetic diversity at these two closely-related immune loci than that observed in Caucasian Canadians. A broader spectrum of infectious pathogens including some that are known specifically to subvert DC-SIGN and DC-SIGNR function to escape the immune system might have played an important role in shaping the genetic repertoire of these genes among Africans. Further studies will be needed to determine the net impact of DC-SIGN and DC-SIGNR genetic variants on the expression, translation and function of the proteins and to understand how these functional polymorphisms may affect immune response capacity or immune escape.
Acknowledgments
This work was supported in part by an operating grant from the Canadian Institutes of Health Research (CIHR) and Réseau Sida et maladies infectieuses du Fonds de la Recherche en Santé du Québec (FRSQ). M. Roger is a scientific scholar receiving support from FRSQ. The ZVITAMBO project is supported by the Canadian International Development Agency (R/C Project 690/M3688), Cooperative Agreement DAN 0045-A-005094-00 between the U.S. Agency for International Development and The Johns Hopkins School of Hygiene and Public Health, and the Rockefeller Foundation. It is a collaborative project of The University of Zimbabwe, The Harare City Health Department, The Johns Hopkins School of Hygiene and Public Health, and the Montreal General Hospital Research Institute, McGill University.
The authors thank all the participants in this study and the ZVITAMBO Study Group: H. Chidawanyika, Jean Humphrey, P. Iliff, A. Mahomva, F. Majo, L. Malaba, L. Moulton, K. Mutasa, J. Mutsambi, K. Nathoo, M. Ndhlovu, L. Propper, A. Ruff, N. Tavengwa, C. Zunguza, and P. Zvandasara. We also thank Claudine Matte and Julie Lacaille for processing the specimens.
References
- 1.Geijtenbeek T.B., Torensma R., van Vliet S.J., van Duijnhoven G.C., Adema G.J., van Kooyk Y., Figdor C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 2.Soilleux E.J., Morris L.S., Leslie G., Chehimi J., Luo Q., Levroney E., Trowsdale J., Montaner L.J., Doms R.W., Weissman D., Coleman N., Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445. [PubMed] [Google Scholar]
- 3.Soilleux E.J., Morris L.S., Rushbrook S., Lee B., Coleman N. Expression of human immunodeficiency virus (HIV)-binding lectin DC-SIGNR: Consequences for HIV infection and immunity. Hum Pathol. 2002;33:652. doi: 10.1053/hupa.2002.124036. [DOI] [PubMed] [Google Scholar]
- 4.Cambi A., Figdor C.G. Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol. 2003;15:539. doi: 10.1016/j.ceb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Geijtenbeek T.B., Krooshoop D.J., Bleijs D.A., van Vliet S.J., van Duijnhoven G.C., Grabovsky V., Alon R., Figdor C.G., van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J., Cornelissen I.L., Nottet H.S., KewalRamani V.N., Littman D.R., Figdor C.G., van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez C.P., Lasala F., Carrillo J., Muniz O., Corbi A.L., Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;6:6841. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halary F., Amara A., Lortat-Jacob H., Messerle M., Delaunay T., Houles C., Fieschi F., Arenzana-Seisdedos F., Moreau J.F., Dechanet-Merville J. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653. doi: 10.1016/s1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- 9.Appelmelk B.J., Van Die I., van Vliet S.J., Vandenbroucke-Grauls C.M., Geijtenbeek T.B., van Kooyk Y. Cutting edge: Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 10.Lozach P.Y., Lortat-Jacob H., de Lacroix D.L., Staropoli I., Foung S., Amara A., Houles C., Fieschi F., Schwartz O., Virelizier J.L., Arenzana-Seisdedos F., Altmeyer R. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem. 2003;278:20358. doi: 10.1074/jbc.M301284200. [DOI] [PubMed] [Google Scholar]
- 11.Tailleux L., Schwartz O., Herrmann J.L., Pivert E., Jackson M., Amara A., Legres L., Dreher D., Nicod L.P., Gluckman J.C., Lagrange P.H., Gicquel B., Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tassaneetrithep B., Burgess T.H., Granelli-Piperno A., Trumpfheller C., Finke J., Sun W., Eller M.A., Pattanapanyasat K., Sarasombath S., Birx D.L., Steinman R.M., Schlesinger S., Marovich M.A. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman M.P., Engering A., Smits H.H., van Vliet S.J., van Bodegraven A.A., Wirth H.P., Kapsenberg M.L., Vandenbroucke-Grauls C.M., van Kooyk Y., Appelmelk B.J. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzi A., Gramberg T., Simmons G., Moller P., Rennekamp A.J., Krumbiegel M., Geier M., Eisemann J., Turza N., Saunier B., Steinkasserer A., Becker S., Bates P., Hofmann H., Pohlmann S. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78:12090. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colmenares M., Puig-Kroger A., Pello O.M., Corbi A.L., Rivas L. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J Biol Chem. 2002;277:36766. doi: 10.1074/jbc.M205270200. [DOI] [PubMed] [Google Scholar]
- 16.Bashirova A.A., Geijtenbeek T.B., van Duijnhoven G.C., van Vliet S.J., Eilering J.B., Martin M.P., Wu L., Martin T.D., Viebig N., Knolle P.A., KewalRamani V.N., van Kooyk Y., Carrington M. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J Exp Med. 2001;193:671. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barreiro L.B., Patin E., Neyrolles O., Cann H.M., Gicquel B., Quintana-Murci L. The heritage of pathogen pressures and ancient demography in the human innate-immunity CD209/CD209L region. Am J Hum Genet. 2005;77:869. doi: 10.1086/497613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin M.P., Lederman M.M., Hutcheson H.B., Goedert J.J., Nelson G.W., van Kooyk Y., Detels R., Buchbinder S., Hoots K., Vlahov D., O’Brien S.J., Carrington M. Association of DC-SIGN promoter polymorphism with increased risk for parenteral, but not mucosal, acquisition of human immunodeficiency virus type 1 infection. J Virol. 2004;78:14053. doi: 10.1128/JVI.78.24.14053-14056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakuntabhai A., Turbpaiboon C., Casademont I., Chuansumrit A., Lowhnoo T., Kajaste-Rudnitski A., Kalayanarooj S.M., Tangnararatchakit K., Tangthawornchaikul N., Vasanawathana S., Chaiyaratana W., Yenchitsomanus P.T., Suriyaphol P., Avirutnan P., Chokephaibulkit K., Matsuda F., Yoksan S., Jacob Y., Lathrop G.M., Malasit P., Despres P., Julier C. A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet. 2005;37:507. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barreiro L.B., Neyrolles O., Babb C.L., Tailleux L., Quach H., McElreavey K., Helden P.D., Hoal E.G., Gicquel B., Quintana-Murci L. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez L.M., Anaya J.M., Sierra-Filardi E., Cadena J., Corbi A., Martin J. Analysis of DC-SIGN (CD209) functional variants in patients with tuberculosis. Hum Immunol. 2006;67:808. doi: 10.1016/j.humimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Liu H., Hwangbo Y., Holte S., Lee J., Wang C., Kaupp N., Zhu H., Celum C., Corey L., McElrath M.J., Zhu T. Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J Infect Dis. 2004;190:1055. doi: 10.1086/423209. [DOI] [PubMed] [Google Scholar]
- 23.Liu H., Carrington M., Wang C., Holte S., Lee J., Greene B., Hladik F., Koelle D.M., Wald A., Kurosawa K., Rinaldo C.R., Celum C., Detels R., Corey L., McElrath M.J., Zhu T. Repeat-region polymorphisms in the gene for the dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin-related molecule: Effects on HIV-1 susceptibility. J Infect Dis. 2006;193:698. doi: 10.1086/499820. [DOI] [PubMed] [Google Scholar]
- 24.Lichterfeld M., Nischalke H.D., van Lunzen J., Sohne J., Schmeisser N., Woitas R., Sauerbruch T., Rockstroh J.K., Spengler U. The tandem-repeat polymorphism of the DC-SIGNR gene does not affect the susceptibility to HIV infection and the progression to AIDS. Clin Immunol. 2003;107:55. doi: 10.1016/s1521-6616(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 25.Nattermann J., Ahlenstiel G., Berg T., Feldmann G., Nischalke H.D., Muller T., Rockstroh J., Woitas R., Sauerbruch T., Spengler U. The tandem-repeat polymorphism of the DC-SIGNR gene in HCV infection. J Viral Hepat. 2006;13:42. doi: 10.1111/j.1365-2893.2005.00652.x. [DOI] [PubMed] [Google Scholar]
- 26.Chan V.S., Chan K.Y., Chen Y., Poon L.L., Cheung A.N., Zheng B., Chan K.H., Mak W., Ngan H.Y., Xu X., Screaton G., Tam P.K., Austyn J.M., Chan L.C., Yip S.P., Peiris M., Khoo U.S., Lin C.L. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat Genet. 2006;38:38. doi: 10.1038/ng1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parham P., Ohta T. Population biology of antigen presentation by MHC class I molecules. Science. 1996;272:67. doi: 10.1126/science.272.5258.67. [DOI] [PubMed] [Google Scholar]
- 28.Stephens M., Smith N.J., Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens M., Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barreiro L.B., Quintana-Murci L. DC-SIGNR neck-region polymorphisms and HIV-1 susceptibility: From population stratification to a possible advantage of the 7/5 heterozygous genotype. J Infect Dis. 2006;194:1184. doi: 10.1086/507845. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi N., Nakamura H.T., Goto M., Nakamura T., Nakamura K., Sugiura W., Iwamoto A., Kitamura Y. Polymorphisms and haplotypes of the CD209L gene and their association with the clinical courses of HIV-positive Japanese patients. Jpn J Infect Dis. 2002;55:131. [PubMed] [Google Scholar]
- 32.Mazumder B., Seshadri V., Fox P.L. Translational control by the 3′-UTR: The ends specify the means. Trends Biochem Sci. 2003;28:91. doi: 10.1016/S0968-0004(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhou T., Chen Y., Hao L., Zhang Y. DC-SIGN and immunoregulation. Cell Mol Immunol. 2006;3:279. [PubMed] [Google Scholar]
- 34.Lekkerkerker A.N., van Kooyk Y., Geijtenbeek T.B. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr HIV Res. 2006;4:169. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- 35.Soilleux E.J., Barten R., Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol. 2000;165:2937. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell D.A., Fadden A.J., Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR: Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 37.Guo Y., Atkinson C.E., Taylor M.E., Drickamer K. All but the shortest polymorphic forms of the viral receptor DC-SIGNR assemble into stable homo- and heterotetramers. J Biol Chem. 2006;281:16794. doi: 10.1074/jbc.M602430200. [DOI] [PubMed] [Google Scholar]


