Highlights
► This initial U.S. study examined genetic and antigenic differences among BoCV. ► Two clades were identified, BoCV1 and BoCV2 using viral DNA sequencing. ► Three subclades of BoCV2 were identified: BoCV2a, BoCV2b, and BoCV2c. ► Antigenic differences among BoCV clades were detected by viral neutralization tests.
Keywords: Bovine, Respiratory disease, Antigenic, Genetic, Bovine coronavirus, Vaccination
Abstract
BoCV isolated from respiratory tract, nasal swab and broncho alveolar washing fluid samples were evaluated for genetic and antigenic differences. These BoCV from the respiratory tract of healthy and clinically ill cattle with BRD signs were compared to reference and vaccine strains based on Spike protein coding sequences and VNT using convalescent antisera. Based on this study, the BoCV isolates belong to one of two genomic clades (clade 1 and 2) which can be differentiated antigenically. The respiratory isolates from Oklahoma in this study were further divided by genetic differences into three subclades, 2a, 2b, and 2c. Reference enteric BoCV strains and a vaccine strain were in clade 1. Currently available vaccines designed to control enteric disease are based on viruses from one clade while viruses isolated from respiratory tracts, in this study, belong to the other clade.
1. Introduction
Bovine respiratory disease (BRD) is associated with infectious agents and often complicated by stress factors including environmental, nutrition, transportation, and commingling with cattle of mixed origins and multiple herd sources [1], [2]. Infectious agents considered in the etiologies of BRD include viruses: bovine herpesvirus-1 (BHV-1), parainfluenza-3 virus (PI-3V), bovine respiratory syncytial virus (BRSV), bovine viral diarrhea virus (BVDV), bovine adenoviruses (BAV), and bovine coronaviruses (BoCV) and bacteria, Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma spp. [1], [2]. For many years, BHV-1, PI-3V, BRSV, and BVDV were the viruses most associated with the viral etiology of BRD. In contrast, BoCV was associated with neonatal enteric disease and winter dysentery of adult cattle [3], [4], [5]. Recently BoCV as a respiratory infection and disease has received attention and has been the subject of multiple reviews [6], [7].
BoCV are enveloped, nonsegmented, positive sense single stranded RNA viruses that are grouped as a species within the Coronavirus genus of the Coronaviridae family [8]. BoCV virions contain a large surface glycoprotein referred to as the spike or S protein, an integral membrane protein (M), a small membrane protein (E), a hemagglutinin-esterase glycoprotein (HE) and a nucleocapsid protein (N). While strong humoral responses are elicited against the S, M, N and HE proteins following natural infection, the predominant antigens involved in virus neutralization are located in the S and HE proteins. Various studies have segregated the Coronavirus genus into groups based on several criteria, including; position and variation of non-structural proteins in the 3′ end of the genome, antigenic cross reactivity, processing of the S protein and host range. However, there are no set guidelines for defining new Coronavirus species or differentiating subgroups within existing species. As stated above BoCV were initially associated with outbreaks of enteric disease. More recently, BoCV have been associated with bovine respiratory disease (BRD) and in cattle pulled for treatment in the feedlot as well as from healthy cattle in numerous studies in the U.S. In these studies BoCV was identified by virus isolation from nasal swabs and bronchoalveolar lavage (BAL) and serotests detecting seroconversions indicating exposure to BoCV in outbreaks of BRD and inapparent infections [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. BoCV have been identified in pneumonic lungs from field cases, often in combination with other viruses and bacteria including Mycoplasma spp. [20], [21], [22]. Experimentally BoCV have caused respiratory tract lesions affecting the epithelium of the turbinates, trachea, and lungs [23]. Based on the observation of two different presentations following BoCV exposure, it has been suggested there is a dual tropism by BoCV for respiratory and digestive tracts of cattle [23], [24].
Control measures for BoCV respiratory disease are limited. The vaccines available for BoCV are licensed to control of the neonatal enteric disease [7], [9], [25]. There are three inactivated vaccines licensed to control of neonatal enteric disease and these are used in pregnant cows/heifers during pregnancy to stimulate humoral immunity for passive immunization of the newborn calf [7], [25]. There is a modified live virus vaccine containing BoCV for administration orally to the newborn calf to provide an active immune response to protect the calf against enteric disease [7], [25]. There are no licensed BoCV vaccines in the U.S. to protect against BRD, nor have effectiveness of the licensed enteric BoCV vaccines been determined for protection against BRD with BoCV challenge. However there is one U.S. report using the MLV vaccine containing BoCV that reduced treatment for BRD [26]. The methods of protection as correlates of immunity were not determined however [9]. To provide optimal immunity, vaccine antigens should be as similar as possible to the circulating viruses. It appears important that the virus or viruses used as immunogens to control BoCV BRD should align antigenically and genetically with the BoCV circulating in the field.
The purpose of this study was to compare BoCV isolates from the respiratory tract in Oklahoma cattle to reference respiratory and enteric strains and enteric vaccine strains by antigenic and genetic procedures. A valid question being asked, “Are there differences between enteric and respiratory strains isolated as BoCV, and if so, what strain or strains should be used to replace or add to existing BCV enteric vaccines”.
2. Materials and methods
2.1. Source of samples
A total of 56 field strains of BoCV were characterized. Included were samples from cattle not manifesting BRD signs at collection (healthy) and from cattle with BRD signs (BRD). There were multiple sources and studies from which the BoCV strains were derived (Table 1 ). There were five studies performed at the Oklahoma State University (OSU) Willard Sparks Beef Research Center (WSBRC) feedlot at the Department of Animal Sciences, including four in 2009 (OSU-1,OSU-2,OSU-3, OSU-4) and a fifth in 2011 (OSU-5). All cattle were test negative for BVDV by ear notch immunohistochemistry using skin samples. These calves were auction market purchased calves that were commingled at the auctions and transported to the OSU WSBRC where they were processed receiving identification and MLV vaccines containing BHV-1, PI-3V, BVDV1a, BVDV2a, and BRSV immunogens. Nasal swabs and in some cases bronchoalveolar lavage (BAL) fluids were collected at processing. Sample collections were repeated at weekly times up to 14 days. [19]. The cattle were placed in pens and a representative [21], [22], [23], [24], [25], [26] group for each study monitored as sentinels. Cattle that were treated for BRD were sampled as well as the sentinel calves. Blood was collected at processing for serums as well as convalescent sera at ≥56 days after arrival. There were in some instances multiple positive BCV samples from the same animal, either from NS and BAL or from sequential collections. In addition NS collected during an OSU study (OSU-6) for a viral challenge study unrelated to BoCV were included in this study. Similarly, nasal swab samples collected from southeastern U.S. sourced cattle that were commingled and delivered to a research facility and monitored for BRD from studies in 1999 (OSU-7) and 2000 (OSU-8) were included. All studies were approved by the OSU Institutional Animal Care and Use Committee (#VM0818 and #VM0819).
Table 1.
Identification of Oklahoma bovine coronaviruses from the respiratory tract of cattle.
| Identification | Health status | Study | BoCV clade |
|---|---|---|---|
| OK 554 BAL | BRD | OSU-1 | BoCV 2c |
| OK 538 BAL | Healthy | OSU-1 | BoCV 2c |
| OK 542 BAL | Healthy | OSU-1 | BoCV 2c |
| OK 563 BAL | Healthy | OSU-1 | BoCV 2c (6) |
| OK 575 NS | BRD | OSU-1 | BoCV 2c (6) |
| OK 603 NS | BRD | OSU-1 | BoCV 2c (6) |
| OK 521 BAL (17) | BRD | OSU-1 | BoCV 2c (6) |
| OK 552 BAL | Healthy | OSU-1 | BoCV 2c (6) |
| OK 591 BAL | BRD | OSU-1 | BoCV 2c (6) |
| OK 513 BAL | Healthy | OSU-1 | BoCV 2c (6) |
| OK 609 BAL | Healthy | OSU-1 | BoCV 2c (6) |
| OK 521 NS | BRD | OSU-1 | BoCV 2c (6) |
| OK 575 BAL (15) | BRD | OSU-1 | BoCV 2c (6) |
| OK 545 BAL | Healthy | OSU-1 | BoCV 2c (6) |
| OK 600 BAL | BRD | OSU-1 | BoCV 2c (6) |
| OK 603 BAL | BRD | OSU-1 | BoCV 2c (6) |
| OK 576 BAL | Healthy | OSU-1 | BoCV 2c (6) |
| OK 592 BAL | Healthy | OSU-1 | BoCV 2c (6) |
| OK 746 NS | Healthy | OSU-2 | BoCV 2b (3) |
| OK 833 NS | Healthy | OSU-2 | BoCV 2b (3) |
| OK 746 BAL | Healthy | OSU-2 | BoCV 2b (3) |
| OK 747 NS | Healthy | OSU-2 | BoCV 2b (3) |
| OK 821 NS | Healthy | OSU-2 | BoCV 2b (1) |
| OK 801 NS | Healthy | OSU-2 | BoCV 2b (1) |
| OK 747 BAL | Healthy | OSU-2 | BoCV 2b (3) |
| OK 778 NS | Healthy | OSU-2 | BoCV 2b (4) |
| OK 778 BAL | Healthy | OSU-2 | BoCV 2b (4) |
| OK 787 NS | BRD | OSU-2 | BoCV 2b (2) |
| OK 802 NS (42) | BRD | OSU-2 | BoCV 2b (2) |
| OK 797 NS | BRD | OSU-2 | BoCV 2b (2) |
| OK 802 NS (53) | BRD | OSU-2 | BoCV 2b (2) |
| OK 766 NS | Healthy | OSU-2 | BoCV 2b |
| OK 665 NS | Healthy | OSU-2 | BoCV 2b |
| OK 834 NS | Healthy | OSU-2 | BoCV 2c |
| OK 3167 NS | Healthy | OSU-3 | BoCV 2b |
| OK 3172 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3162 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3169 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3165 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3163 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3181 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3174 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3168 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3170 NS | Healthy | OSU-3 | BoCV 2b (1) |
| OK 3175 NS | Healthy | OSU-3 | BoCV 2b |
| OK 3171 NS | Healthy | OSU-3 | BoCV 2b |
| OK 3166 NS | Healthy | OSU-3 | BoCV 2b |
| OK 1817 NS | Healthy | OSU-4 | BoCV 2b (1) |
| OK 1776 NS | Healthy | OSU-4 | BoCV 2b |
| OK 776 NS | BRD | OSU-5 | BoCV 2c |
| OK 717 NS | BRD | OSU-5 | BoCV 2b |
| OK 43 NS | Healthy | OSU-6 | BoCV 2b (5) |
| OK 45 NS | Healthy | OSU-6 | BoCV 2b (5) |
| OK AN 3 NS | Healthy | OSU-7 | BoCV 2a |
| OK AN 5 NS | Healthy | OSU-7 | BoCV 2a |
| OK TN 10 NS | Healthy | OSU-8 | BoCV 2a |
| BCV NVSL | Reference strain USDA | BoCV 1 |
(1) identical; (2) identical; (3) identical; (4) identical; (5) identical; (6) identical.
2.2. Virus isolation
The BoCV in this study were isolated in human rectal tumor (HRT) monolayer cultures from filtered nasal swabs and/or BAL as described [19].
2.3. Viral serology
A microtitration virus neutralization test (VNT) was performed in 96-well plates using the HRT cells to quantitate antibodies to BoCV using duplicate rows for the serum dilutions [19]. Initially the challenge virus was a cytopathic BoCV (USDA APHIS NVSL, Ames, IA), the BoCV NVSL strain. The endpoint was the lowest final/virus tested (1:4) which completely neutralized the viral CPE. The titers were expressed as the reciprocal of the endpoint dilution. Positive and negative controls were utilized. During the study other BoCV were used in the VNT for the serotest comparing different OSU strains isolated from the respiratory tract (Table 1). A monoclonal antibody, lot WR99316 BC 28 HI.2C against N protein served as the positive control [9], [19].
2.4. Antigenic studies
The antibody titers to several OSU strains in addition to the BoCV NVSL reference strain were compared to determine if the calves’ serums were able to neutralize the viral CPE for the respective virus. There were three sera from calves from which BoCV2b was isolated, two sera from calves from which BoCV2c was isolated and three sera from animals from which BoCV2a was isolated. Viruses used in the NVT as challenge virus included five BoCV2b, two BoCV2c, three BoCV2a, and one BoCV1 strain. In Fig. 2, the error bars represent standard error of mean. A reference antibody, described above, was used in the study in the VNT with each respective OSU virus. Serums were convalescent compared to determine if the calves’ sera were able to neutralize the viral CPE for the respective virus.
Fig. 2.
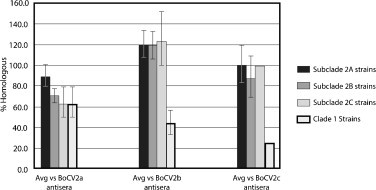
Convalescent serology with each subclade strains against serums from calves with respective subclade virus.
2.5. Genetic sequencing
The BoCV respiratory viruses from the OSU studies along with reference strains and MLV vaccine strain were examined for genomic diversity based on comparison of a region of the S gene. In brief, RNA from the BoCV viruses from the OSU studies along with reference enteric and respiratory and MLV vaccine strains was prepared using the Qiagen Viral RNA mini kit for Qiacube per the manufacturers instructions (Qiagen Inc. USA, Valencia, CA). A 10 μl aliquot of the extracted RNA was amplified using the primer set published by Kanno et al. [33]. The 1194 nucleotide section of the S protein gene that was amplified included the polymorphic region of the S protein gene that was used for phylogenetic analysis in previous studies of Coronavirus [27], [28], [29], [30], [31], [32], [33], [34]. Amplification was confirmed based on size of amplicon as visualized by agarose gel electrophoresis. Amplicons were purified and concentrated using a Geneclean Spin Kit per manufacturers instructions (MP Biomedicals, Solon, Ohio) followed by quantification using the Pico Green assay for ds DNA (Invitrogen Corporation, Carlsbad, CA) and the appropriate quantity of dsDNA was labeled in both directions using Big Dye terminator chemistries (Applied Biosystems Inc., Foster City, CA) according to the manufacturer's instructions. The labeled products were sequenced using an ABI 3100 genetic analyzer (Applied Biosystems Inc.). All sequences were confirmed by sequencing both strands and all sequencing reactions were run in duplicate. Sequences were edited and aligned using Sequencher 4.2 (Gene Codes Corporation, Ann Arbor, MI). Final phylogenetic and molecular evolutionary analyses were conducted using Mega version 5.0 (Tamura K, Peterson D, Peterson N, Stecher G, Nei M, and Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution doi:10.1093/molbev/msr121). Analysis was done using Clustal-W based on unpaired geometric mean analysis supported by bootstrapping (5000 replicates). Reference sequences from Genbank used in analysis are listed in Table 2 . GenBank accession numbers for generated sequences are listed in Table 3 .
Table 2.
Reference bovine coronavirus strains for sequencing.
| Ref. strain | GenBank Accession Number | Clinical presentation | Ref. |
|---|---|---|---|
| 182 NS | DQ320764.1 | Respiratory disease | Virus Res 2002;84:101–109 |
| 232 NS | DQ320763.1 | Respiratory disease | Virus Res 2002;84:101–109 |
| DB2 | DQ811784.2 | Winter dysentery | Virus Res 2002;84:101–109 |
| BcoV-LUN | AF391542.1 | Respiratory disease | J Gen Virol 2001;82(Pt 12):2927–2933 |
| G95 | M80844.1 | Respiratory disease | Arch Virol 1994;134(3–4):421–426 |
| 43806-TN-50 | EU814648.1 | Respiratory disease | New Microbiol 2009;32 (January (1)):109–113 |
| BCQ.20 | UO6092.1 | Epidemic diarrhea | Arch Virol 1994;135(3–4):319–331 |
| KWD1 | AY935637 | Winter dysentery | Virus Res 2005;108(1–2):207–212 |
| KWD10 | AY935646 | Winter dysentery | Virus Res 2005;108(1–2):207–212 |
| OK-0514-3 | AF058944 | Respiratory disease | Virus Genes 1998;17(1):33–42 |
| LSU-94LSS-051-2 | AF058943 | Respiratory disease | Virus Genes 1998;17(1):33–42 |
| F15 | D00731 | Enteritis | J Gen Virol 1990;71(Pt 2):487–492 |
| LY138 | AF058942 | Enteritis | Virology 1991;183(1):397–404 |
| Nebraska (NVSL) | JQ741969 | Enteritis | Strain received from C.A. Mebus and used by NVSL/APHIS as standard reference strain |
| Quebec | AF220295 | Enteritis | Adv Exp Med Biol 2001;494:73–76 |
| CalfGuard-Pfizer | JQ741970 | MLV vaccine | |
| Mebus | U00735 | Calf diarrhea | Am J Vet Res 1972;33:1147–1156 |
Table 3.
GenBank Accession Numbers for generated sequences.
| Sample number | GenBank submission name | Accession # | Isolates with identical sequences |
|---|---|---|---|
| OK AN 3 NS | OSU-AN3-00 | JQ741947 | |
| OK TN 10 NS | OSU-TN10-99 | JQ741948 | |
| OK AN 5 NS | OSU-AN5-00 | JQ741949 | |
| OK 45 NS | OSU-43NS-08 | JQ741950 | OK 43 NS |
| OK 717 NS | OSU-717NS-11 | JQ741951 | |
| OK 665 NS | OSU-665NS-09 | JQ741952 | |
| OK 766 NS | OSU-766NS-11 | JQ741953 | |
| OK 3171 NS | OSU-3171NS-09 | JQ741954 | |
| OK 1776 NS | OSU-1776NS-09 | JQ741955 | |
| OK 778 NS | OSU-778NS-09 | JQ741956 | OK 778 BAL |
| OK 787 NS | OSU-787NS-09 | JQ741957 | OK 802 NS (53), OK 797 NS, OK 802 NS (42) |
| OK 3175 NS | OSU-3175NS-09 | JQ741958 | |
| OK 746 BAL | OSU-746BAL-09 | JQ741959 | OK 746 NS, OK 833 NS, OK 747 NS |
| OK 3167 NS | OSU-3167NS-09 | JQ741960 | |
| OK 747 BAL | OSU-747BAL-09 | JQ741961 | |
| OK 3174 NS | OSU-3174NS-09 | JQ741962 | OK 3172 NS, OK 3162 NS, OK 3169 NS, OK 3165 NS, OK3163 NS, OK 3181 NS, OK 3168 NS, OK 3170 NS, OK 801 NS, OK 747 BAL |
| OK 776 NS | OSU-776NS-09 | JQ741963 | |
| OK 834 NS | OSU-834NS-09 | JQ741964 | |
| OK 554 BAL | OSU-554BAL-09 | JQ741965 | |
| OK 538 BAL | OSU-538BAL-09 | JQ741966 | |
| OK 542 BAL | OSU-542BAL-09 | JQ741967 | |
| OK 576 BAL | OSU-576BAL-09 | JQ741968 | OK 592 BAL, OK 603 BAL, OK 600 BAL, OK 545 BAL, OK 575 BAL (15), OK 521 NS, OK 609 BAL, OK 513 BAL, OK 591 BAL, OK 552 BAL, OK 551 BAL (17), OK 1817 NS, OK 603 NS, OK 575 NS, OK 563 BAL |
| OK 3166 NS | OSU-3166NS-09 | JX536392 | |
| Reference strain | Nebraska (NVSL)] | JQ741969 | |
| Vaccine strain | CalfGuard A609860 | JQ741970 |
2.6. Determination of serological relatedness
Endpoint dilutions reflected the highest dilution of serum that inhibited the growth of virus. The serological relatedness was expressed by calculating the ratio (P, stated as percentage) of the heterologous VN value as compared to the homologous VN value using the following formula.
where BA is the VN titer against strain B using antiserum A and AA is the VN titer against strain A using antiserum A. Statistical significance was evaluated using analysis of variance (ANOVA) and least significant difference (LSD) method. The level of significance was P ≤ 0.05
3. Results
3.1. Phylogenetic analysis
In conjunction with the 56 field strains, Spike protein coding sequences were amplified and sequenced from one reference strain received from the National Veterinary Services Laboratory (Nebraska) and one vaccine strain amplified from a vial of modified live vaccine (CalfGuard-Pfizer BCV). Alignment of generated sequences revealed 100% sequence identity between among some strains (Table 1). Multiple isolates from the same animal, in all cases, had 100% sequence identity. In addition six clusters of viruses with 100% sequence identity were noted (clusters identified in Table 1). The dendogram shown in Fig. 1 was constructed using sequences of one representative from each of the six clusters (shown in bold lettering) and sequences from the remaining 21 BoCV which did not have 100% sequence identity with any of other BoCV characterized in this study. Phylogenetic analysis, using the 27 sequences generated in this study from field strains of BoCV and sequences from reference strains and a vaccine strain, resulted in a dendogram with two major clades. Clade 1 was composed of reference strains associated with enteric disease and a vaccine strain from a multivalent vaccine for the prevention of scours in calves. Clade 2 was composed of the BoCV field strain panel assembled for this study and reference strains associated with respiratory disease or winter dysentery. Three subgroups (A, B, C in Fig. 1) were evident in clade 2. Subgroup A was made up of reference strains from previous studies and three isolates from the BoCV field strain panel. The other two subgroups were made up entirely of isolates from the BoCV field strain panel.
Fig. 1.
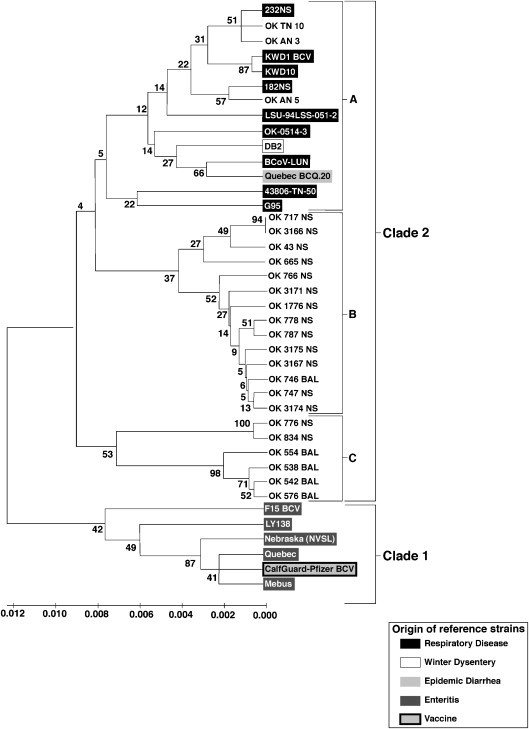
Dendogram of representative BoCV of the six clusters and the reference strains.
In the study described, there were instances where there were BoCV that were identical (Table 1, Table 3). There were identical viruses from different studies over time. However there were instances where there were calves with nonidentical BoCV in the same subclade such as in OSU-2. Also in OSU-2 there were mixtures of multiple BoCV subclades including BoCV2b and BoCV2c. Because these calves in the OSU studies 1–5 were cattle purchased at auction markets, these isolates likely reflect exposure to multiple sources of viruses.
3.2. Determination of serological relatedness
Convalescent sera collected from animals exposed to BoCV strains from clade 2, subgroup A, could not be used to differentiate viruses from clades 1 and 2. (Fig. 2 ) The error bars in Fig. 2 represent standard error of the mean. In contrast, convalescent sera collected from animals exposed to BoCV strains from clade 2, subgroups B and C, did have a statistically significant (P < 0.05) greater neutralizing power for strains from clade 2 as opposed to one strain from clade 1. There was no statistically significant difference between neutralization of any of the clade 2 subgroups using any of the sera evaluated.
4. Discussion
BoCV were initially identified as enteric pathogens and vaccines are available for control of BoCV in calves. However, more recently, BoCV has been isolated in conjunction with BRD in feeder and stockyard cattle. This raises several questions. Is there is a difference between BoCV associated with enteric disease and BoCV associated with respiratory disease? Are BoCV evolving over time so that recent isolates are detectably different from reference strains isolated 50–60 years ago? Are differences between recently isolated strains and vaccine strains of practical significance?
Previously, the S protein coding sequences were compared from nine BoCV isolates that were isolated from dysentery cases in Korean cattle between 2002 and 2003 were compared to previously characterized BoCV strains [35]. These nine isolates were more closely related to the more recently isolated respiratory BCoV strain OK and the enteric BCoV strain LY-138 than to the prototype BoCV reference strain Mebus. They hypothesized that BoCV were evolving over time and may be diverging from an enteric tropism to a dual respiratory and enteric tropism. Similarly, Kanno et al. compared the S protein coding sequences from 55 BoCV collected in Japan from 1999 to 2000 to reference strains [33]. They concluded that these 55 isolates had distinctive genetic divergence from the prototype enteric BoCV strains (Mebus, Quebec, Kakegawa, F15 and LY138). The study reported herein expands on these observations. In addition to comparing S protein coding sequences of recent U.S. isolates to reference strains, we have also compared these strains to a vaccine strain and have conducted an antigenic comparison using convalescent sera from cattle exposed to BoCV. Our results suggest that BoCV strains currently circulating in the U.S., similar to recent Japanese and Korean strains, are divergent from prototype BoCV strains isolated, in some cases 50 to 60 years ago. Further, there are antigenic differences between these more recent strains and a prototype strain Mebus. These results are consistent with a divergence of BoCV over time. Whether this divergence is associated with an increased tropism for respiratory tissue resulting in an increased association with respiratory disease is a matter for future study. Differences in cell tropism, that result in different clinical presentations, have been observed with other coronaviruess. Transmissible gastroenteritis virus (TGEV) and porcine respiratory coronavirus (PRCV) are both pathogens of swine that co-circulate in swine herds [36], [37]. TGEV replicates primarily in the enteric tract and PRCV replicates almost exclusively in the respiratory tract. In the case of porcine coronaviruses, changes in cell tropism are associated with variations of the S gene. It appears that PRCV may be a deletion mutant of TGEV [38]. In comparison to TGEV, PRCV has a large deletion in the 5′ region of the S gene and minor deletions in genes 3/2a and 3–1/3b [39]. It is theorized, based on studies using TGEV mutants, that mutations located in the S gene affect tissue tropism and virulence [40], [41].
Differences, observed in this study, between the strain used in a vaccine for the control of BoCV associated enteric disease, and more recently isolated BoCV suggest that the vaccine may not be fully protective against BoCV isolates currently in circulation. Further research is needed to determine if BoCV are diverging from an enteric tropism to a dual respiratory and enteric tropism and if control of BoCV will reduce BRD losses.
In this study diversity of the BoCV subclades in the cattle was observed in calves that were sampled at arrival. These cattle were most likely purchased from multiple herds at the auction markets. This diversity points out the need to determine if potential immunizations provide broad range of protection to these heterologous BoCV. Another point is that these cattle in the OSU1-5 studies were found to be shedding viruses at arrival or short after arrival [19]. Thus immunizations for control of BoCV should be performed prior to the cattle being exposed to the virus in the marketing and shipping channels. BoCV control programs using vaccination would appear best done at the originating breeding herd.
If BoCV has become a respiratory pathogen, differences between the strain used in vaccines for enteric disease may not provide the protection expected of immunization. Studies should be performed to determine if the current vaccine (BoCV clade 1) provides adequate protection against the BoCV subclades 2a,2b,or 2c. Or potentially the BoCV clade 2 strains might be developed as immunogens including subclades 2a,2b and 2c as well. Ideally vaccine efficacy would utilize animal challenge with clinical signs and lesions due to virulent challenge demonstrating protection. Another approach with approval might utilize reduction of viral shedding as a measure of vaccine efficacy. Also surveillance programs should be maintained to determine if additional BoCV clades are present in the cattle populations.
5. Conclusions
This study was the initial study in the U.S. to examine genetic differences among BoCV isolated from the respiratory tract in cattle from various Oklahoma studies. There were two clades identified (BoCV 1 and 2), and there were three subclades for the BoCV2 clade (a,b, and c). There were antigenic differences detected based on virus neutralization tests using multiple strains from each subclade. Our results indicate genetic and antigenic differences, and thus these results have potential application for immunogens. The MLV vaccine available in the U.S. is a member of the BoCV clade 1, and our respiratory isolates were in BoCV clade 2. The current MLV vaccine should be tested for protection against the BoCV clade 2 viruses, or the BoCV clade 2 viruses might be incorporated into the existing BoCV clade 1 vaccine.
Conflict of interest statement
Dr. Robert Fulton has had prior research support from Pfizer Animal Health, and has been a consultant on bovine respiratory disease. Pfizer Animal Health has not had access to this study's results and has not provided financial support for these antigenic and genetic studies. They have not had influence over this study.
Acknowledgement
This research was supported by the Center for Veterinary Health Sciences and The Noble Foundation, Ardmore, OK USA.
References
- 1.Fulton R.W. Bovine respiratory disease research (1983–2009) Anim Health Res Rev. 2009;10:130–139. doi: 10.1017/S146625230999017X. [DOI] [PubMed] [Google Scholar]
- 2.Fulton RW. Viral diseases of bovine respiratory tract: bovine herpesvirus-1, parainfluenza -3 virus, bovine respiratory syncytial virus, bovine adenoviruses, bovine coronavirus, and bovine viral diarrhea viruses. In: Anderson DE and Rings DM, editors. Current veterinary therapy-food animal practice. Saunders Elsevier, St. Louis, MO: 2008, p. 171–191.
- 3.Saif L.J. Coronaviruses of domestic livestock and poultry: interspecies transmission, pathogenesis and immunity. In: Perlman S., Gallagher T., Snijder E., editors. vol. 18. ASM; Washington, DC: 2007. pp. 279–296. (The nidoviruses). [Google Scholar]
- 4.Saif L.J., Heckert R.A. Enteric coronaviruses. In: Saif L.J., Theil K.W., editors. Viral diarrheas of man and animals. CRC Press; Boca Raton, FL: 1990. pp. 185–252. [Google Scholar]
- 5.Saif L.J. A review of evidence implicating bovine coronavirus in the etiology of winter dysentery in cows: an enigma resolved. Cornell Vet. 1990;80:303–311. [PubMed] [Google Scholar]
- 6.Boileau M.J., Kapil S. Bovine coronavirus associated syndromes. Vet Clin Food Anim. 2010;26:123–146. doi: 10.1016/j.cvfa.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saif L.J. Bovine respiratory coronavirus. Vet Clin Food Anim. 2010;26:349–364. doi: 10.1016/j.cvfa.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spann W.J.M., Brian D., Cavanagh D., De Groot R.J., Eujuanes L., Gorbalenya A.E. Chapter, “family Coronaviridae”. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselbeerger U., Ball L.A., editors. Eighth report of the international committee on taxonomy of viruses. Elsevier/Academic Press; San Diego, CA: 2005. pp. 947–964. [Google Scholar]
- 9.Cho K.O., Hoet A.E., Loerch S.C., Loech S.C., Wittum T.E., Saif L.J. Evaluation of concurrent shedding of bovine coronavirus via the respiratory tract and enteric route in feedlot cattle. Am J Vet Res. 2001;62:1436–1441. doi: 10.2460/ajvr.2001.62.1436. [DOI] [PubMed] [Google Scholar]
- 10.Decaro N., Campolo M., Desario C., Cirone F., D’Abramo M., Lorusso E. Respiratory disease associated with bovine coronavirus infection in cattle herds in Southern Italy. J Vet Diagn Invest. 2008;20:28–32. doi: 10.1177/104063870802000105. [DOI] [PubMed] [Google Scholar]
- 11.Hasokusz M., Lathrop S.L., Gadfield K.L., Saif L.J. Isolation of bovine respiratory coronaviruses from feedlot cattle and comparison of their biological and antigenic properties with bovine enteric coronavirus. Am J Vet Res. 1999;60:1227–1233. [PubMed] [Google Scholar]
- 12.Lathrop S.L., Wittum T.E., Loerch S.C., Perino L.J., Saif L.J. Antibody titers against bovine coronavirus and shedding of the virus via the respiratory tract in feedlot cattle. Am J Vet Res. 2000;61:1057–1061. doi: 10.2460/ajvr.2000.61.1057. [DOI] [PubMed] [Google Scholar]
- 13.Lathrop S.L., Wittum T.E., Brock K.V., Loerch S.C., Perino L.J., Bingham H.R. Association between infection of the respiratory tract attributable to bovine coronavirus and health and growth performance of cattle in feedlots. Am J Vet Res. 2000;61:1062–1066. doi: 10.2460/ajvr.2000.61.1062. [DOI] [PubMed] [Google Scholar]
- 14.Lin X., O’Reilly K.L., Burrell M.L., Storz J. Infectivity-neutralizing and hemagglutinin-inhibiting antibody responses to respiratory coronavirus infections of cattle in pathogenesis of shipping fever pneumonia. Clin Diagn Lab Immunol. 2001;8:357–362. doi: 10.1128/CDLI.8.2.357-362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin S.W., Nagy E., Shewen P.E., Harland R.J. The association of titers to bovine coronavirus with treatment for bovine respiratory disease and weight gain in feedlot calves. Can J Vet Res. 1998;62:257–261. [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor A., Martin S.W., Nagy E. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in 3 Ontario feedlots. Can J Vet Res. 2001;65:137–142. [PMC free article] [PubMed] [Google Scholar]
- 17.Storz J., Purdy C.W., Lin X., Burrell M., Truax R.E., Briggs R.E. Isolation of respiratory bovine coronavirus, other cytocidal viruses, and Pasteurella spp from cattle involved in two natural outbreaks of shipping fever. J Am Vet Med Assoc. 2000;216:1599–1604. doi: 10.2460/javma.2000.216.1599. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C.J., Hoet A.R., Sreevatsan S., Wittum T.E., Briggs R.E., Duff G.C. Transmission of bovine coronavirus and serologic responses in feedlot calves under field conditions. Am J Vet Res. 2006;67:1412–14420. doi: 10.2460/ajvr.67.8.1412. [DOI] [PubMed] [Google Scholar]
- 19.Fulton R.W., Step D.L., Wahrmund J., Burge L.J., Payton M.E., Cook B.J. Bovine coronavirus (BCV) infections in transported and commingled beef cattle and sole-source calves. Can J Vet Res. 2011;75:191–199. [PMC free article] [PubMed] [Google Scholar]
- 20.Gagea M., Bateman K.G., vanDreumel T., McEwen B.J., Carman S., Archambault M. Diseases and pathogens associated with mortality in Ontario beef feedlots. J Vet Diagn Invest. 2006;18:18–28. doi: 10.1177/104063870601800104. [DOI] [PubMed] [Google Scholar]
- 21.Storz J., Lin X., Purdy C.W., Chouljenko V.N., Kousoulas K.G., Enright F.M. Coronavirus and Pasteurella infections in bovine shipping fever and Evans criteria for causation. J Clin Microbiol. 2000;38:3291–3298. doi: 10.1128/jcm.38.9.3291-3298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulton R.W., Blood K.S., Panciera R.G., Payton M.E., Ridpath J.F., Confer A.W. Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. J Vet Diagn Invest. 2009;21:464–467. doi: 10.1177/104063870902100407. [DOI] [PubMed] [Google Scholar]
- 23.Park S.J., Kim G.Y., Choy H.E., Hong Y.J., Saif L.J., Jeong J.H. Dual enteric and respiratory tropisms of winter dysentery bovine coronavirus in calves. Arch Virol. 2007;152:1885–1900. doi: 10.1007/s00705-007-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds D.J., Debney T.G., Hall G.A., Thomas L.H., Parsons K.B. Studies on the relationship between coronaviruses from the intestinal and respiratory tracts of calves. Arch Virol. 1985;85:71–83. doi: 10.1007/BF01317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.12th ed. North American Compendiums; Port Huron, MI: 2010. Compendium of veterinary products. p. 1–1848. [Google Scholar]
- 26.Plummer P.J., Rohrbach B.W., Daugherty R.A., Daugherty R.A., Thomas K.V., Wilkes R.P. Effect of intranasal vaccination against bovine enteric coronavirus on the occurrence of respiratory tract disease in a commercial backgrounding feedot. J Am Vet Med Assoc. 2004;225:726–731. doi: 10.2460/javma.2004.225.726. [DOI] [PubMed] [Google Scholar]
- 27.Aita T., Kuwabara M., Murayama K., Sasagawa Y., Yabe S., Higuchi R. Characterization of epidemic diarrhea outbreaks associated with bovine torovirus in adult cows. Arch Virol. 2012;157:423–431. doi: 10.1007/s00705-011-1183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decaro N., Cirone F., Mari V., Nava D., Tinelli A., Elia G. Characterisation of bubaline coronavirus strains associated with gastroenteritis in water buffalo (Bubalus bubalis) calves. Vet Microbiol. 2010;145:245–251. doi: 10.1016/j.vetmic.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorusso A., Desario C., Mari V., Campolo M., Lorusso E., Elia G. Molecular characterization of a canine respiratory coronavirus strain detected in Italy. Virus Res. 2009;141:96–100. doi: 10.1016/j.virusres.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.J., Lim G.K., Park S.I., Kim H.H., Koh H.B., Cho K.O. Detection and molecular characterization of calf diarrhoea bovine coronaviruses circulating in South Korea during 2004–2005. Zoonoses Public Health. 2007;54:223–230. doi: 10.1111/j.1863-2378.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Guy J.S., Snijder E.J., Denniston D.A., Timoney P.J., Balasuriya U.B. Genomic characterization of equine coronavirus. Virology. 2007;369:92–104. doi: 10.1016/j.virol.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin L., Cebra C.K., Baker R.J., Mattson D.E., Cohen S.A., Alvarado D.E. Analysis of the genome sequence of an alpaca coronavirus. Virology. 2007;365:198–203. doi: 10.1016/j.virol.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanno T., Hatama S., Ishihara R., Uchida I. Molecular analysis of the S glycoprotein gene of bovine coronaviruses isolated in Japan from 1999 to 2006. J Gen Virol. 2007;88:1218–1224. doi: 10.1099/vir.0.82635-0. [DOI] [PubMed] [Google Scholar]
- 34.Erles K., Shiu K.B., Brownlie J. Isolation and sequence analysis of canine respiratory coronavirus. Virus Res. 2007;124:78–87. doi: 10.1016/j.virusres.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park S.J., Jeong C., Yoon S.S., Choy H.E., Saif L.J., Park S.H. Detection and characterization of bovine coronaviruses in fecal specimens of adult cattle with diarrhea during the warmer seasons. J Clin Microbiol. 2006;44:3178–3188. doi: 10.1128/JCM.02667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim L., Hayes J., Lewis P., Parwani A.V., Chang K.O., Saif L.J. Molecular characterization and pathogenesis of transmissible gastroenteritis coronavirus (TGEV) and porcine respiratory coronavirus (PRCV) field isolates co-circulating in a swine herd. Arch Virol. 2000;145:1133–1147. doi: 10.1007/s007050070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saif LJ, Wesley RD. Transmissable gastroenteritis and porcine respiratory coronavirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine, 8th ed., Iowa State University Press, Ames, IA, 1999, p. 295–325.
- 38.Laude H., Van Reeth K., Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet Res. 1993;24:125–150. [PubMed] [Google Scholar]
- 39.Vaughn E.M., Halbur P.G., Paul P.S. Sequence comparison of porcine respiratory coronavirus isolates reveals heterogeneity in the S, 3, and 3-1 genes. J Virol. 1995;69:3176–3184. doi: 10.1128/jvi.69.5.3176-3184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballesteros M.L., Sanchez C.M., Enjuanes L. Two amino-acid changes at the N-terminus of transmissible gastroenteritis coronavirus spike protein result in loss of enteric tropism. Virology. 1997;227:378–388. doi: 10.1006/viro.1996.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard S., Laude H. Site-specific alteration of transmissible gastroenteritis virus spike protein results in markedly reduced pathogenicity. J Gen Virol. 1995;76:2235–2241. doi: 10.1099/0022-1317-76-9-2235. [DOI] [PubMed] [Google Scholar]


