Highlights
-
•
We assessed the immunogenicity of A(H1N1)pdm09 vaccine (1 dose) with/without adjuvant.
-
•
Four thousands and forty-eight adults received one dose of either the adjuvanted or non-adjuvanted vaccine.
-
•
Both vaccines induced protective HI antibody levels at Day 21.
-
•
At Month 6 immunogenicity guidance criteria were met in subjects 18–64 years of age.
Abbreviations: ATP, according to protocol; BARDA, Biomedical Advanced Research and Development Authority; BMI, body mass index; CBER, Center for Biologics Evaluation & Research; CHMP, Committee for Medicinal Products for Human Use; CI, confidence interval; GMFR, geometric mean fold rise; GMT, geometric mean titer; HA, hemagglutinin; HI, hemagglutination inhibition; HHS, United States Department of Health and Human Services; ILIs, Influenza-Like-Illnesses; pIMDs, potential immune mediated diseases; RVP, respiratory viral panel; SAEs, serious adverse events; SCR, seroconversion rate; SD, standard deviation; SPR, seroprotection rate; TVC, total vaccinated cohort; VEI, vaccine effectiveness improvement; WHO, World Health Organization
Keywords: H1N1, Pandemic, Influenza, Adjuvant, Vaccine
Abstract
Background
This study (NCT00979602) evaluated the immunogenicity and relative protective efficacy of one dose of influenza A(H1N1)pdm09 vaccine with or without AS03 (an α-tocopherol oil-in-water emulsion based Adjuvant System).
Methods
Four thousands and forty-eight healthy adults aged ≥18 years were randomized (1:1) to receive one dose of either the adjuvanted split virion (3.75 μg hemagglutinin antigen [HA]/AS03) or non-adjuvanted (15 μg HA) vaccine. Hemagglutination inhibition [HI] antibody response was evaluated before vaccination and at Days 21, 42 and 182 (Month 6). Safety of the study vaccines was evaluated during the entire study duration.
Results
At Day 21, both study vaccines induced HI immune responses meeting the US regulatory criteria in subjects 18–64 years (seroprotection rate [SPR]: 98.0% [97.1–98.6]; seroconversion rate [SCR]: 89.7% [88.0–91.2] in the AS03-adjuvanted group; SPR: 91.4% [89.9–92.8]; SCR: 74.6% [72.3–76.9] in the non-adjuvanted group) and >64 years of age (SPR: 86.0% [82.5–89.0]; SCR: 75.3% [71.1–79.2] in the AS03-adjuvanted group; SPR: 69.1% [64.6–73.3]; SCR: 56.7% [52.0–61.3] in the non-adjuvanted group). The AS03-adjuvanted vaccine induced higher HI geometric mean titers than the non-adjuvanted vaccine at all time points. At Month 6, only subjects 18–64 years of age from both vaccine groups still met the US regulatory criteria (SPR: 82.1% [80.0–84.1]; SCR: 62.3% [59.6–64.8] in the AS03-adjuvanted group; SPR: 75.3% [72.9–77.5]; SCR: 53.7% [51.0–56.4] in the non-adjuvanted group). Protective efficacy was not evaluated due to low number of RT-qPCR-confirmed A(H1N1)pdm09 influenza cases. Through Month 12, 216 serious adverse events (in 157 subjects: 84 in the AS03-adjuvanted and 73 in the non-adjuvanted group) and 12 potentially immune mediated diseases (5 in the AS03-adjuvanted and 7 in the non-adjuvanted group) were reported.
Conclusion
A single dose of either adjuvanted or non-adjuvanted influenza A(H1N1)pdm09 vaccine induced protective HI antibody levels against the A/California/7/2009 strain that persisted through Month 6 in the 18–64 years population.
1. Introduction
Mass immunization is considered to be an effective prophylactic method of mitigating influenza pandemic-associated morbidity and mortality [1], [2], [3], [4]. Due to the novel antigenic characteristics of the swine-origin influenza A(H1N1) 2009 pandemic virus [influenza (A(H1N1)pdm09] [5], [6], the seasonal influenza vaccines available at the time of the 2009–2010 H1N1 pandemic were unlikely to confer protection against the novel virus [5], [7], [8].
The World Health Organization (WHO) encouraged the development and use of adjuvanted influenza A(H1N1)pdm09 vaccines [9], [10], with the aim of dose-reduction, antigen-sparing and to potentially provide broader vaccine efficacy against drifted strains through cross-reactive immunity [11]. Based on the experience of developing a pre-pandemic A/H5N1 influenza vaccine utilizing AS03 (an α-tocopherol oil-in-water emulsion based Adjuvant System) [12], [13] that was well-tolerated and highly immunogenic in adults [14], [15], [16], an AS03-adjuvanted influenza A(H1N1)pdm09 vaccine with 3.75 μg hemagglutinin (HA) content was developed [17], [18], [19].
This large-scale, randomized study in subjects ≥18 years of age assessed whether one dose of AS03-adjuvanted 3.75 μg HA influenza A(H1N1)pdm09 vaccine elicited immune response that met the US and European regulatory criteria. Additionally, non-inferiority and superiority of this vaccine protective efficacy versus a non-adjuvanted 15 μg HA influenza A(H1N1)pdm09 vaccine were evaluated.
2. Materials and methods
2.1. Study design and participants
In this phase III, observer-blind, randomized study (NCT00979602), adults ≥18 years of age were enrolled across 25 centers in the US and 13 in Canada between November 2009 and December 11, 2009. They were randomized (allocation ratio 1:1) to receive one dose of either a monovalent AS03-adjuvanted 3.75 μg HA A/California/7/2009 pandemic influenza vaccine or a non-adjuvanted 15 μg HA A/California/7/2009 pandemic influenza vaccine. The enrolment stratification was by age (1:1:1:1; 18–30 years, 31–40 years, 41–64 years, ≥65 years). The subjects and study personnel involved in evaluating end points were blinded to the intervention administered. Double blinding was not possible because the vaccine preparation required mixing of AS03 and A(H1N1)pdm09 antigen from two vials. Randomization was performed using a central, internet-based system that balanced groups with respect to center, age strata and previous seasonal influenza vaccination.
Adults were excluded from enrolment: if they had a history of physician-confirmed A(H1N1)pdm09 influenza infection or vaccination, those who received any vaccination other than a seasonal influenza vaccine within 30 days preceding study start, those with confirmed or suspected immunosuppressive or immunodeficient conditions, diagnosed with or undergoing treatment for cancer, and/or with a history of allergic/anaphylactic reactions following previous influenza vaccination. In addition, laboratory screening was performed to exclude those with results outside of protocol-specified normal ranges. The following safety laboratory parameters were tested to evaluate the participants’ eligibility: hepatic aminotransferases, total and direct bilirubin, alkaline phosphatase, creatinine, serum urea nitrogen, hemoglobin, hematocrit, white blood cell count and platelet count.
Active surveillance of influenza-like infections (ILIs: defined as fever ≥38.0 ˚C/100.4 ˚F or new or worsening myalgia accompanied by new or worsening cough or sore throat) was done during study visits and through bi-weekly telephonic contact through Day 385 (12 months after the initially planned administration of the second study vaccine dose). Additionally, the subjects were instructed to contact the study sites if they develop any ILI symptoms. Once the study site had been notified of a possible ILI episode, a visit for nasal and throat swab sample collection was scheduled within 5 days of symptom onset and before initiating any antimicrobial/influenza antiviral therapy. If an ILI episode was reported more than 5 days after onset, no swab specimen was collected.
Written informed consent was obtained from all subjects prior to conducting any study-related procedures. The study was conducted in accordance with the Good Clinical Practice guidelines, the Declaration of Helsinki and local regulations. All study-related documents were approved by institutional review boards.
2.2. Study vaccines
The influenza A(H1N1)pdm09 vaccine was a monovalent, inactivated, split-virion antigen suspension (A/California/07/2009 strain) adjuvanted with AS03 (Arepanrix™, a trademark of GlaxoSmithKline Vaccines) or administered as plain antigen. The H1N1 viral seed for the vaccine was prepared as per WHO recommendations [20]. AS03 is an oil-in-water emulsion based Adjuvant System containing squalene (10.69 mg per dose), DL-α-tocopherol (11.86 mg) and polysorbate 80 (4.86 mg). The AS03-adjuvanted influenza A(H1N1)pdm09 vaccine doses were prepared by mixing the A(H1N1)pdm09 antigen and AS03 (1:1) from separate multi-dose vials. 0.5 ml of the assigned study vaccine was administered into the deltoid muscle within 30 min after mixing the antigen and the adjuvant.
2.3. Study objectives and end points
The first co-primary objective of the study was to evaluate HI antibody responses 21 days after vaccination in the AS03-adjuvanted vaccine group based on the Center for Biologics Evaluation and Research (CBER) and Committee for Medicinal Products for Human Use (CHMP) criteria for pandemic influenza vaccines in adults [21], [22].
At least 360 RT-qPCR-confirmed A/California influenza cases were required to evaluate the second co-primary objective on non-inferior protective efficacy followed by superiority. As only three RT-qPCR-confirmed A/California influenza cases were diagnosed during the study, descriptive analyses of the influenza attack rate and vaccine efficacy improvement (VEI) were computed only for ILI and pneumonia cases.
The study also assessed whether the non-adjuvanted 15 μg HA influenza A(H1N1)pdm09 vaccine elicited immune responses that met the US and European regulatory criteria, 21 days after vaccination and whether these criteria were met for either study vaccines at Day 42 (in a small subset of subjects) and at Day 182 (Month 6).
2.4. Laboratory assays
Hemagglutination inhibition (HI) antibody levels in serum samples were assessed at GlaxoSmithKline Vaccines central laboratory using a validated in-house assay [cut-off: ≥1:10] that used chicken erythrocytes, as previously described [23]. The A/California/7/2009 strain was used as the antigen strain.
RT-qPCR was performed on viral RNA from the clinical samples as described previously [24]. Viral load values were quantified and the sample was considered positive when the measured viral load was equal to or above the assay cut-off [24].
2.5. Immunological assessment
Serum samples were collected before vaccination (Day 0), at Days 21, 42 (in a subset of subjects) and 182 (Month 6) for assessment of humoral immune response and for clinical chemistry and hematology assessments at Days 0, 7 and 21.
The immunological assessment was based on HI antibody seroconversion rates (SCR), seroprotection rate (SPR) and geometric mean fold rise (GMFR), against the vaccine homologous strain.
Post hoc exploratory analyses included the assessment of possible correlation of HI antibody response with body mass index (BMI) and with previous influenza vaccination history. Further assessments were performed to identify the respiratory viruses isolated from swab samples from ILI cases using xTAG Respiratory Viral Panel (RVP) Fast assay (Luminex Molecular Diagnostics Inc., Toronto, Canada) [25], [26].
2.6. Safety and reactogenicity assessment
Subjects used diary cards to record the solicited local and general symptoms occurring within 7 days following vaccination and the unsolicited adverse events occurring within 42 days following vaccination. Potential immune-mediated diseases (pIMDs: subset of AEs that include both autoimmune diseases and other inflammatory and/or neurologic disorders which may/may not have an autoimmune etiology) and serious adverse events (SAEs) were recorded throughout the study period. The intensity of all solicited adverse events except fever was graded on a scale of (0–3), Grade 1 being those that did not interfere with normal activities and Grade 3 being those that prevented normal activities (Grade 3 redness and swelling: diameter >100 mm; Grade 3 fever: temperatures ≥39.0–≤40.0 °C. Fever was graded on a scale of (0–4), Grade 4 being temperatures >40.0 °C. Based on clinical judgment, the investigators assessed whether the AEs/SAEs were potentially related/not related to the study vaccine.
Serum samples for the analysis of clinical safety laboratory parameters were collected at Days 7 and 21. The following laboratory parameters were tested: hepatic aminotransferases, total and direct bilirubin, alkaline phosphatase, creatinine, serum urea nitrogen, hemoglobin, hematocrit, white blood cell count and platelet count.
2.7. Statistical analyses
The sample size was calculated taking into consideration the co-primary objectives. Overall, 1900 evaluable subjects (1800 for VEI evaluation) in each of the two treatment groups (accounting for 5% and 10% drop-out rates for the co-primary objectives) was estimated to provide a power of 91.85% to meet the co-primary objectives, assuming 90%/74% as reference for SPR/SCR in subjects 18–64 years and >64 years of age, respectively, 40% vaccine efficacy for the non-adjuvanted influenza A(H1N1)pdm09 vaccine, and an attack rate of 20% in subjects who do not receive any H1N1 vaccine (PASS 2005; one-sided test, one-sided alpha = 2.5%).
The SCR, SPR and GMFR and incidence rates of solicited and unsolicited adverse events were calculated with 95% confidence interval (CI). The analyses of immunogenicity were performed on the according to protocol (ATP) cohort which included evaluable subjects meeting eligibility criteria and adhering to protocol-defined procedures. A Cox regression model, including the vaccine group as a fixed effect, age and baseline antibody titer as covariates was used to estimate the VEI for the any ILI cases and any pneumonia cases (the first event was considered if multiple events were reported by a subject). All statistical analyses were performed using Statistical Analysis Software (SAS) version 9.1.
3. Results
3.1. Study population
A total of 5660 subjects were screened, 4048 received vaccine, and 3770 completed the study through Day 385. The reasons for withdrawals and elimination of subjects from the analyses at different time points are presented in Fig. 1 .
Fig. 1.
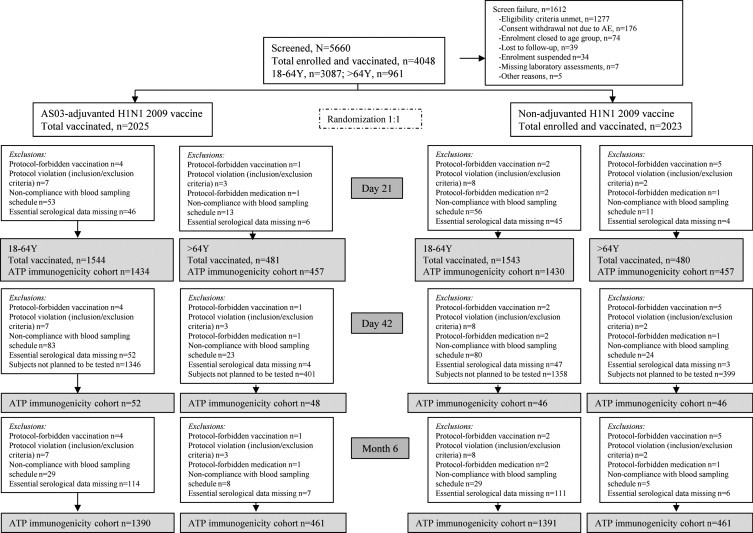
Participant flow diagram.
The mean age of subjects in the TVC at the time of vaccination in the 18–64 years age group was 37.4 years (range: 18–64 years); >64 years age group was 71.2 years (range: 65–92 years). Overall, 59.2% and 56.3% of subjects in the respective two age groups were female and the majority of subjects were Caucasians (86.9% and 93.0%, respectively).
3.2. Immune response
Co-primary objectives: The first co-primary objective was met. A single dose of the AS03-adjuvanted 3.75 μg HA influenza A(H1N1)pdm09 vaccine elicited HI immune responses in the 18–64 years and >64 years age groups that met the CBER regulatory criteria at Day 21 (Table 1 ). The CHMP criteria were met in the 18–60 years and >60 years age groups (data not presented).
Table 1.
Hemagglutination inhibition antibody response to the A/California/7/2009 (H1N1) strain in the 18–64 years and >64 years age groups at all time points (according to protocol cohort for immunogenicity).
| Immune response | Time point | AS03A/3.75 μg HAa |
Non-adjuvanted 15 μg HA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 18–64 years | >64 years | 18–64 years | >64 years | ||||||
| Nc | Percentage or value (95% CIb) | N | Percentage or value (95% CI) | N | Percentage or value (95% CI) | N | Percentage or value (95% CI) | ||
| Seroconversion rate | Day 21 | 1426 | 89.7% (88.0–91.2) | 454 | 75.3% (71.1–79.2) | 1414 | 74.6% (72.3–76.9) | 455 | 56.7% (52.0–61.3) |
| Day 42 | 52 | 98.1% (89.7–100) | 48 | 70.8% (55.9–83.0) | 46 | 78.3% (63.6–89.1) | 46 | 41.3% (27.0–56.8) | |
| Day 182 (Month 6) | 1383 | 62.3% (59.6–64.8) | 458 | 34.9% (30.6–39.5) | 1380 | 53.7% (51.0–56.4) | 458 | 26.6% (22.6–30.9) | |
| Seroprotection rate | Pre-vaccination | 1428 | 24.5% (22.3–26.8) | 454 | 13.4% (10.4–16.9) | 1420 | 24.4% (22.2–26.7) | 456 | 17.1% (13.8–20.9) |
| Day 21 | 1432 | 98.0% (97.1–98.6) | 457 | 86.0% (82.5–89.0) | 1424 | 91.4% (89.9–92.8) | 456 | 69.1% (64.6–73.3) | |
| Day 42 | 52 | 98.1% (89.7–100) | 48 | 75.0% (60.4–86.4) | 46 | 93.5% (82.1–98.6) | 46 | 58.7% (43.2–73.0) | |
| Day 182 (Month 6) | 1388 | 82.1% (80.0–84.1) | 461 | 51.4% (46.7–56.1) | 1391 | 75.3% (72.9–77.5) | 459 | 43.1% (38.6–47.8) | |
| Geometric mean titer | Pre-vaccination | 1428 | 14.7 (13.8–15.7) | 454 | 10.9 (10.0–11.9) | 1420 | 14.7 (13.7–15.7) | 456 | 12.0 (11.0–13.2) |
| Day 21 | 1432 | 396.2 (373.8–419.9) | 457 | 128.6 (114.6–144.3) | 1424 | 217.6 (203.3–232.9) | 456 | 75.2 (65.9–85.9) | |
| Day 42 | 52 | 276.5 (207.1–369.0) | 48 | 105.9 (70.2–159.9) | 46 | 170.0 (117.7–245.4) | 46 | 47.2 (32.1–69.3) | |
| Day 182 (Month 6) | 1388 | 109.5 (102.3–117.1) | 461 | 37.1 (33.2–41.5) | 1391 | 83.3 (77.5–89.5) | 459 | 29.2 (26.0–33.0) | |
| Geometric mean fold rise | Day 21 | 1345 | 27.7 (25.7–29.9) | 535 | 12.5 (11.2–13.9) | 1338 | 15.5 (14.2–16.8) | 531 | 6.4 (5.7–7.1) |
| Day 42 | 50 | 26.8 (20.0–35.8) | 50 | 11.1 (7.7–16.0) | 44 | 13.7 (9.1–20.6) | 1304 | 5.9 (5.4–6.4) | |
| Day 182 (Month 6) | 1302 | 7.7 (7.2–8.4) | 539 | 3.5 (3.2–3.9) | 48 | 4.0 (2.9–5.5) | 534 | 2.5 (2.3–2.8) | |
Bolded value = did not meet CBER criteria; SCR: percentage of subjects with pre-vaccination titer <1:10 and post-vaccination titer ≥1:40, or pre-vaccination titer >1:10 and at least four-fold increase in post-vaccination titer, SPR: percentage of subjects with a post-vaccination titer ≥1:40; GMFR: post-vaccination fold increase in geometric mean titers (GMTs) in terms of HI antibodies against the vaccine homologous strain; Center for Biologics Evaluation and Research (CBER) criteria in adults <65 years of age: lower bound of 95% confidence interval [CI] for HI antibody SCR: ≥40% and SPR: ≥70%; CBER criteria in adults ≥65 years of age: lower bound of 95% CI for HI antibody for SCR: ≥30% and SPR: ≥60%; Committee for Medicinal Products for Human Use (CHMP) criteria in adults 18–60 years of age: point estimates for HI antibody SCR: >40%, SPR: >70% GMFR: >2.5 [data not presented]; CHMP criteria in adults >60 years of age: point estimates for HI antibody SCR: >30%, SPR: >60% GMFR: >2 [data not presented].
HA = hemagglutinin.
CI = confidence interval.
N = number of subjects with available results.
The second co-primary objective was not evaluated as only three RT-qPCR-confirmed A/California influenza cases were identified (AS03-adjuvanted: 1; non-adjuvanted: 2).
Secondary objectives: In the Day 42 subset (N = 192) which received the AS03-adjuvanted 3.75 μg HA influenza A(H1N1)pdm09 vaccine, the CBER criteria were met in the 18–64 years age group and >64 years age group (Table 1). At Day 182 (Month 6), the CBER criteria were met only for subjects 18–64 years of age (Table 1). Subjects >64 years of age had a LL of the 95% CI for SPR of 47.7%, thus not fulfilling the CBER criteria at this time point.
At Day 21, a single dose of the non-adjuvanted 15 μg HA influenza A(H1N1)pdm09 vaccine elicited HI immune responses in subjects 18–64 years and >64 years of age that met the CBER regulatory criteria (Table 1). Only those in the 18–64 years age group met the CBER criteria at Day 42 and at Day 182 (Month 6). At this time points subjects >64 years of age had a LLs of the 95% CI for SPR of 43.2 and 38.6%, respectively and LLs of the 95% CI for SCR of 27.0% and 22.6%, respectively, thus not fulfilling the CBER criteria.
The CHMP criteria were met at Day 21 and Day 42 in the 18–60 years and >60 years age groups for both study vaccines. At Day 182, the CHMP criteria were met in the 18–60 years age group but not in the >60 years age group for both study vaccines (data not presented).
HI antibody GMTs in both age groups were higher at all post-vaccination time points for those who received the AS03-adjuvanted influenza A(H1N1)pdm09 vaccine compared to those who received the non-adjuvanted vaccine; GMTs were generally lower in the >64 years compared to the 18–64 years age group at all time points (Table 1). Persistence of HI antibody response at Day 182 (Month 6) was observed for both study vaccines, although at lower levels compared to that observed at Day 21 (Table 1). Overall, the immune response against the vaccine homologous strain appeared to decrease with advancing age (Fig. 2 /Web-appendix Table 1).
Fig. 2.
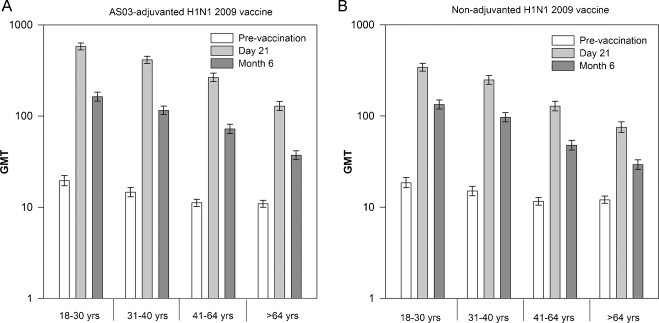
Geometric mean titers for influenza A(H1N1)pdm09 hemagglutination inhibition antibodies by age strata at Days 21 (A) and 182 (Month 6) (B) (according to protocol cohort for immunogenicity). Footnotes: HI, hemagglutination inhibition; GMTs, geometric mean titers; ATP, according to protocol.
Post hoc exploratory analyses showed that HI antibody responses were mostly comparable across healthy weight, overweight and obese subjects. No clear patterns emerged due to the modest number of subjects in the underweight category (Web-appendix Table 2). A higher HI antibody was observed among influenza vaccine-naïve subjects, compared with those with previous seasonal influenza vaccination, in terms of HI antibody GMTs and GMFRs (Web-appendix Table 2).
Relative efficacy outcomes: The attack rates and VEIs for ILI cases and VEI for pneumonia cases Day 0–Day 385 (Month 12) and Day 14–Day 385 (Month 12) are presented in Table 2 . For the efficacy analysis of ILIs, 429 ILI cases (195 cases [10.01%] and 234 cases [11.95%] in the AS03-adjuvanted and non-adjuvanted treatment groups) were reported and 337 were sampled during the study. Of these, 290 samples were tested and only three cases (0.7%) of A(H1N1)pdm09 were confirmed by RT-qPCR (one and two cases respectively). The incidence of ILI cases was comparable between the two treatment groups, except through Day 28 (10 versus 18 [Day 0–Day 14] and 10 versus 23 [Day 14–Day 28] ILI cases in the respective treatment groups). Twenty-nine pneumonia cases were reported during the entire study period: 8 [0.41%] and 21 [1.07%] cases in the AS03-adjuvanted and non-adjuvanted treatment groups, respectively. Of these, 2 cases were diagnosed during the first 14 days post-vaccination in the non-adjuvanted vaccine group, 1 between Days 14 and 28 in the non-adjuvanted vaccine group, and 26 between Days 28 and 365 (8 in the AS03-adjuvanted group and 18 in the non-adjuvanted group). The VEI was 100% from Day 0 to Day 28, 62.52% (95% CI: 15.19–83.44) from Day 0 to Day 385 (Month 12) and 58.69% (95% CI: 5.35–81.97) from Day 14 through Day 385 (Month 12).
Table 2.
Attack rate and vaccine efficacy increase for ILI cases and vaccine efficacy increase for pneumonia cases occurring during the post study starts periods, Day 0–Day 385 (Month 12) and Day 14–Day 385 (Month 12) (according to protocol cohort for efficacy).
| ARa | VEIb | ||||
|---|---|---|---|---|---|
| Day 0–Day 385 (Month 12) | |||||
| Event type | Group | N | n | % (95% CIe) | % (95% CI) |
| ILIc | AS03A/3.75 μg HAd | 1950 | 171 | 8.77 (7.55–10.11) | 11.44 (−8.85–27.95) |
| Non-adjuvanted 15 μg HA | 1954 | 191 | 9.77 (8.49–11.18) | – | |
| Pneumonia | AS03A/3.75 μg HA | 1950 | 8 | 0.41 (0.18–0.81) | 62.52 (15.19–83.44) |
| Non-adjuvanted 15 μg HA | 1954 | 21 | 1.07 (0.67–1.64) | – | |
| Day 14–Day 385 (Month 12) | |||||
| Event type | Group | N | n | % (95% CI) | % (95% CI) |
| ILI | AS03A/3.75 μg HA | 1950 | 164 | 8.41 (7.22–9.73) | 7.41 (−14.54–25.15) |
| Non-adjuvanted 15 μg HA | 1954 | 176 | 9.01 (7.77–10.36) | – | |
| Pneumonia | AS03A/3.75 μg HA | 1950 | 8 | 0.41 (0.18–0.81) | 58.69 (5.35–81.97) |
| Non-adjuvanted 15 μg HA | 1954 | 19 | 0.97 (0.59–1.51) | – | |
N = number of subjects in each group without missing values; n = number of subjects reporting at least one event in each group; attack rate = percentage of subjects reporting at least one ILI case; VEI = relative risk of ILI and pneumonia cases in subjects who received the AS03-adjuvanted 3.75 μg HA influenza A(H1N1)pdm09 vaccine versus subjects who received the non-adjuvanted 15 μg HA influenza A(H1N1)pdm09 vaccine.
AR = attack rate.
VEI = vaccine efficacy increase.
ILI = influenza-like infection.
HA = hemagglutinin.
CI = confidence interval.
Respiratory viruses: Rhinovirus, identified from 74 (25.5%) nasopharyngeal swabs, was the most frequently determined respiratory virus (Table 3 ).
Table 3.
Distribution of other respiratory viruses identified on swab samples collected during ILIs (total vaccinated cohort).
| na | % | ||
|---|---|---|---|
| Number of samples tested | 290 | 100.0 | |
| No virus detected | 162 | 55.9 | |
| One virus detected | Rhinovirus | 74 | 25.5 |
| Human metapneumovirus (hMPV) | 14 | 4.8 | |
| Human Coronavirus HKU1 | 13 | 4.5 | |
| Respiratory syncytial virus (RSV) | 5 | 1.7 | |
| Human Coronavirus 229E | 5 | 1.7 | |
| Human Coronavirus OC43 | 4 | 1.4 | |
| Parainfluenzavirus 3 | 3 | 1.0 | |
| Parainfluenzavirus 4 | 3 | 1.0 | |
| Parainfluenzavirus 1 | 3 | 1.0 | |
| Adenovirus | 2 | 0.7 | |
| Influenza B | 1 | 0.3 | |
| Bocavirus | 1 | 0.3 | |
| Two viruses detected | Parainfluenzavirus 1 + Bocavirus | 1 | 0.3 |
n = number of subjects/samples in a given category.
3.3. Safety and reactogenicity
Solicited adverse events: Pain at the injection site was the most frequently reported solicited local adverse event. It was reported for 82.1% and 29.9% of subjects in the 18–64 years age group who received the AS03-adjuvanted and the non-adjuvanted influenza A(H1N1)pdm09 vaccine, respectively (p < 0.0001) and for 56.7% and 9.4% of subjects in the >64 years group who received the adjuvanted and the non-adjuvanted influenza A(H1N1)pdm09 vaccine, respectively (p < 0.0001) (Fig. 3 /Web-appendix Table 3). Additionally, in both age groups, a statistically significant higher percentage of subjects receiving the adjuvanted vaccine reported redness and swelling compared with non-adjuvanted vaccine group (p < 0.05 for both). Muscle ache (AS03-adjuvanted/non-adjuvanted: 18–64 years: 39.2%/18.4%, p < 0.0001; >64 years: 19.4%/7.3%, p < 0.0001), fatigue (AS03-adjuvanted/non-adjuvanted: 18–64 years: 30.9%/23.6%, p < 0.0001; >64 years: 17.5%/12.3%, p = 0.03) and headache (AS03-adjuvanted/non-adjuvanted: 18–64 years: 29.6%/27.5%, p = 0.21; >64 years: 15.2%/11.5%, p = 0.11) were the most frequently reported solicited general adverse events (Fig. 3/Web-appendix Table 3). In the 18–64 years age group, a higher percentage on subjects receiving the adjuvanted vaccine reported joint pain, shivering and sweating compared with non-adjuvanted group (p < 0.05 for all). In the >64 years group joint pain was reported by a higher percentage of subjects receiving adjuvanted vaccine compared with the subjects receiving the non-adjuvanted vaccine (p = 0.005). In this age group, no statistically significant differences were observed between vaccine groups in terms of shivering, sweating and fever (p > 0.05). Solicited local and general adverse events of Grade 3 intensity were reported for ≤3.6% of subjects. In the 18–64 years age group, the incidence of pain at the injection site, joint pain and muscle aches of Grade 3 intensity was significantly higher in the adjuvanted vaccine group compared with the non-adjuvanted group (p < 0.0001 for pain at the injection site; p = 0.03 for joint pain; p = 0.006 for muscle aches). The incidence of other solicited symptoms of Grade 3 intensity in this age group, as well as in the >64 years age group, was not statistically significant different between the adjuvanted and the non-adjuvanted vaccine groups (p > 0.05). Reporting of solicited adverse events was higher in the 18–64 years age group.
Fig. 3.
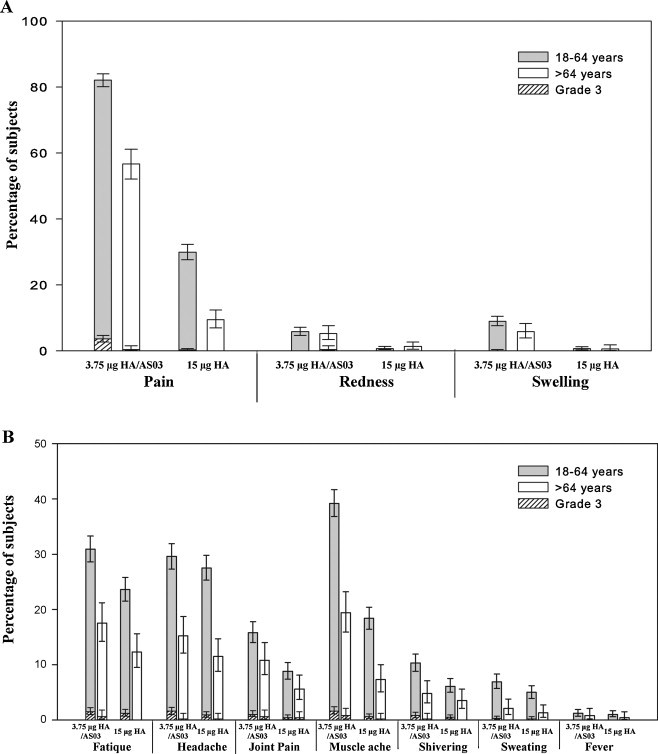
The incidence of solicited local (A) and general (B) adverse events reported during the 7-day post-vaccination period (total vaccinated cohort).
Unsolicited adverse events: A total of 181 subjects (4.5%; AS03-adjuvanted: 18–64 years: 87 [5.6%], >64 years: 14 [2.9%]; non-adjuvanted: 18–64 years: 65 [4.2%], >64 years: 15 [3.1%]) reported at least one unsolicited adverse event causally related to vaccination, during the 42-day post-vaccination follow-up period.
Overall, 216 SAEs were reported in 157 subjects (3.9%) through Day 385 (Month 12) (Web appendix Table 4); 84 subjects in the AS03-adjuvanted treatment group, 18–64 years: 2.9%, >64 years: 8.1%, and 73 subjects in the non-adjuvanted treatment group, 18–64 years: 2.1%, >64 years: 8.5%. Two of these events, intestinal obstruction (AS03-adjuvanted treatment group) and multiple sclerosis (non-adjuvanted treatment group) were considered by the investigator to be possibly related to study vaccine and were also considered pIMDs. Through Day 385 (Month 12), 12 pIMDs according to the predefined list of pIMD preferred terms were reported, with 5 and 7 in AS03-adjuvanted and non-adjuvanted influenza treatment groups, respectively. Seven fatal SAEs were reported, 6 and 1 in AS03-adjuvanted and non-adjuvanted treatment groups, respectively. All were assessed by investigators as not related to vaccination. A detailed description of all fatal SAEs is provided in Web appendix Table 5. Overall, 32 samples had laboratory values for the hematological and biochemical parameters outside the normal laboratory reference range at Days 7 and 21. Of these, 14 were from subjects in the adjuvanted vaccine group and 18 were from subjects in the non-adjuvanted vaccine group.
4. Discussion
Data from this large, controlled study in adults 18 years of age and older demonstrated that a single dose of AS03-adjuvanted or non-adjuvanted influenza A(H1N1)pdm09 vaccine elicited strong HI immune responses 21 days later that met the CHMP and the more stringent CBER criteria for pandemic influenza vaccines. The HI antibody response persisted through six months after vaccination for both vaccines, although the CBER criteria were met only in the 18–64 years age group and CHMP criteria in the 18–60 years age group.
The co-primary objective concerning relative vaccine efficacy against influenza was not evaluated due to the small number of RT-qPCR-confirmed H1N1/09 influenza cases. The low number of cases observed may be partially due to the timing of the study; the start of study vaccination followed the peak of A(H1N1)pdm09 virus transmission in the US and Canada by a week or more (last week of October, 2009), by which time A(H1N1)pdm09 circulation had diminished considerably. Published estimates of AS03-adjuvanted influenza A(H1N1)pdm09 vaccine effectiveness against influenza range from 62.0% to 100.0% [27], [28], [29], [30].
Overall, the incidence of ILI cases was comparable between the two groups, except in the first 28 days after vaccination (20 versus 41 ILI cases in the AS03-adjuvanted and non-adjuvanted treatment groups, respectively). This study was not sufficiently powered to detect statistical significance in this analysis.
The data for elderly subjects from the present study are in agreement with observations made in previous studies that one dose of the AS03-adjuvanted 3.75 μg HA influenza A(H1N1)pdm09 vaccine may be insufficient to meet CBER criteria at 6 months in elderly [31] and two doses of vaccine administered 21 days apart induce long-term persistence of HI antibodies at putatively protective levels [32], [33], [34]. Nicholson et al. demonstrated that two doses of a different AS03-adjuvanted influenza A(H1N1)pdm09 vaccine elicited HI immune responses that persisted at seroprotective levels in >70% of subjects ≥65 years of age, up to six months after vaccination, although at lower levels compared to younger adults (p < 0.0001) [34].
Similar to other observations [11], [17], [18], [35], [36], [37], [38], [39], [40], our results showed that previous seasonal vaccination appeared to negatively influence the strength of the immune response elicited by the influenza A(H1N1)pdm09 vaccines, especially in terms of long-term immunogenicity. There are conflicting reports on whether previous seasonal influenza vaccination increases the risk of subsequently contracting A(H1N1)pdm09 infection requiring medical attention [30], [41]. The effect of BMI on immune response was also studied. Consistent with previous trials [42], [43], in the present study, high BMI did not appear to impair HI antibody response shortly after vaccination. However, Sheridan et al. reported a decrease in HI antibody titers in obese subjects 12 months after vaccination [43], an observation also made in the present study.
The reactogenicity and safety profile was in agreement with available data in adults and children [19], [32], [44]. The frequency of solicited local adverse events in this study was higher in the AS03-adjuvanted versus the non-adjuvanted treatment group and the frequency of solicited adverse events were comparatively lower in the >64 years age group. Previous clinical trials of influenza A(H1N1/)pdm09 vaccines [17], [45], [2], [46] comparing safety outcomes between adjuvanted and non-adjuvanted vaccines reported similar observations, with higher frequency of both local and general adverse events with adjuvanted vaccines compared with non-adjuvanted vaccines. In our study, we did not observe any differences between the two vaccine groups in terms of SAEs considered as possibly related to vaccination (1 in each group). Although an imbalance in the number of fatal SAEs was observed between the adjuvanted and non-adjuvanted group (6 versus 1), none were considered to be related to vaccination and they all occurred in subjects with a relevant medical history.
A gradual decrement in the HI antibody GMTs elicited by both study vaccines against the A(H1N1)pdm09 vaccine strain in older subjects was observed and this could be attributed to “immunosenescence” [47], [48]. A decreasing trend with advancing age was also observed in the frequency of solicited adverse events.
A possible limitation of this study was the absence of blood samples collection for assessment of the immune response after Day 182 (Month 6). This period of six months was anticipated to cover the period of transmission of influenza virus during one season. A recently published study enrolling 240 subjects randomized to receive one or two doses of the same adjuvanted vaccine and followed up to 12 months, showed that regulatory criteria were met 6 months after the administration of the last vaccine dose in subjects aged 18–60 years receiving either one or two vaccine doses and in subjects aged >60 years receiving two vaccine doses [49]. At Day 385 (Month 12) the regulatory criteria were still met only in subjects aged 18–60 years who received two vaccine doses.
In conclusion, a single dose of either adjuvanted or non-adjuvanted influenza A(H1N1)pdm09 vaccines elicited protective levels of HI antibodies against the vaccine homologous A/California/7/2009 strain that persisted up to Day 182 (Month 6) in the 18–64 years population. Adjuvantation potentially offers the opportunity for antigen-sparing, making this AS03-adjuvanted influenza A(H1N1)pdm09 vaccine a candidate to help meet the demands for the large number of vaccine doses required to mitigate pandemic influenza.
Acknowledgements
We are grateful to the New York Medical College, New York for providing the vaccine virus strain. The authors are indebted to the participating study volunteers, clinicians, nurses and laboratory technicians at the study sites. We are grateful to the principal investigators, Drs. Steven Kaster, James Borders, Peter Dzongowski, John Ervin, Charles Fogarty, David Fried, Steven Kaster, Pierre Lachance, James Lawless, Andrew Lewin, Christopher Lucasti, Lew Pliamm, Terry Poling, Calvin Powell, Anthony Puopolo, Bruce Rankin, Paul Rheault, Stephan Sharp, Gerard Shockey, Eric St-Amour, Anne Crawley, and Linda Fisher, from the study sites, to all teams of GlaxoSmithKline Vaccines for their contribution to this study, especially the clinical and serological laboratory teams, Grace Cole, Jacqueline McCormick, Natalie McCloskey, Deseree Wong and Caroline Gesualdi for clinical study management, Janine Linden for preparation of the study protocol and related study documentation, Dorothy Slavin, Clinical Safety Representative, Rosalia Calamera, Clinical Data Coordinator, Karl Walravens lab manager and Clinical Readout. Finally, we thank Avishek Pal (GlaxoSmithKline Vaccines) and Adriana Rusu (XPE pharma and Science) who provided medical writing services and Dr. Santosh Mysore (XPE Pharma and Science, c/o GlaxoSmithKline Vaccines) for editorial assistance and manuscript coordination.
Financial disclosure: The study was funded by the US Department of Health and Human Services (HHS), Assistant Secretary of Preparedness and Response (ASPR), Biomedical Advanced Research and Development Authority (BARDA) and GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data. The corresponding author had final responsibility to submit for publication. Conflict of interest: All investigators received compensation for study involvement and travel related to this study. Ping Li, Miguel Madariaga, Olivier Godeaux and David Vaughn are/were employees of GlaxoSmithKline group of companies and report receiving restricted shares of the company. Contributorship: W.Y., M.D., M.K. and N.A. contributed to the data collection, data interpretation and critical review of the manuscript drafts. P.L., M.M., O.G. and D.W.V. contributed to the study design, data analysis and interpretation as well as to the critical review of all drafts of the manuscript. Trade mark statement: Arepanrix is a trade mark of GlaxoSmithKline group of companies.
Footnotes
ClinicalTrials.gov Identifier: NCT00979602.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2013.07.007.
Appendix A. Supplementary data
The following is supplementary data to this article:
References
- 1.Clark T.W., Pareek M., Hoschler K., Dillon H., Nicholson K.G., Groth N. Trial of influenza A(H1N1) 2009 monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 2.Liang X.F., Wang H.Q., Wang J.Z., Fang H.H., Wu J., Zhu F.C. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 3.Vajo Z., Tamas F., Sinka L., Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009–2010 influenza season: a multicentre, randomised controlled trial. Lancet. 2010;375:49–55. doi: 10.1016/S0140-6736(09)62039-0. [DOI] [PubMed] [Google Scholar]
- 4.Madhun A.S., Akselsen P.E., Sjursen H., Pedersen G., Svindland S., Nøstbakken J.K. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine. 2011;29:266–273. doi: 10.1016/j.vaccine.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Dawood F.S., Jain S., Finelli L., Shaw M.W., Lindstrom S., Garten R.J., Virus Investigation Team Novel Swine-Origin Influenza A(H1N1). Emergence of a novel swine-origin influenza A(H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [erratum in: N Engl J Med 2009;361(1):102] [DOI] [PubMed] [Google Scholar]
- 6.Garten R.J., Davis C.T., Russell C.A., Shu B., Lindstrom S., Balish A. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz J., Hancock K., Veguilla V., Zhong W., Lu X.H., Sun H. Serum cross-reactive antibody response to a novel influenza A(H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:521–524. [PubMed] [Google Scholar]
- 8.Hancock K., Veguilla V., Lu X., Zhong W., Butler E.N., Sun H. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO). Global Alert and Response (GAR). Pandemic influenza A(H1N1) 2009 virus vaccine – conclusions and recommendations from the October 2009 meeting of the immunization Strategic Advisory Group of Experts, http://www.who.int/csr/disease/swineflu/meetings/sage_oct_2009/en/; December 04, 2009 [accessed 16.11.11].
- 10.World Health Organization (WHO), WHO recommendations on pandemic (H1N1) 2009 vaccines. Pandemic (H1N1) 2009: briefing note 2, http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090713/en/index.html; July 13, 2009 [accessed 16.11.11].
- 11.Jackson L.A., Chen W.H., Stapleton J.T., Dekker C.L., Wald A., Brady R.C. Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 Adjuvant System in healthy adults and older persons. J Infect Dis. 2012;206:811–820. doi: 10.1093/infdis/jis427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel S., Didierlaurent A., Bourquiqon P., Delhaye S., Baras B., Jacob V. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Garcon N., Vaughn D.W., Didierlaurent A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines. 2012;11:349–366. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 14.Chu D.W.S., Hwang S.J., Lim F.S., Oh H.M., Thongcharoen P., Yang P.C. Immunogenicity and tolerability of an AS03A-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009;27:7428–7435. doi: 10.1016/j.vaccine.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 15.Nagai H., Ikematsu H., Tenjinbaru K., Maeda A., Dramé M., Roman F.P. A phase II, open-label, multicentre study to evaluate the immunogenicity and safety of an adjuvanted prepandemic (H5N1) influenza vaccine in healthy Japanese adults. BMC Infect Dis. 2010;10:338. doi: 10.1186/1471-2334-10-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leroux-Roels I., Bernhard R., Gérard P., Dramé M., Hanon E., Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS ONE. 2008;3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman F., Vaman T., Gerlach B., Markendorf A., Gillard P., Devaster J.M. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03(A)-trial. Adjuvant: preliminary report of an observer-blind, randomized controlled trial. Vaccine. 2010;28:1740–1745. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Roman F., Clement F., Dewe W., Walravens K., Maes C., Willekens J. Effect on cellular and humoral immune responses of the AS03 Adjuvant System in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol. 2011;18:835–843. doi: 10.1128/CVI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikematsu H., Nagai H., Kawashima M., Kawakami Y., Tenjinbaru K., Maeda A. Immunogenicity and safety of a novel AS03A-adjuvanted H1N1 2009 pandemic influenza vaccine in adults in Japan. Hum Vaccin. 2010;6:888–893. doi: 10.4161/hv.6.11.12851. [DOI] [PubMed] [Google Scholar]
- 20.WHO Expert Committee on Biological Standardization: 56th report, Geneva, World Health Organization. Annex 5: WHO biosafety risk assessment and guidelines for the production and quality control of human influenza pandemic vaccines, http://www.who.int/biologicals/publications/trs/areas/vaccines/influenza/Annex%205%20human%20pandemic%20influenza.pdf; 2007 [accessed 19.04.13].
- 21.US Food and Drug Administration (FDA) for Industry, Clinical data needed to support the licensure of pandemic influenza vaccines. US Food and Drug Administration, http://www.fda.gov/cber/gdlns/panfluvac.htm; May 2007 [accessed 16.11.10].
- 22.European Committee for Proprietary Medicinal Products (CHMP), Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006). European Agency for the Evaluation of Medicinal Products, January 24, 2007.
- 23.Hehme N.W., Künzel W., Petschke F., Gisela T., Carmen R., Christian van H. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Invest. 2002;22:751–769. [Google Scholar]
- 24.Vesikari T., Beran J., Durviaux S., Stainier I., El Idrissi M., Walravens K. Use of real-time polymerase chain reaction (rtPCR) as a diagnostic tool for influenza infection in a vaccine efficacy trial. J Clin Virol. 2012;53:22–28. doi: 10.1016/j.jcv.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Pabbaraju K., Wong S., Tokaryk K.L., Fonseca K., Drews S.J. Comparison of the Luminex xTAG Respiratory Viral Panel with xTAG Respiratory Viral Panel Fast for diagnosis of respiratory virus infections. J Clin Microbiol. 2011;49:1738–1744. doi: 10.1128/JCM.02090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadsby N.J., Hardie A., Claas E.C.J., Templeton K.E. Comparison of the Luminex Respiratory Virus Panel Fast Assay with In-House Real-Time PCR for respiratory viral infection diagnosis. J Clin Microbiol. 2010;48:2213–2216. doi: 10.1128/JCM.02446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmud S., Hammond G., Elliott L., Hilderman T., Kurbis C., Caetano P. Effectiveness of the pandemic H1N1 influenza vaccines against laboratory-confirmed H1N1 infections: population-based case–control study. Vaccine. 2011;29:7975–7981. doi: 10.1016/j.vaccine.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 28.Skowronski D.M., de Serres G., Crowcroft N.S., Janjua N.Z., Boulianne N., Hottes T.S. Association between the 2008 and 2009 seasonal influenza vaccine and pandemic H1N1 illness during spring–summer 2009: four observational studies from Canada. PLoS Med. 2010;7:e1000258. doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Buynder P.G., Dhaliwal J.K., van Buynder J.L., Couturier C., Minville-Leblanc M., Garceau R. Protective effect of single-dose adjuvanted pandemic influenza vaccine in children. Influenza Other Respi Viruses. 2010;4:171–178. doi: 10.1111/j.1750-2659.2010.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews N., Waight P., Yung C.F., Miller E. Age-specific effectiveness of an oil-in-water adjuvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England. J Infect Dis. 2011;203:32–39. doi: 10.1093/infdis/jiq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikematsu H., Tenjinbaru K., Li P., Madan A., Vaughn D. Evaluation of immune response following one dose of an AS03A-adjuvanted H1N1 2009 pandemic influenza vaccine in Japanese adults 65 years of age or older. Hum Vaccin Immunother. 2012;8:1119–1125. doi: 10.4161/hv.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson M., Risi G., Davis M., Sheldon E., Baron M., Li P. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis. 2012;205:733–744. doi: 10.1093/infdis/jir641. [DOI] [PubMed] [Google Scholar]
- 33.Ikematsu H., Nagai H., Kawashima M., Kawakami Y., Tenjinbaru K., Li P. Characterization and long-term persistence of immune response following two doses of an AS03A-adjuvanted H1N1 influenza vaccine in healthy Japanese adults. Hum Vaccin Immunother. 2012;8:1–7. doi: 10.4161/hv.18469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson K.G., Abrams K.R., Batham S., Clark T.W., Hoschler K., Lim W.S. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A(H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis. 2011;11:91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 35.Nolan T., McVernon J., Skeljo M., Richmond P., Wadia U., Lambert S. Immunogenicity of a monovalent 2009 Influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 36.Nolan T., Richmond P.C., Formica N.T., Höschler K., Skeljo M.V., Stoney T. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus infl uenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–6391. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 37.Andrews N.J., Walker W.T., Finn A. Predictors of immune response and reactogenicity to AS03B-adjuvanted split virion and nonadjuvanted whole virion H1N1 (2009) pandemic influenza vaccines. Vaccine. 2011;29:7913–7919. doi: 10.1016/j.vaccine.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 38.Ohfuji S., Fukushima W., Deguchi M. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis. 2011;203:1301–1308. doi: 10.1093/infdis/jir026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohmit S.E., Petrie J.G., Malosh R.E., Cowling B.J., Thompson M.G., Shay D.K., Monto A.S. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013 doi: 10.1093/cid/cit060. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McElhaney J.E., Beran J., Devaster J.M., Esen M., Launay O., Leroux-Roels G. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(13)70046-X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Mahmud S.M., van Caeseele P., Hammond G., Kurbis C., Hilderman T., Elliott L. No association between 2008 and 2009 influenza vaccine and influenza A(H1N1)pdm09 virus infection, Manitoba, Canada, 2009. Emerg Infect Dis. 2012;18:801–810. doi: 10.3201/eid1805.111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talbot H.K., Coleman L.A., Crimin K., Zhu Y., Rock M.T., Meece J. Association between obesity and vulnerability and serologic response to influenza vaccination in older adults. Vaccine. 2012;30:3937–3943. doi: 10.1016/j.vaccine.2012.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheridan P.A., Paich H.A., Handy J., Karlsson E.A., Hudgens M.G., Sammon A.B. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obesity (Lond) 2011:1–6. doi: 10.1038/ijo.2011.208. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saitoh A., Tamura S., Nagai A., Tsuchida N., Sako M., Maekawa T. Clinical evaluation of an AS03-adjuvanted pandemic influenza H1N1 2009 vaccine in children (preliminary report) J Jap Pediatr Soc. 2011;115:578–584. [Japanese] [Google Scholar]
- 45.Clark T.W., Pareek M., Hoschler K., Dillon H., Nicholson K.G., Groth N., Stephenson I. Trial of 2009 influenza A(H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 46.Wu J., Li W., Wang H.Q., Chen J.T., Lv M., Zhou J.C. A rapid immune response to 2009 influenza A(H1N1) vaccines in adults: a randomized, double-blind, controlled trial. J Infect Dis. 2010;202:675–680. doi: 10.1086/655226. [DOI] [PubMed] [Google Scholar]
- 47.McElhaney J.E., Zhou X., Talbot H.K., Soethout E., Bleackley R.C., Granville D.J. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–2067. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talbot H.K.B., Libster R., Edwards K.M. Influenza vaccination for older adults. Human Vaccin Immunother. 2012;8:1–6. doi: 10.4161/hv.8.1.18129. [DOI] [PubMed] [Google Scholar]
- 49.van Damme P., Kafeja F., Bambure V., Hanon E., Moris P., Roman F. Long-term persistence of humoral and cellular immune responses induced by an AS03A-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized study in adults aged 18–60 years and older. Hum Vaccin Immunother. 2013;9 doi: 10.4161/hv.24504. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


