Abstract
Objective:
To perform a cost-utility analysis on the treatment of attention deficit hyperactivity disorder (ADHD) with methylphenidate immediate-release (MPH-IR) in children and adolescents from Brazil.
Method:
A Markov model was constructed to compare MPH-IR vs. no treatment. A 24-week naturalistic study was conducted to collect transition probabilities and utility data. Effectiveness was expressed as quality-adjusted life-years (QALY), and costs reported in 2014 international dollars (I$). The perspective was the Brazilian Unified Health System as payer, and the time horizon was 6 years.
Results:
Of 171 patients, 73 provided information at baseline, and 56 at week 24. Considering the MPH-IR monthly cost of I$ 38, the incremental cost-effectiveness ratio (ICER) of treatment was I$ 9,103/QALY for children and I$ 11,883/QALY for adolescents. In two-way sensitivity analysis, considering one Gross National Product per capita (I$ 11,530) as willingness-to-pay, a cost of no-treatment lower than I$ 45/month would render MPH-IR a cost-saving strategy.
Discussion:
MPH-IR treatment of children and adolescents is cost-effective for ADHD patients from the Brazilian public health system perspective. Both patients and the healthcare system might benefit from such a strategy.
Trial registration number:
Keywords: Attention deficit hyperactivity disorder, cost-utility analysis, methylphenidate
Introduction
Methylphenidate immediate release (MPH-IR) is one of the most scientifically supported stimulant drugs for the treatment of attention deficit hyperactivity disorder (ADHD) in children and adolescents.1 2-3 Nevertheless, MPH is not an available treatment for many people in low/middle-income countries (LMICs), such as Brazil, where it is estimated that around 257,662 individuals are not receiving ADHD treatment.4 The decision to provide treatment in the Brazilian public health system lies with healthcare managers.5
ADHD is characterized by non-episodic inattention and/or a hyperactivity/impulsivity behavior pattern occurring more frequently than expected for the patient’s age.6 Thus, it is usual to find economic losses resulting from academic difficulties,7 accidents,8 or even criminality,9 requiring expensive care. Moreover, comorbidities, such as conduct disorder (CD), oppositional defiant disorder (ODD), major depression (MD), bipolar disorder (BD), anxiety disorders (AD), and enuresis, are extremely frequent,10 increasing difficulties for patients and their families. Additionally, untreated hyperactive boys with ADHD are at increased risk of substance use disorders and impairment.11
The burden of ADHD must be analyzed not only in an individual context, but also from a collective standpoint. The economic consequences of the lack of ADHD treatment for society can result in very high losses. An ADHD patient can cost the State approximately US$ 4,181/year due to special academic needs,7 US$ 7,424 to 36,076/year due to criminal activities,9 and about US$ 1,151/year due to health care utilization.12 Recently, the incremental cost of ADHD in all age groups was estimated to range from US$ 143 to 266 billion/year in the United States alone. The educational and health care fractions of this cost for children ranged from US$ 15 to 25 billion/year and from US$ 21 to 44 billion/year, respectively.13 In Brazil, an extremely conservative analysis, considering an ADHD prevalence of 0.9% (the lowest described in the country), reveals an estimated annual cost of R$ 1.6 billion/year in two main areas: grade repetition and emergency department use.4 In the same study, the authors predicted annual savings of around R$ 1 billion/year if patients could have access to treatment with MPH-IR 30 mg/day, as suggested by the World Health Organization (WHO).14 In contrast, economic analyses in the Brazilian mental health system are infrequent; a literature search found only one cost-effectiveness study in the mental health field in the country, on adult schizophrenia.15 Moreover, no cost-utility analyses (CUA) for medications to treat ADHD in LMICs are found in the literature.
In contrast, countries like the UK,16 the U.S.,17 Australia,18 and Spain19 are largely focusing on economic analysis and health policy for ADHD treatment. To date, a systematic review found 14 studies from different countries on the theme.20 Unfortunately, the applicability of these data to LMICs is limited because these studies are country-specific, as is the case for utilities measures that are derived from health-related quality of life instruments for which cross-cultural differences have a clear impact on the kind of information that can be obtained.21 In addition, the transferability of findings of multinational studies to an individual health care context is limited, due to differences in population and methodological characteristics.22
Within this context, the objective of this study is to conduct a CUA considering the perspective of the public health system of a LMIC as the payer. To implement this analysis, we conducted a 24-week naturalistic study, a Delphi panel of Brazilian experts on ADHD treatment, and constructed a deterministic Markov model portraying the pathway of an ADHD patient.
Method
The study was performed in six stages: 1) definition of the base-case; 2) a 24-week naturalistic study; 3) a Delphi panel; 4) decision tree; 5) determination of variable scores; and 6) CUA with a Markov analytical model. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement was used.23 The CHEERS is an update of health economic evaluation guidelines which was created to better report economic analysis and to disseminate information in the field (for more information, please visit: http://www.ispor.org/TaskForces/EconomicPubGuidelines.asp).
Base case
For the base case, we chose a MPH-IR dose of 30 mg/day as recommended by the WHO14 for both children and adolescents. A 6-year time horizon was proposed, and an incremental cost of I$ 7.93/month was assigned to patients whose treatment was interrupted (further information in the “Costs” item; one I$ is one 2014 international dollar, a unit of currency with the same purchasing power parity as the U.S. dollar; one I$ would buy in the country of interest the same items or services a U.S. dollar would buy in the United States). The choice of a 6-year time horizon for the base case was supported by our intention to reflect the public health system scenario of ADHD treatment, and, due to utility measures, could be different between children and adolescents.24 The perspective adopted was that of a publicly funded health system as payer.
24-week naturalistic study
The main objective of the naturalistic study was to collect information on transition probabilities and utilities to be applied in the decision model. The sample was selected by telephone calls to former ADHD patients from the dataset of Hospital de Clínicas de Porto Alegre (HCPA), Brazil, who were on MPH-IR treatment for no less than 24 weeks and no more than 1 year, and to patients who sought psychiatric evaluation due to ADHD symptoms. Patients aged 6 to 17 years were invited to participate. The requirements for inclusion were ADHD diagnosis (any subtype) according to the DSM-IV criteria25 and intelligence quotient > 80. The only exclusion criteria were hypersensitivity to MPH or a medical condition precluding MPH-IR use. To increase external validity for a public health system perspective, psychiatric comorbidities were not considered as exclusion criteria.
Parents of children and adolescents enrolled in the study provided written informed consent for participation, while the children provided verbal assent for inclusion. The study was approved by the HCPA Institutional Review Board (IRB) (approved as such by the U.S. Office for Human Research Protections - IRB 00000921).
The evaluation process was composed of a five-step protocol at baseline: 1) clinical evaluation by three experienced child and adolescent psychiatrists; 2) psychometric assessment with the Wechsler Intelligence Scale for Children (WISC-III), a continuous performance test (CPT), and the Stroop test; 3) a psycho-pedagogical evaluation to detect learning disabilities; 4) a semi-structured interview consisting of the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (KSADS-PL),26 administered by a trained research assistant; 5) completion of the following scales: Child Behavior Checklist (CBCL),27 Barkley Side-effect rating scale (SERS),28 and Swanson, Nolan, and Pelham-IV Questionnaire (SNAP-IV) (parents).29 At baseline and on the 24th week of the study protocol, all parents/caregivers were asked to complete the Health Utility Index (HUI®) questionnaire (proxy assessment)30 (more details below).
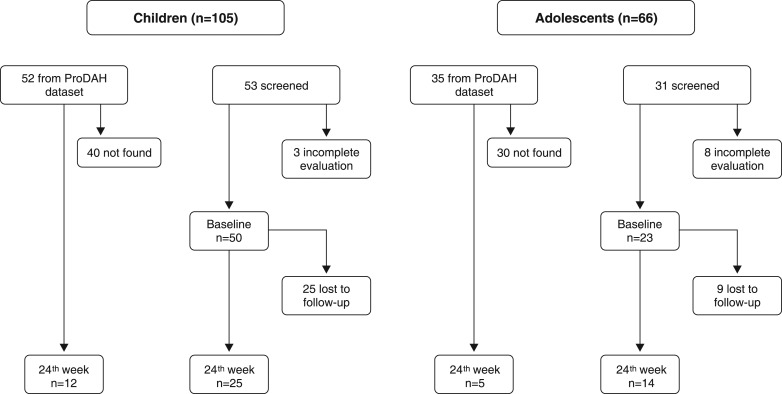
Treatment with MPH-IR was initiated after ADHD diagnosis, and doses were progressively increased until there was no more room for improvement or significant adverse events were present. Medication costs were paid by the parents/caregivers, and no financial benefit was offered for participation. After baseline, patients were evaluated at two time points: week 4 and week 24. The SNAP-IV and Barkley SERS were completed by the parents/caregivers at all visits. The study flowchart is presented in Figure 1.
Figure 1. The flowchart for the naturalistic study.
ProDAH = Programa de Déficit de Atenãço/Hiperatividade do Hospital de Clínicas de Porto Alegre (ADHD Outpatient Program).
Probabilities and utility data were both estimated according to the treatment effect/adherence and adverse events detected. Treatment effect was defined as a symptom reduction ≥ 30% in the SNAP-IV rating scale29 (inattention and hyperactivity/impulsivity) between baseline and week 4, and from week 4 to week 24 of follow-up. Similarly, adverse events were evaluated by means of the Barkley SERS. An adverse event was rated as positive when a difference of two or more points in its score was detected between two visits.
Delphi panel
Since there were not enough subjects in the natural course (NC) condition to provide transition probabilities at week 24, a Delphi panel of 26 ADHD Brazilian experts was created. All had experience in the Unified Health System and/or had experience supervising residents who treated patients in the same conditions. An anonymous questionnaire was created in the SurveyMonkey.com platform and sent by email to each expert. The questionnaire comprised four items about the estimated frequency of MPH-IR treatment, frequency of symptom remission and maintenance of treatment with or without adverse events, and frequency of no treatment and disease following its NC.
Decision tree model structure
The economic evaluation employed a CUA to calculate the incremental cost by quality-adjusted life-year (QALY) gained with MPH-IR therapy for 6 years vs. no treatment. At this point, a Markov model, a resource that allows the evaluation of chronic diseases, was used. This is a statistical tool which incorporates the time cycle of a disease, allowing simulations that would not be feasible in a real-life process due to the complexity of the disease and long time periods involved. The model was created with two hypothetical cohorts of ADHD patients: one for children (aged 6 to 12 years) and one for adolescents (aged 13 to 18 years). The decision model was as close as possible to the daily service of a public outpatient center.
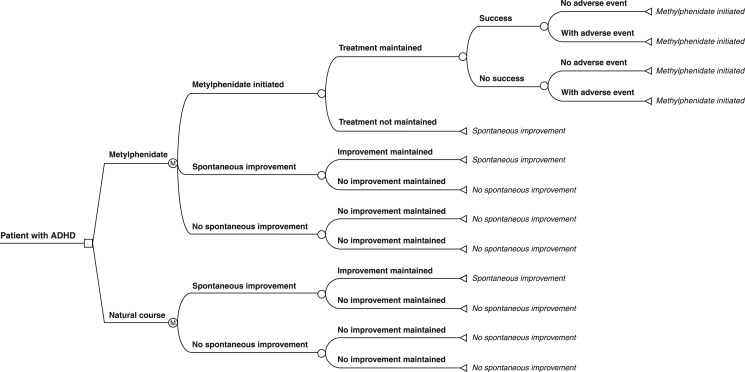
Patients entered the model after the complete evaluation mentioned above, and pharmacotherapy was then initiated (Figure 2). It was assumed that all ADHD patients received the initial MPH-IR treatment. After this point, the patient could stay on treatment or decide to stop using MPH-IR, whether due to spontaneous improvement or to other factors; patients who chose the latter would stay in this situation, which is called the “natural course” (NC). For NC patients experiencing a negative outcome, a health state node was considered due to the chronic condition – all stayed at this stage until the end of the cycle. If spontaneous improvement was identified, the patient could stay at this stage or migrate to the opposite node – no spontaneous improvement.
Figure 2. The decision tree.
The model used stipulates that, once in treatment, patients should remain on MPH-IR for at least 4 weeks. If they persist with treatment, patients can experience success or not, with or without adverse events. In case of an adverse event, the patient could choose whether to stay in treatment.
TreeAge Pro 2013 software (TreeAge Software, Inc., Williamstown, MA, USA) was used to construct the decision tree and conduct CUA.
Model variables
Costs
The perspective was from the Brazilian publicly funded Unified Health System as payer. Prices for medications were obtained from the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA), which is the Brazilian federal agency in charge of supervising and regulating all health products. MPH-IR 10 mg (20 pills) is acquired by the public health system at a maximum price of I$ 8.43 (including taxes), which corresponds to I$ 0.42/pill (distribution costs not included). Considering the WHO suggestion on MPH-IR doses (3 pills/day), the treatment cost for a patient was estimated at I$ 37.8/month.
The cost of one psychiatric outpatient visit, as informed by the Brazilian public health system, was I$ 5.37.
Costs for the NC were obtained from the most recent data published in the literature. Braun et al.31 described the resource utilization and treatment costs of German patients aged 6 to 17 years. Cost of disease was considered to be the difference between the median of 12-month resource consumption spent by an ADHD patient who was drug treatment-persistent (I$ 1,999) and one who was not (I$ 2,094).31 The total amount was I$ 7.93/month after conversion from euros to 2014 I$. A discount was applied for the 6-year follow-up period, corresponding to 5% per year for both groups of children and adolescents.
Transition probabilities
Transition probabilities for the Markov model are presented in Table 1 as percentages. Data were derived from the 24-week naturalistic study and from the Delphi panel, both described herein.
Table 1. Sociodemographic characteristics, utility measures, and transition probabilities for children and adolescents in the base-case analysis.
| Children (n=62)* | Adolescents (n=28)† | |
|---|---|---|
| Age, mean ± standard deviation | 8.62±1.7 | 13.67±1.61 |
| Intelligence quotient, mean ± standard deviation | 96.46±11.8 | 92.5±10.2 |
| Male | 46 (74.2) | 25 (89.3) |
| Ethnicity | ||
| White | 44 (71) | 21 (80.8) |
| Stimulant-naïve | 45 (72.6) | 16 (59.3) |
| ADHD subtype | ||
| Combined | 43 (69.4) | 13 (46.4) |
| Inattentive | 13 (21) | 10 (35.7) |
| Hyperactivity/impulsivity | 3 (4.8) | 1 (3.6) |
| Subthreshold | 3 (4.8) | 4 (14.3) |
| Comorbidity | ||
| Oppositional defiant disorder | 25 (40.3) | 14 (50.0) |
| Conduct disorder | 3 (4.8) | 3 (10.7) |
| Anxiety disorder | 5 (8.1) | 5 (17.9) |
| Mood disorder | 1 (1.6) | 1 (3.6) |
| Transition probabilities | ||
| MPH-IR initiation | 1.0 | 1.0 |
| MPH-IR use, 4th week | 0.7769 | 0.7697 |
| MPH-IR success, 4th week | 0.8941 | 0.9422 |
| MPH-IR, probability of AE in non-success patients/24th week | 0.406‡ | 0.321§ |
| MPH-IR, probability of AE in success patients/24th week | 0.333‡ | 0.091§ |
| Spontaneous improvement, 24th week | 0.09 | 0.09 |
| Utility measures | ||
| Baseline utility | 0.69 | 0.66 |
| Utility gained with MPH-IR | 0.10 | 0.09 |
| Utility gained for spontaneous improvement | 0.04 | 0.04 |
| Disutility for AE | ‐0.04 | ‐0.04 |
| Costs per month, I$ (range) | ||
| MPH-IR (WHO) | 37.8 (0.00-150.00) | |
| One consultation | 5.37 (10.00-25.00) | |
| Natural course | 7.93 (0.00-150.00) | |
| Discount per month | 0.004265319 | |
Data presented as n (%), unless stated otherwise.
ADHD = attention deficit hyperactivity disorder; AE = adverse event; MPH-IR = methylphenidate immediate release; WHO = World Health Organization.
All costs were converted to I$. The 2013 conversion factor is R$ 1.86. For more information, please visit The World Bank website (http://www.worldbank.org/).
62 children are the sum of 50 (baseline) plus 12 from ProDAH data set; † 28 adolescents are the sum of 23 (baseline) plus 5 from ProDAH data set; ‡ number of children in the 24th week: 37; § number of adolescents in the 24th week: 19.
The statistical analysis was performed in SPSS version 21.0.32
Utility data
The HUI® is a paper-and-pencil questionnaire to be completed by parents or caregivers. It is a multi-attribute health status classification system, which collects information for health-related quality of life and generates utility measurements for calculation of QALYs. It is composed of the HUI® Mark 2 (HUI®2) and HUI® Mark 3 (HUI®3) instruments. The HUI®2 includes seven attributes (sensation, mobility, emotion, cognition, self-care, pain, and fertility), with three to five levels, which describe 24,000 health states. The HUI®3 has eight attributes (vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain) with five to six levels, which define 972,000 health states.30,33 Both instruments were translated and validated for use in Brazilian children and parents/caregivers by Shimoda et al.34,35 The instrument was administered along with the other questionnaires at baseline and again at the 24-week visit. Parents/caregivers were encouraged to consider the symptoms of the previous 4 weeks when filling in the instruments. The instruments were coded using an algorithm, and utility was calculated with a specific formula provided by HUI Inc. (Toronto, Ontario, Canada). If any question was left empty or a duplicate answer was detected, the instrument was discarded from the study. Again, statistical analysis was performed in SPSS version 21.0.32
Sensitivity analysis
One-way sensitivity analyses were performed to assess the effect of cost of MPH-IR and NC, MPH compliance, and MPH effectiveness on the incremental cost-effectiveness ratio (ICER, or the cost difference between a conventional treatment and a new treatment to be tested/implemented, divided by the difference of effectiveness between conventional treatment and the new one). Two-way sensitivity analyses for MPH-IR cost and NC were also performed, considering the willingness-to-pay (WTP) of one Brazilian gross national product (GNP) per capita = I$ 11,530. The TreeAge Pro 2013 software was used for all sensitivity analyses.
Results
Naturalistic study
The medical records of 87 subjects with ADHD (52 children and 35 adolescents) were retrospectively reviewed. Of these, 70 could not be found, resulting in 17 subjects available for reevaluation. All were on MPH-IR for more than 24 weeks. Regarding those who were initially evaluated in the program, 11 did not return to complete the diagnostic process, resulting in 73 patients at baseline. Of this total, 34 were lost to follow-up with no information available, resulting in 39 children and adolescents at week 24. Sociodemographic characteristics, utility measures, transition probabilities, and costs are presented in Table 1.
Delphi panel
Of 26 ADHD experts, 14 completed the online questionnaire. The probability of being diagnosed with ADHD and remaining untreated and without spontaneous improvement by the 24th week was 91%, and that of spontaneous improvement, 9%.
Base-case analysis
The introduction of MPH-IR in doses as recommended by the WHO resulted in total costs of I$ 629.85 and 3.53 per QALY for children, considering an incremental cost for NC of I$ 7.93. As a result, the ICER to implement pharmacological treatment for this age group was I$ 9,103/QALY. For adolescents, although cost differences (Delta) were lower than those for children, the utility measure also exhibited a decrease, yielding an ICER of I$ 11,883/QALY. One-way sensitivity analyses and base-case comparisons between age groups are described in Table 2.
Table 2. One-way sensitivity analysis for MPH-IR vs. natural course (I$ 7.93) in 6 years* .
| MPH-IR | Natural course | Δ | |
|---|---|---|---|
| Children | |||
| Total cost (I$) | 629.85 | 491.77 | 138.08 |
| Total QALY | 3.53 | 3.52 | 0.01 |
| ICER (I$/QALY) | 9,103 | - | - |
| Adolescents | |||
| Total cost (I$) | 625.05 | 491.77 | 133.28 |
| Total QALY | 3.42 | 3.41 | 0.01 |
| ICER (I$/QALY) | 11,883 | - | - |
ICER = incremental cost-effectiveness ratio; MPH-IR = methylphenidate immediate-release; QALY = quality-adjusted life-years.
Mean dose for cost estimation: 30 mg/day corresponds to I$ 37.8/month. Highest price of one box of 10 mg MPH-IR (20 tablets), government as payer, with 19% tax = I$ 8.43 (http://portal.anvisa.gov.br/wps/wcm/connect/67eaaa004702351980f69341cdd33a01/LISTA+CONFORMIDADE_2014-11-20.pdf?MOD=AJPERES).
For the base case, a NC with no incremental cost (I$ 0.00) was also tested. As result, the ICER for treatment with MPH-IR increased, reaching I$ 11,151/QALY and I$ 14,556/QALY for children and adolescents, respectively.
Sensitivity analysis
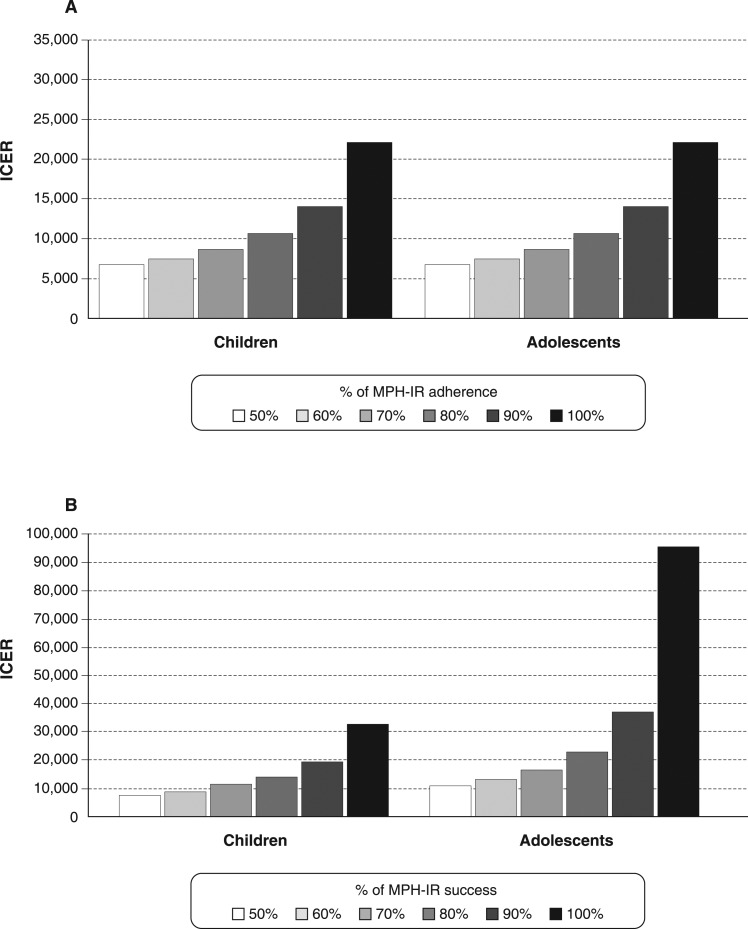
One-way sensitivity analyses for MPH-IR tested the 6-year adherence rate (from 50 to 100%) for both age groups, with NC fixed at I$ 7.93. All (Figure 3A) conditions presented an ICER under I$ 15,000/QALY when the adherence rate ranged from 70 to 100%.
Figure 3. A) One-way sensitivity analysis of ICER for probability of MPH adherence over 6 years; B) one-way sensitivity analysis of ICER for probability of MPH success over 6 years. ICER = incremental cost-effectiveness ratio; MPH-IR = methylphenidate immediate-release.
Additionally, as the success of MPH-IR therapy decreased, the ICER became less favorable, reaching I$ 95,164/QALY (Figure 3B) for adolescents with 50% treatment success. In common, both one-way sensitivity analyses demonstrated that, if MPH-IR treatment reaches a success or use rate > 60%, the ICER will be less than I$ 34,590/QALY (three times the Brazilian GDP), which is considered cost-effective. In addition, even with a lower rate of adherence (50%), the cost of treatment with MPH-IR was I$ 28,698 per QALY.
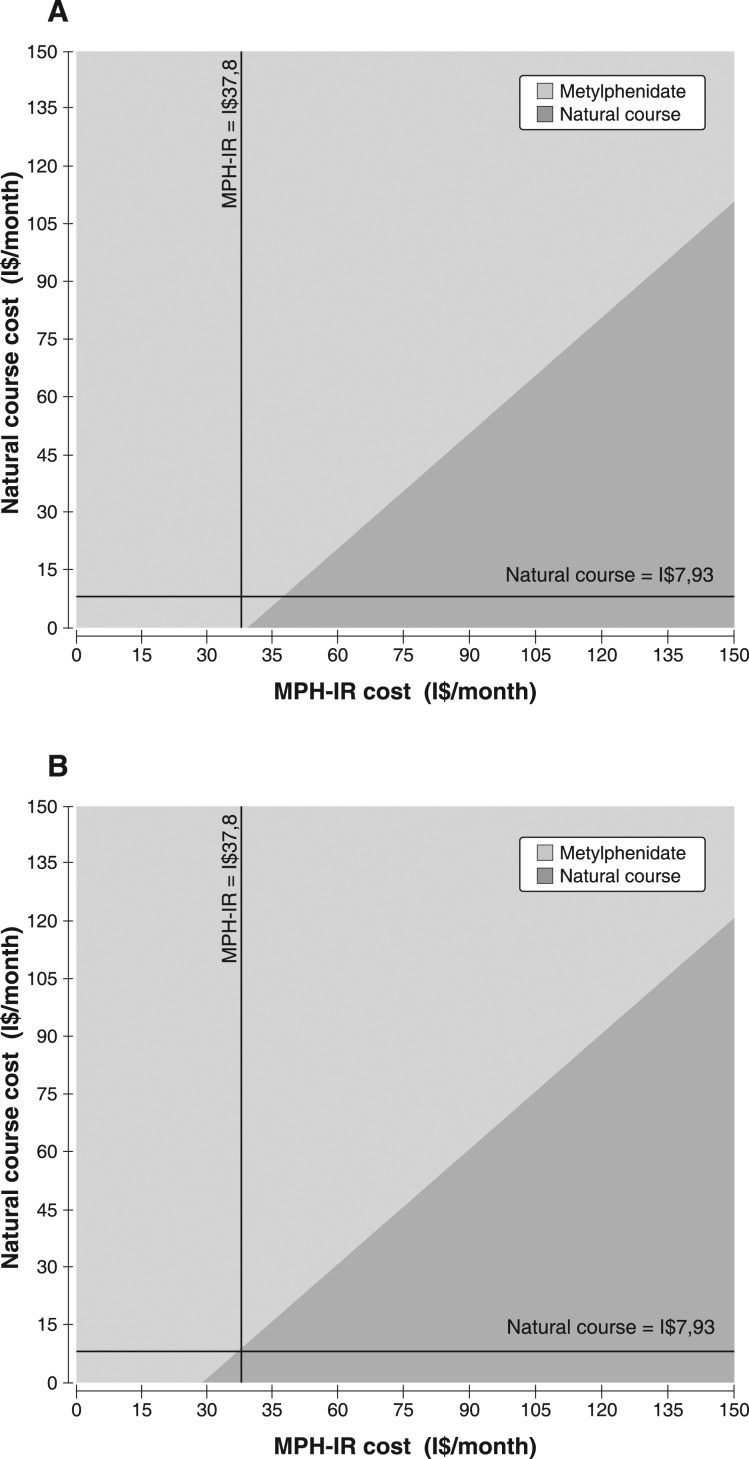
Considering a WTP threshold of I$ 11,530 and a cost variation for both NC and MPH-IR from I$ 0 to 150 in the base-case, two-way sensitivity analyses demonstrated a similar pattern for children and adolescents. For MPH-IR costs under I$ 37/month (children) or I$ 30/month (adolescents), any cost attributed to the NC seems to be cost-effective, as shown in Figure 4A and 4B.
Figure 4. Two-way sensitivity analyses of 6-year cost of MPH-IR vs. natural course of ADHD for A) children and B) adolescents, considering a willingness-to-pay of I$ 11,530. The dark gray area represents the natural cost variability per month, and the light gray area, the MPH-IR cost variability per month. ADHD = attention deficit hyperactivity disorder; MPH-IR = methylphenidate immediate-release.
Discussion
This study evaluated the cost-utility of MPH-IR treatment of ADHD in children and adolescents in the context of a LMIC, such as Brazil. In the base-case analysis, adding MPH-IR would represent an ICER of I$ 9,103/QALY and I$ 11,883/QALY for children and adolescents, respectively. When treatment success was taken into account, the results ranged from I$ 7,624/QALY to I$ 95,164/QALY.
Usually, the base case is defined by researchers as that which best represents the intervention and choices under analysis.36 The base case in this study fulfills almost all of these precepts, except treatment regimen (mg/day). In the ADHD outpatient program sample, the maximum MPH-IR mean dose at one of the three time points was 21.14 mg/day for children and 22 mg/day for adolescents. However, it is important to consider that most patients did not reach their best dose due to the failure to sustain the financial cost of MPH-IR treatment. As a result, the sensitivity analysis demonstrated a higher ICER for both age groups due to the increased number of patients who migrated to NC.
Some difficulties inherent to the sample characteristics led to a low rate of MPH-IR use, as reflected in the 6-year sensitivity analysis. For probability values, it was assumed that the most appropriate prevalence would be the highest number of dropouts during the 24th week of treatment, which was 77% for children and adolescents. For purposes of comparison, previous studies have reported dropout rates of 20 to 65% with stimulant treatments, including MPH-IR.37 In a 12-month study of MPH-IR, only 74% of patients took 50% or more of the medication and only 52% continued the treatment for 36 months.38
There is a scarcity of economic analyses of child and adolescent mental health in LMICs. Indeed, no such studies were found in a search of the literature. In contrast, a number of developed countries have demonstrated the cost-effectiveness of ADHD treatment. It may sound incoherent that LMICs, which should be saving money, are probably those that are wasting it most.39 Recent studies have pointed out the necessity of cost-effectiveness studies for LMICs,40 but, to the best of our knowledge, the present work is the first to show an advantageous economic option for a chronic disease which can be treated with MPH-IR even in low-resource settings.41 In addition, our study demonstrated that, in LMICs, investments in such analyses can reveal a favorable scenario in contrast with wealthy countries. To illustrate, the incremental cost per QALY gained with MPH-IR treatment seems to be much more attractive for the Brazilian public health system when compared to that of the UK, where the ICER was estimated at I$ 13,904/QALY,42 or to the U.S. (I$ 18,717/QALY),43 and even better if compared to atomoxetine treatment in Spain (I$ 43,427/QALY)19 (original costs were £ 9,177, US$ 14,758, and € 34,308, respectively).
Some limitations of this study should be taken into account. First, a Markov model with transition probabilities from a naturalistic study was used to simulate a real-world cohort, but it is expected that people would behave quite differently from a decision model. In addition, it was assumed that patients who did not benefit from treatment would not have a second chance. Despite that, the probabilities of the Markov model represent the local population, with high dropout rates, prevalent comorbidities as shown in Table 1, and low rates of patients in treatment in the first few years. Furthermore, the model adopted in this study was more complete than those used in previous investigations.16,18 Second, the high ICER values for treatment success could be even higher than those presented in this analysis. Due to the high dropout rates, most patients may leave treatment, for a variety of reasons, including lack of efficacy. Third, to supplement probabilities from the NC, we surveyed a group of experts (Delphi panel) by means of an online questionnaire. This was done due to the very small number of patients in the NC condition in our sample. A possible alternative solution would have been to assume transition probabilities derived from different countries. However, this approach would deviate from the intention of more accurately representing the Brazilian Unified Health System and its users. It should be borne in mind that this method is widely tested and recommended in psychiatric studies.44 Fourth, the study addressed only MPH-IR treatment; however, extended-release stimulants are available in the Brazilian market, and behavioral treatments were not considered at all. In fact, according to international guidelines, combinations of treatment modalities are preferable to medication alone.41 The main reasons for our choice of MPH-IR could be summarized as: the high costs of extended-release presentations, the absence of a specific policy for ADHD treatment by the Brazilian authorities,45 and the high costs of adding combined treatments, as demonstrated by the MTA.17 Lastly, the MPH-IR prices adopted are exclusively for the Brazilian government, which means they are lower than wholesale prices. To test the difference, an additional analysis for the base case with the highest price on the Brazilian market at the time of writing (I$ 0.56/pill, which represents I$ 50.4/month) was conducted. In the base case, the ICER was I$ 12,357/QALY for children and I$ 16,131/QALY for adolescents, which is still cost-effective for Brazil. In addition, it is very important to highlight that MPH-IR treatment was cost-effective with a willingness-to-pay threshold for the two-way sensitivity analysis set as one GNP per capita (I$ 11,530).
Cost-utility studies of ADHD treatment are becoming increasingly necessary to guide decision makers on the rational use of healthcare resources. The importance of economic analyses has become greater, especially for countries with limited resources, where the majority of the world’s population of children is found.46 The main contribution of this first CUA of ADHD treatment in a LMIC is to offer the opportunity for underprivileged countries to prevent financial losses and impairments. Herein, we demonstrated that the incremental cost-effectiveness of ADHD treatment with MPH-IR is affordable, particularly when adherence and success with treatment are taken into account. Future research on creative and cost-effective procedures to reduce dropout rates in ADHD treatment should be encouraged by the Unified Health System.
Disclosure
CRM has served as speaker and developed educational material for Novartis. FVK has served as a speaker for Shire Pharmaceuticals. LAR has served on the speakers’ bureau/advisory board and/or acted as consultant for Eli Lilly, Janssen-Cilag, Novartis, and Shire in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by him received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Abbott, Eli Lilly, Janssen-Cilag, Novartis, and Shire. He also receives authorship royalties from ArtMed and Oxford Press. CAP has served as a speaker and/or consultant to Eli Lilly, Novartis, Janssen-Cilag, and Shire Pharmaceuticals; has developed educational material for Janssen-Cilag; has received travel fees from Shire to take part in two scientific meetings; receives authorship royalties from Manole Conteúdo; and has received unrestricted research support from Novartis. The other authors report no conflicts of interest.
Acknowledgements
This study has received partial financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Edital MCT/CNPq/CT-Saúde/MS/SCTIE/DECIT N° 067/2009, research grant awarded to GVP and LAR), and Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, Brazil. CRM receives financial research support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); has received travel fees from Instituto de Avaliação de Tecnologia em Saúde (IATS), Universidade Federal do Rio Grande do Sul (UFRGS); and received travel and registration support for the 4th World Congress on ADHD from the World Federation of ADHD. CAP is the recipient of a CNPq research scholarship. The HUI® instruments were provided through a Health Utilities, Inc. grant.
References
- 1.Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, et al. European clinical guidelines for hyperkinetic disorder -- first upgrade. Eur Child Adolesc Psychiatry. 2004;13:I7–30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- 2.Charach A, Dashti B, Carson P, Booker L, Lim CG, Lillie E, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Oct. Report No.: 12-EHC003-EF. AHRQ Comparative Effectiveness Reviews. 0000 [PubMed] [Google Scholar]
- 3.Pliszka S, AACAP Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 4.Maia CR, Stella SF, Mattos P, Polanczyk GV, Polanczyk CA, Rohde LA. The Brazilian policy of withholding treatment for ADHD is probably increasing health and social costs. Rev Bras Psiquiatr. 2015;37:67–70. doi: 10.1590/1516-4446-2014-1378. [DOI] [PubMed] [Google Scholar]
- 5.Vianna C, Caetano R, Ugá M. Diretrizes metodológicas. Estudos de avaliação econômica de tecnologias em saúde. Brasília: Ministério da Saúde; 2009. [Google Scholar]
- 6.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 7.Robb JA, Sibley MH, Pelham WE, Jr, Foster EM, Molina BS, Gnagy EM, et al. The estimated annual cost of ADHD to the US education system. School Ment Health. 2011;3:169–77. doi: 10.1007/s12310-011-9057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange H, Buse J, Bender S, Siegert J, Knopf H, Roessner V. Accident proneness in children and adolescents affected by ADHD and the impact of medication. J Atten Disord. 2014 Jan 27 doi: 10.1177/1087054713518237. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher J, Wolfe B. Long-term consequences of childhood ADHD on criminal activities. J Ment Health Policy Econ. 2009;12:119–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Shreeram S, He JP, Kalaydjian A, Brothers S, Merikangas KR. Prevalence of enuresis and its association with attention-deficit/hyperactivity disorder among U.S. children: results from a nationally representative study. J Am Acad Child Adolesc Psychiatry. 2009;48:35–41. doi: 10.1097/CHI.0b013e318190045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huntley Z, Maltezos S, Williams C, Morinan A, Hammon A, Ball D, et al. Rates of undiagnosed attention deficit hyperactivity disorder in London drug and alcohol detoxification units. BMC Psychiatry. 2012;12:223–223. doi: 10.1186/1471-244X-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan E, Zhan C, Homer CJ. Health care use and costs for children with attention-deficit/hyperactivity disorder: national estimates from the medical expenditure panel survey. Arch Pediatr Adolesc Med. 2002;156:504–11. doi: 10.1001/archpedi.156.5.504. [DOI] [PubMed] [Google Scholar]
- 13.Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51:990–1002 e2. doi: 10.1016/j.jaac.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO), Collaborating Centre for Drug Statistics Methodology (WHOCC). 2009 Dec 17 [cited 2014 Jan]. DDD definition and general consideration [Internet]. http://www.whocc.no/ddd/definition_and_general_considera/ [Google Scholar]
- 15.Lindner LM, Marasciulo AC, Farias MR, Grohs GE. Economic evaluation of antipsychotic drugs for schizophrenia treatment within the Brazilian Healthcare System. Rev Saude Publica. 2009;43:62–9. doi: 10.1590/s0034-89102009000800010. [DOI] [PubMed] [Google Scholar]
- 16.King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al. A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents. Health Technol Assess. 2006;10:iii–iv. xiii–146. doi: 10.3310/hta10230. [DOI] [PubMed] [Google Scholar]
- 17.Jensen PS, Garcia JA, Glied S, Crowe M, Foster M, Schlander M, et al. Cost-effectiveness of ADHD treatments: findings from the multimodal treatment study of children with ADHD. Am J Psychiatry. 2005;162:1628–36. doi: 10.1176/appi.ajp.162.9.1628. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly M, Haby MM, Carter R, Andrews G, Vos T. Cost-effectiveness of dexamphetamine and methylphenidate for the treatment of childhood attention deficit hyperactivity disorder. Aust N Z J Psychiatry. 2004;38:592–601. doi: 10.1080/j.1440-1614.2004.01422.x. [DOI] [PubMed] [Google Scholar]
- 19.Hong J, Dilla T, Arellano J. A modelled economic evaluation comparing atomoxetine with methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder in Spain. BMC Psychiatry. 2009;9:15–15. doi: 10.1186/1471-244X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maia CRM, Cruz LN, Rosa T, Polanczyk GV, Polanczyk CA, Rohde LA. 8th Annual Meeting HTAi; Utility measures for ADHD: a systematic review; Rio de Janeiro, Brazil: 2011. p. p. 145. [Google Scholar]
- 21.Ravens-Sieberer U, Erhart M, Wille N, Wetzel R, Nickel J, Bullinger M. Generic health-related quality-of-life assessment in children and adolescents: methodological considerations. Pharmacoeconomics. 2006;24:1199–220. doi: 10.2165/00019053-200624120-00005. [DOI] [PubMed] [Google Scholar]
- 22.Reinhold T, Brüggenjürgen B, Schlander M, Rosenfeld S, Hessel F, Willich SN. Economic analysis based on multinational studies: methods for adapting findings to national contexts. J Pub Health. 2010;18:327–35. [Google Scholar]
- 23.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Pharmacoeconomics. 2013;31:361–7. doi: 10.1007/s40273-013-0032-y. [DOI] [PubMed] [Google Scholar]
- 24.Petrou S. Methodological issues raised by preference-based approaches to measuring the health status of children. Health Econ. 2003;12:697–702. doi: 10.1002/hec.775. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Arlington: American Psychiatric Publishing; 2000. [Google Scholar]
- 26.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Costello EJ, Edelbrock CS, Costello AJ. Validity of the NIMH Diagnostic Interview Schedule for Children: a comparison between psychiatric and pediatric referrals. J Abnorm Child Psychol. 1985;13:579–95. doi: 10.1007/BF00923143. [DOI] [PubMed] [Google Scholar]
- 28.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–92. [PubMed] [Google Scholar]
- 29.Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–79. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braun S, Russo L, Zeidler J, Linder R, Hodgkins P. Descriptive comparison of drug treatment-persistent, -nonpersistent, and nondrug treatment patients with newly diagnosed attention deficit/hyperactivity disorder in Germany. Clin Ther. 2013;35:673–85. doi: 10.1016/j.clinthera.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 32.IBM Corp . IBM SPSS Statistics for Windows, Version 21.0. Armonk: IBM Corp; 2012.. Released. [Google Scholar]
- 33.Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40:113–28. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Shimoda S, Horsman J, Furlong W, Barr R, de Camargo B. Disability and health-related quality of life in long-term survivors of cancer in childhood in Brazil. J Pediatr Hematol Oncol. 2008;30:563–70. doi: 10.1097/MPH.0b013e31816e231c. [DOI] [PubMed] [Google Scholar]
- 35.Shimoda S, de Camargo B, Horsman J, Furlong W, Lopes LF, Seber A, et al. Translation and cultural adaptation of Health Utilities Index (HUI) Mark 2 (HUI2) and Mark 3 (HUI3) with application to survivors of childhood cancer in Brazil. Qual Life Res. 2005;14:1407–12. doi: 10.1007/s11136-004-6127-3. [DOI] [PubMed] [Google Scholar]
- 36.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–7. [PubMed] [Google Scholar]
- 37.Swanson J. Compliance with stimulants for attention-deficit/hyperactivity disorder: issues and approaches for improvement. CNS Drugs. 2003;17:117–31. doi: 10.2165/00023210-200317020-00004. [DOI] [PubMed] [Google Scholar]
- 38.Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:922–8. doi: 10.1097/00004583-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Lima AF, Cruz LN, Polanczyk CA, Maia CR. Economic evaluation in the field of mental health: conceptual basis. Rev Bras Psiquiatr. 2013;35:186–92. doi: 10.1590/1516-4446-2012-0989. [DOI] [PubMed] [Google Scholar]
- 40.Cruz L, Lima AF, Graeff-Martins A, Maia CR, Ziegelmann P, Miguel S, et al. Mental health economics: insights from Brazil. J Ment Health. 2013;22:111–21. doi: 10.3109/09638237.2012.759193. [DOI] [PubMed] [Google Scholar]
- 41.Flisher AJ, Sorsdahl K, Hatherill S, Chehil S. Packages of care for attention-deficit hyperactivity disorder in low- and middle-income countries. PLoS Med. 2010;7:e1000235. doi: 10.1371/journal.pmed.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmore A, Milne R. Methylphenidate in children with hyperactivity: review and cost-utility analysis. Pharmacoepidemiol Drug Saf. 2001;10:85–94. doi: 10.1002/pds.564. [DOI] [PubMed] [Google Scholar]
- 43.Narayan S, Hay J. Cost effectiveness of methylphenidate versus AMP/DEX mixed salts for the first-line treatment of ADHD. Expert Rev Pharmacoecon Outcomes Res. 2004;4:625–34. doi: 10.1586/14737167.4.6.625. [DOI] [PubMed] [Google Scholar]
- 44.Ferri C, Chisholm D, Van Ommeren M, Prince M. Resource utilisation for neuropsychiatric disorders in developing countries: a multinational Delphi consensus study. Soc Psychiatry Psychiatr Epidemiol. 2004;39:218–27. doi: 10.1007/s00127-004-0729-5. [DOI] [PubMed] [Google Scholar]
- 45.Vieira FS, Zucchi P. [Judicial demands and therapeutic assistance in the Brazilian Public Health System]. Rev Assoc Med Bras. 2009;55:672–83. doi: 10.1590/s0104-42302009000600011. [DOI] [PubMed] [Google Scholar]
- 46.Kieling C, Baker-Henningham H, Belfer M, Conti G, Ertem I, Omigbodun O, et al. Child and adolescent mental health worldwide: evidence for action. Lancet. 2011;378:1515–25. doi: 10.1016/S0140-6736(11)60827-1. [DOI] [PubMed] [Google Scholar]