Abstract
Objectives:
To compare hair cortisol concentrations (HCC) in drug-naïve first-episode psychosis (FEP) patients and healthy controls and to investigate the correlations between HCC and psychopathology.
Methods:
Twenty-four drug-naïve FEP patients and 27 gender- and age-matched healthy control subjects were recruited. The Structured Clinical Interview for DSM-IV (SCID-1) was used to confirm/rule out diagnoses, and the Positive and Negative Symptoms Scale (PANSS) was used to assess symptom severity. Hair samples (2-3 cm long) obtained from the posterior vertex region of the scalp were processed in 1-cm segments considering a hair growth rate of 1 cm per month. The 1-cm segments were classified according to their proximity to the scalp: segment A was the closest to the scalp and referred to the month prior to inclusion in the study. Segments B and C referred to the 2nd and 3rd months prior to the time of evaluation respectively. Hair steroid extraction was performed using a known protocol.
Results:
Two-way analysis of covariance (ANCOVA) with gender and age as covariates revealed a group effect (F1.106 = 4.899, p = 0.029) on HCC. Between-segment differences correlated with total PANSS score and with PANSS General Psychopathology subscale and total score.
Conclusions:
Our findings suggest that hypothalamic-pituitary-adrenal (HPA) axis, as assessed by long-term (3-month) cortisol concentration, is abnormal in the early stages of psychosis. The magnitude of changes in HCC over time prior to the FEP correlates to psychopathology. HPA axis abnormalities might begin prior to full-blown clinical presentation requiring hospital admission.
Keywords: First-episode psychosis, schizophrenia, cortisol, stress
Introduction
According to the diathesis-stress model, psychosis is caused by an interaction between pre-existing vulnerability (diathesis) and exposure to acute and chronic psychosocial and biological stress.1 The hypothalamic-pituitary-adrenal (HPA) axis is the main biological system involved in stress responses.1,2 The HPA axis is activated by neural stimuli related to the perception and interpretation of threats, and its action is triggered by the release of corticotropin-releasing hormone (CRH) and vasopressin (AVP), which stimulates the secretion of adrenocorticotropic hormone (ACTH) from the pituitary, which in turn stimulates the secretion of cortisol from the adrenal gland.2 Cortisol binds with its receptors in multiple target tissues, including the HPA axis, where its feedback inhibits the secretion of ACTH from the pituitary and CRH from the hypothalamus.2 Cortisol exhibits a circadian rhythm, pulsatile secretion, diurnal variation, and reactivity to acute transient stress.3
The link between the HPA axis and the onset of psychosis might be related to the interaction between cortisol and dopamine.1 It is well established that dopaminergic system abnormalities are related to psychotic symptoms.1 Recent studies have shown that glucocorticoid secretion augments dopamine in the brain, particularly in the mesolimbic area, as reviewed by Walker et al.4 Compromised cognition and impairments in approach-avoidance behavior due to blunted cortisol response are other possible mechanisms involved in psychosis.5 Although studies have suggested that basal cortisol levels are elevated in first-episode psychosis (FEP), reactivity to acute stress is also impaired.6-8 A third biological pathway in psychosis is related to the influence of glucocorticoids on neuroplasticity.1 Increased concentrations of cortisol have been shown to reduce neurotrophin levels (such as brain derived neurotrophic factor, BDNF), which can lead to volumetric changes in the brain (e.g., reduced hippocampal volume). This finding has reliably been reported in FEP.9,10
Several methods have been proposed to assess HPA axis functions, including assessments of ACTH levels, dexamethasone suppression tests, and assessments of cortisol levels in body fluids, serum, saliva, and urine.11 Saliva and serum measures reflect circulating cortisol, and urine samples provide data about cortisol secretion over periods no longer than 24 h.11 As short-term measures, these levels are strongly affected by acute events, such as the stress of evaluation and the collection of the sample itself.11
Hair cortisol concentration (HCC) is a feasible and easily obtained measure for long-term assessment of HPA axis function.11,12 Hair grows at an average of 1 centimeter per month.12 There are different growth patterns across sites, and the posterior vertex region of the scalp exhibits the most uniform growth rates.3,12 The exact mechanism by which cortisol is deposited in hair is not fully understood, but the main hypothesis involves passive diffusion from blood capillaries into the growing hair cells and incorporation through sweat and sebum.3,12 In the last decade, HCC has been studied in many disorders, such as posttraumatic stress disorder,13 and drug abstinence,14 among other conditions.3,15 Nevertheless, HCC has never been investigated in individuals with first episodes of psychosis.
The objective of this study was to investigate differences in the HCC of FEP patients and healthy controls. Additionally, in the FEP group, we evaluated correlations between HCC and psychopathology and between the magnitude of changes in HCC occurring over the 3 months prior to admission and the expression of symptoms.
Methods
This study was approved by the ethics committees of Universidade Federal de São Paulo (UNIFESP) and Faculdade de Ciências Médicas da Santa Casa de São Paulo (FCMSCSP). All participants provided written informed consent prior to enrollment in the study. This study is part of a large cohort program aiming to investigate the neurobiology of FEP in drug-naïve individuals.
Participants
A total of 24 FEP patients were recruited at admission to the Psychiatric Emergency Unit. For the purposes of this study, psychosis was defined as the fulfillment of DSM-IV criteria for any of the following conditions: schizophrenia, schizoaffective disorder, manic episode with psychotic features, and depressive episode with psychotic features. The diagnoses were based on the Semi-Structured Clinical Interview for DSM-IV (SCID-1). Inclusion criteria were never having used an antipsychotic medication, not having experienced another psychiatric disorder during lifetime, having hair of at least 2 cm in length, and age between 18 and 45 years. Comorbid substance abuse, pregnancy, being in the post-partum period, general medical diseases, and inability to understand the research procedures were causes for exclusion.
Twenty-seven age- and gender-matched healthy control subjects with no previous history of mental illness according to the SCID-1 and with no family history of psychosis were selected for this study. Controls were recruited among university staff and undergraduate or graduate students. The same exclusion factors applied to the FEP group were also applied to the healthy control group.
All participants provided a socio-demographic history. The Positive and Negative Symptoms Scale (PANSS), Calgary Depression Scale, Young Mania Rating Scale (YMRS), Clinical Global Impression-Schizophrenia (CGI-S) and Global Assessment of Functioning (GAF) were applied. All of these instruments have been translated and validated for Brazilian Portuguese.16-20
Hair cortisol analysis
Collection
The samples were obtained from the posterior vertex region of the scalp using surgical scissors to cut the hair as close to the scalp as possible. Because hair was not plucked, the hair follicle was not included. The posterior vertex was chosen because it has the least hair variation among individuals, and because hair removal in this region has minimal esthetic effect. After collection, the scalp end was clearly marked and the samples were stored at room temperature. Considering a hair growth rate of 1 cm per month, the collected strands were cut into 1-cm segments. Each 1-cm segment was further divided into pieces not larger than 1 mm. The 1-cm segments were classified according to their proximity to the scalp: segment A was the closest to the scalp and therefore referred to the month prior to the first contact with the service. Segments B and C referred to the 2nd and 3rd months prior to the time of evaluation respectively. In subjects with hair shorter than 3 cm, we conducted the analysis for A and B only.
Extraction
Hair steroid extraction was performed using the protocol described by Van Uum et al.15 adapted by our laboratory.14 At least 10 mg of powdered hair per 1-cm section were weighed and separated into different glass vials, to which 1 mL of 50 °C methanol was added. The vials were then sealed. The samples were sonicated for 30 min and incubated overnight at 52 °C for 16 h. After incubation, 0.75 mL of the supernatant methanol was removed, placed into disposable glass tubes, and evaporated under a constant stream of nitrogen at 50 °C. Residues were dissolved in 250-μL phosphate-buffered saline (pH 8.0) and vortexed for 1 min. To enable the double-blinded measurement of cortisol in the extracts, we used a commercially available high-sensitivity salivary cortisol enzyme-linked immunosorbent assay (ELISA) (Salimetrics LLC, State College, PA, USA) according to the manufacturer’s instructions.
Statistical analyses
All statistical analyses were conducted using SPSS version 20.0. Continuous variables were reported as means and standard deviation, and categorical measures were reported as counts and percentages. Spearman’s rho correlation tests were applied to the HCC from each 1-cm hair segment corresponding to 1 month of the 3-month period, and also to psychopathology scale scores. Absolute HCCs were log transformed due to the non-normal distribution of this variable. Additionally, separate two-way analyses of covariance (ANCOVA) were performed using segment (time) and group as factors, gender and age as covariates, and log-transformed HCC as dependent variable.
The mathematical symbol Δ (delta) represents the results of subtraction between HCCs for different segments. Absolute differences between HCCs in each segment were assessed to investigate the potential relevance of concentration variation over time for psychopathology.
To investigate the course of mean HCCs over the 3 months prior to admission and the correlation of HCCs with symptom severity, we subdivided the sample into three groups according to ΔC-A: positive ΔC-A was obtained when the HCC of the 3rd month prior to admission was larger than that of the 1st month, indicating a reduction in mean HCC over time; negative ΔC-A was obtained when 3rd month HCC was larger than 1st month HCC, indicating an increase in HCC over time; and zero difference represented no variation over time.
Results
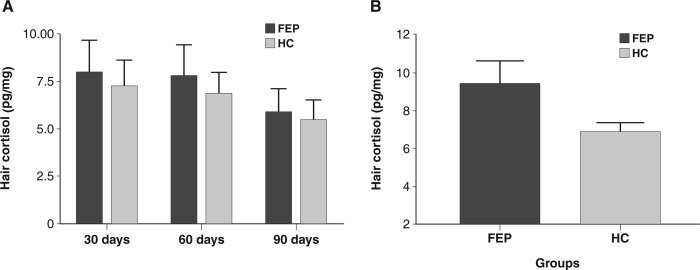
The sociodemographic and hair-related data of the sample are shown in Table 1. The proportion of smokers and unemployed individuals was significantly larger in the FEP group compared to healthy controls. The proportion of individuals with higher educational levels was also higher in the control group. There were no statistically significant differences between the groups in mean HCC in segments A (Mann-Whitney U = 280.000, p = 0.901), B (Mann-Whitney U = 228.000, p = 1.000) or C (Mann-Whitney U = 156.000, p = 0.899). However, a two-way ANCOVA revealed a group effect, but not a time effect, on HCC for the whole group and for each segment (Figure 1).
Table 1. Clinical and sociodemographic characteristics of the sample.
| FEP (n=24) | Healthy controls (n=27) | p-value | |
|---|---|---|---|
| Age in years* | 27.88±7.652 | 26.41±6.530 | 0.463 |
| Gender, female† | 15 (62.5) | 16 (59.26) | 0.813 |
| Secondary education diploma† | 5 (23.8) | 22 (81) | < 0.001 |
| Caucasian† | 13 (54.16) | 15 (55.55) | 0.100 |
| Employed† | 11 (45.83) | 9 (33.33) | 0.006 |
| Smokers† | 6 (25) | 0 | 0.021 |
| Hair-related variables | |||
| Washes per week‡ | 5.167±4.40 | 5.407±2.859 | 0.299 |
| Hair chemical treatment (%)‡ | 3 (12.5) | 3 (11.11) | 0.878 |
| Coloring (%)‡ | 5 (20.83) | 5 (18.51) | 0.835 |
FEP = first-episode psychosis.
Data presented as mean ± standard deviation or n (%) unless otherwise specified.
Student’s t
chi-square, and
Mann-Whitney tests.
Figure 1. Hair cortisol means for FEP and controls: A) according to hair segment (segment A = 30 days; segment B = 60 days; segment C = 90 days); B) pooled data from all segments. Two-way analysis of covariance (ANCOVA) revealed a group effect of F1,106 = 4.899 (p = 0.029). Raw data. FEP = first-episode psychosis, 9.376±8.8420 pg/mg; HC = healthy controls, 6.816±4.348 pg/mg.
The positive correlations between absolute HCCs and psychopathology are shown in Table 2. Fewer individuals were included in this analysis because some hair segments were lost during processing and some participants had hair shorter than 3 cm, especially in the case of male subjects. Analyses of between-segment variations in HCC were conducted. PANSS items N1, G3, G6, G11, and G14 were correlated with HCC differences between segments as shown in Table 2. ΔC-A was positively correlated with total PANSS score. The ratio of the absolute ΔB-A and the absolute ΔC-A was negatively correlated with the PANSS General Psychopathology subscale (r = -0.615; p = 0.044; n=11) and the PANSS total score (r = -0.661; p = 0.027; n=11).
Table 2. Correlation between cortisol concentration in hair segments and Positive and Negative Symptom Scale (PANSS) items.
| Variables | n | r | p-value |
|---|---|---|---|
| Correlation between absolute values of cortisol and PANSS items | |||
| B vs. P4 - excitation | 16 | 0.727 | 0.002 |
| B vs. G10 - disorientation | 16 | 0.524 | 0.031 |
| C vs. G10 - disorientation | 14 | 0.648 | 0.009 |
| A vs. G13 - ambivalence | 19 | 0.454 | 0.044 |
| Correlation between PANSS items and between-segment differences | |||
| ΔC-B vs. N1 - blunted affect | 13 | 0.622 | 0.023 |
| ΔC-B vs. G6 - depression | 13 | 0.554 | 0.049 |
| ΔC-B vs. G11 - attention | 13 | 0.636 | 0.019 |
| ΔC-B vs. G14 - poor impulse control | 13 | 0.655 | 0.015 |
| ΔC-B vs. PANSS total score | 13 | 0.576 | 0.039 |
| ΔB-A vs. G3 - guilt feelings | 15 | 0.528 | 0.043 |
A = Hair cortisol concentration (HCC) in 1st segment (30 days); B = HCC in 2nd segment (60 days); C= HCC in 3rd segment (90 days). Δ = between-measures subtraction, test statistic: Spearman’s rho.
The smaller number of participants in this analysis reflects the loss of hair segments during processing or participants with hair shorter than 3 cm.
Further analyses of the patterns of variation across the segments were conducted for the FEP group. In the subgroup in which cortisol levels decreased, negative correlations were observed between A, B, C and the GAF score (r = -0.870, r = -0.943; r = -0.900; p = 0.024; p = 0.005; p = 0.037 respectively).
Discussion
The results of this study suggest that HCC is increased in drug-naïve FEP patients. Moreover, differences in hair cortisol between segments representing different time points were correlated with the severity of psychopathology.
A relatively small number of studies of FEP subjects have addressed the HPA axis through cortisol measurements, with heterogeneous results. In the present study, covariance analysis suggested that HCC might be higher in FEP patients than in healthy controls, which is in line with the studies of Abel et al.,21 Ryan et al.,22-24 Walsh et al.,25 Spelman et al.,26 Kale et al.,27 and Mondelli et al.6 Conversely, other studies report no differences in plasma and salivary basal measures between FEP individuals and healthy controls.8,28-30 Those studies, however, assessed short-term cortisol measurements, which are known to be more susceptible to acute influences, such as the distress caused by the collection of blood or saliva itself.
Since psychosis is a developmental condition,10 it is reasonable to expect different biological findings at different stages of clinical presentation. As HCC might provide long-term data regarding cortisol secretion,1 analyses of HCC are interesting for more comprehensive studies of possible abnormalities. In the present study, HCC in hair segments corresponding to the period of 2 months prior to hospitalization were correlated with the PANSS items of excitation, and HCC in hair segments corresponding to the month prior to admission was correlated with disorientation and ambivalence. Although it is not clear whether cortisol plays a role in the brain mechanisms that are related to these psychopathological phenomena, our findings suggest that HPA axis abnormalities begin at least two months prior to the full-blown clinical presentation requiring inpatient treatment.
Blunted affect and depression were positively correlated with a greater decrease in HCC in hair segments corresponding to the period between the third and second months prior to hospitalization. The PANSS general psychopathology subscale and total score were negatively correlated with the change in HCC between the third and first months. These findings suggest that larger differences in HCC (both increases and decreases) are associated with less severe patient conditions. Previous studies have found correlations between PANSS subscales and blood cortisol even in the absence of differences between the cortisol levels of patients and controls.28,31 Taken together, these data suggest that the dynamics of cortisol concentration over time might be more important than the concentrations per se in predicting psychopathology.
Functional impairment might be present in psychotic patients beginning in the premorbid period.32 Such impairment may lead to chronic hyporesponsiveness of the HPA axis. We showed a negative correlation between HCC and GAF score in the subgroup of patients in whom HCC decreased over time. We hypothesize that, in a subgroup of FEP patients, HPA axis hyporesponsivity underlies premorbid functional impairment. This hypothesis is supported by findings of blunted cortisol stress responses in FEP individuals and by the correlation of this blunted response with cognitive impairment.6,33
The present study has limitations. First, the fact that not all eligible candidates accepted having their hair cut resulted in a relatively small sample, with possible selective non-inclusion of severe cases who might have been too paranoid to willingly engage in this study. The hair length required to measure cortisol limited the number of male subjects in the sample because young men commonly shave their heads. The duration of untreated psychosis (DUP) might have been incorrectly or imprecisely informed in some cases. As we conducted a cross-sectional study, later confirmation of the DUP data was not feasible, and we did not analyze this data. Finally, imprecise information on life events made the assessment of stress exposure not feasible. Therefore, the data obtained does not allow us to infer whether cortisol was involved in the development of psychosis or whether cortisol changes and psychosis both resulted from stress.
Our choice to select gender- and age-matched controls by convenience sampling resulted in some heterogeneity in terms of educational status, employment, and smoking habits. Nevertheless, functional impairments in the premorbid period and psychopathologies in the early phase of the psychotic outbursts might have been responsible for the lower educational status and employment rates of the FEP patients.33 However, it is unclear in the literature whether these factors can cause significant changes in HCC.
This study included only drug-naïve individuals. Cortisol secretion might be altered by antipsychotics.34 As a direct result of their pharmacological actions, antipsychotics inhibit HPA axis activity.34 Moreover, we were also careful to only include patients without other psychiatric comorbidities such as depression and PTSD, which induce abnormalities in HPA axis.3,14,15 Therefore, we were able to demonstrate HPA axis abnormalities in the absence of major confounders, and these findings strengthen the idea that the stress response system is independently involved in the pathophysiology of psychotic illness.
This is the first study to assess long-term HPA axis function through hair cortisol analysis. Future studies addressing this matter should clarify the complex relations between cortisol and psychosis. Also, it would be interesting to understand the possible relation between short- and long-term measurements in the same sample and its correlations to clinical presentation. Our data suggest that FEP individuals make up a heterogeneous group with more than one profile of cortisol change during time, which may be related to differences in symptoms domains.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This study was funded by Fundação de Amparo è Pesquisa do Estado de São Paulo (FAPESP; grant 11/23918-8).
References
- 1.Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38:603–11. doi: 10.1016/j.psyneuen.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–8. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38:1220–35. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 5.Roelofs K, Bakvis P, Hermans EJ, van Pelt J, van Honk J. The effects of social stress and cortisol responses on the preconscious selective attention to social threat. Biol Psychol. 2007;75:1–7. doi: 10.1016/j.biopsycho.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D'albenzio A, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116:234–42. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garner B, Phassouliotis C, Phillips LJ, Markulev C, Butselaar F, Bendall S, et al. Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. J Psychiatr Res. 2011;45:249–55. doi: 10.1016/j.jpsychires.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 8.van Venrooij JA, Fluitman SB, Lijmer JG, Kavelaars A, Heijnen CJ, Westenberg HG, et al. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr Bull. 2012;38:272–9. doi: 10.1093/schbul/sbq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondelli V, Cattaneo A, Belvederi Murri M, Di Forti M, Handley R, Hepgul N, et al. Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry. 2011;72:1677–84. doi: 10.4088/JCP.10m06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahn W, Rais M, Stigler FP, van Haren NE, Caspers E, Hulshoff Pol HE, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19:147–51. doi: 10.1016/j.euroneuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Stalder T, Kirschbaum C. Analysis of cortisol in hair--state of the art and future directions. Brain Behav Immun. 2012;26:1019–29. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Int. 2000;107:5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 13.Steudte S, Kirschbaum C, Gao W, Alexander N, Schönfeld S, Hoyer J, et al. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013;74:639–46. doi: 10.1016/j.biopsych.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Grassi-Oliveira R, Pezzi JC, Daruy-Filho L, Viola TW, Francke ID, Leite CE, et al. Hair cortisol and stressful life events retrospective assessment in crack cocaine users. Am J Drug Alcohol Abuse. 2012;38:535–8. doi: 10.3109/00952990.2012.694538. [DOI] [PubMed] [Google Scholar]
- 15.Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11:483–8. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 16.Versiani M. Tradução da Structured Clinical Interview for DSM-IV Axis I/Patient. Rio de Janeiro: Programa de Ansiedade e Depressão: Instituto de Psiquiatria, UFRJ; 1996. Entrevista Clínica Estruturada - DSM-IV Transtornos do Eixo I. [Google Scholar]
- 17.Vessoni AL. Adaptação e estudo de confiabilidade da escala de avaliação das síndromes positiva e negativa para a esquizofrenia no Brasil [dissertação] São Paulo: Escola Paulista de Medicina. 1993 [Google Scholar]
- 18.Bressan RA, Chaves AC, Shirakawa I, de Mari J. Validity study of the Brazilian version of the Calgary Depression Scale for Schizophrenia. Schizophr Res. 1998;32:41–9. doi: 10.1016/s0920-9964(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 19.Lima MS, Soares BG, Paoliello G, Machado Vieira R, Martins CM, Mota Neto JI da, et al. The Portuguese version of the Clinical Global Impression-Schizophrenia Scale: validation study. Rev Bras Psiquiatr. 2007;29:246–9. doi: 10.1590/s1516-44462007000300010. [DOI] [PubMed] [Google Scholar]
- 20.Associação Americana de Psiquiatria. Manual Diagnóstico e Estatístico de Transtornos Mentais, 4a edição, Texto revisado (DSM-IV-TR) Porto Alegre: Artmed. 2002 [Google Scholar]
- 21.Abel KM, O’Keane V, Murray RM. Enhancement of the prolactin response to d-fenfluramine in drug-naive schizophrenic patients. Br J Psychiatry. 1996;168:57–60. doi: 10.1192/bjp.168.1.57. [DOI] [PubMed] [Google Scholar]
- 22.Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160:284–9. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- 23.Ryan MC, Flanagan S, Kinsella U, Keeling F, Thakore JH. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug-naive patients with schizophrenia. Life Sci. 2004;74:1999–2008. doi: 10.1016/j.lfs.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–70. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Walsh P, Spelman L, Sharifi N, Thakore JH. Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide-induced AVP release. Psychoneuroendocrinology. 2005;30:431–7. doi: 10.1016/j.psyneuen.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24:481–5. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 27.Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never- medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175:47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Strous RD, Maayan R, Lapidus R, Goredetsky L, Zeldich E, Kotler M, et al. Increased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatology. Schizophr Res. 2004;71:427–34. doi: 10.1016/j.schres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Justicia A, Parellada E, Bernardo M, et al. Prolactin concentrations in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Res. 2012;134:16–9. doi: 10.1016/j.schres.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruessner M, Vracotas N, Joober R, Pruessner JC, Malla AK. Blunted cortisol awakening response in men with first episode psychosis: relationship to parental bonding. Psychoneuroendocrinology. 2013;38:229–40. doi: 10.1016/j.psyneuen.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol. 2010;24:91–118. doi: 10.1177/1359786810385491. [DOI] [PubMed] [Google Scholar]
- 32.Liu P, Parker AG, Hetrick SE, Callahan P, de Silva S, Purcell R. An evidence map of interventions across premorbid, ultra-high risk and first episode phases of psychosis. Schizophr Res. 2010;123:37–44. doi: 10.1016/j.schres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Aas M, Dazzan P, Mondelli V, Toulopoulou T, Reichenberg A, Di Forti M, et al. Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol Med. 2011;41:463–76. doi: 10.1017/S0033291710001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohrs S, Röher C, Jordan W, Meier A, Huether G, Wuttke W, et al. The atypical antipsychotics olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology (Berl) 2006;185:11–8. doi: 10.1007/s00213-005-0279-x. [DOI] [PubMed] [Google Scholar]