Abstract
Background
China's immunization program is one of the oldest and largest in the world. Rates of vaccine-preventable diseases (VPD) are comparable to those in high-income countries. The program's evolution has been characterized by ambitious target setting and innovative strategies that have not been widely described.
Methods
We reviewed national and provincial health department archives; analyzed disease surveillance, vaccination coverage, and serosurvey data from 1950 through 2016; and, conducted in-depth interviews with senior Chinese experts involved early VPD control efforts.
Results
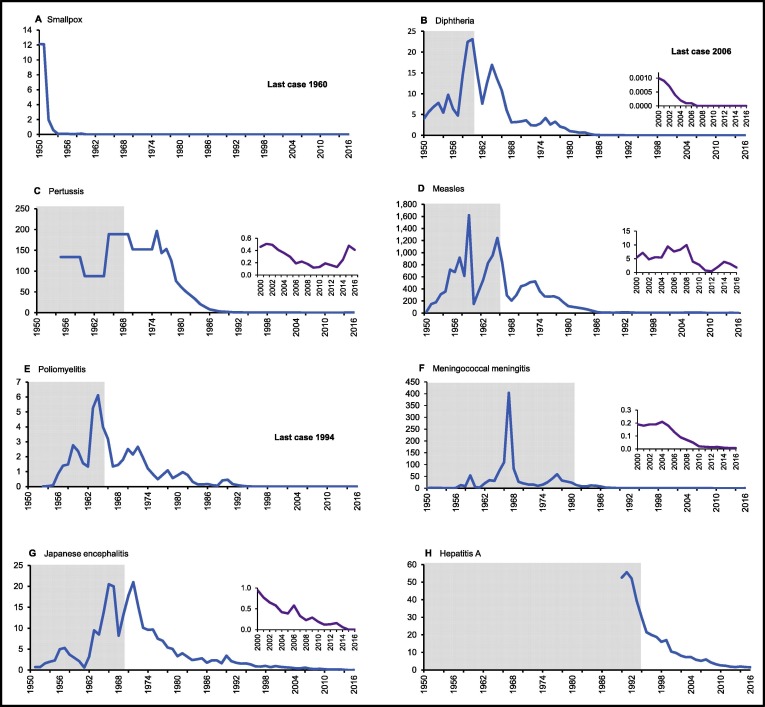
Widespread immunization began in the 1950s with smallpox, diphtheria, and Bacillus-Calmette Guerin vaccines, and in the 1960s with pertussis, tetanus, polio, measles, and Japanese encephalitis (JE) vaccines. The largest drops in absolute VPD burden occurred in the 1970s with establishment of the Rural Cooperative Medical System and a cadre of trained peasant health workers whose responsibilities included vaccinations. From 1970 to 1979, incidence per 100,000 population dropped 48% from 3.3 to 1.75 for diphtheria, 50% from 152.2 to 49.4 for pertussis, 77% from 2.5 to 0.6 for polio, 60% from 450.5 to 178.3 for measles, and 72% from 18.0 to 5.1 for JE, averting an average of 4 million VPD cases each year. Until the early 1980s, vaccines were delivered through annual winter campaigns using a coordinated ‘rush-relay’ system to expedite transport while leveraging vaccine thermostability. Establishment of the cold chain system during in the 1980s allowed bi-monthly vaccination rounds and more timely vaccination resulting in rates of diphtheria, pertussis, measles and meningitis falling over 90% from 1980 to 1989, while polio and JE rates fell 40–50%. In the 1990s, progress stalled as financing for public health was weakened by broad market reforms. Large investments in public health and immunizations by the central government since 2004 has led to further declines in VPD burden and increased equity. During 2011–2016, the incidence per 100,000 population was <2.0 for measles and <0.2 for pertussis, JE, meningococcal meningitis, and hepatitis A. From 1992 to 2014, the prevalence of chronic hepatitis B infection in children <5 years fell from 9.7% to 0.3%, a 97% decline. China was certified polio-free in 2000 and diphtheria was last reported in 2006.
Conclusions
Long-term political commitment to immunizations as a basic right, ambitious targets, use of disease incidence as the primary metric to assess program performance, and nationwide scale-up of successful locally developed strategies that optimized use of available limited resources have been critical to China's success in controlling vaccine-preventable diseases.
Keywords: China, Vaccine-preventable disease, Immunization, Incidence
1. Introduction
China has one of the largest and oldest immunization programs in the world with over 16 million infants vaccinated each year [1]. Incidence rates of vaccine-preventable diseases (VPD) are similar to those in high-income countries and vaccination coverage is uniformly high. These achievements are the result of enormous effort and evolving responses to new challenges and opportunities over the past 65 years.
When the People’s Republic of China was founded in 1949, the new government faced immense health problems. Most of the 550 million population lived in rural areas and in extreme poverty with little access to health care. Infant mortality exceeded 200 per 1000 births and average life expectancy was only 35 years [2]. Lack of United Nations' recognition limited international support and scientific exchange. Despite these challenges, the government set ambitious health goals and positioned immunizations as a central to their achievement.
Prior to Liberation, vaccines were prohibitively expensive in China and largely inaccessible except to the very wealthy. In 1946, constitutional principles of the Communist Party in the Shaanxi-Ningxia-Gansu Border Region affirmed “people’s rights to freedom from ill health”, and in line with this progressive policy, free mass vaccination campaigns against smallpox and cholera were implemented in liberated areas [3]. This was the first time that large numbers of the rural poor in China benefitted from vaccination. Since then, China’s public health system has undergone numerous reforms with continued strengthening of VPD control efforts through sustained high-level political commitment to immunizations, strong supportive legal frameworks, increased public finance, dedicated efforts of many scientists and public health professionals and grass-roots health workers, and national scale-up of successful innovative delivery approaches that optimized use of available material and human resources. This article describes China’s work on VPD control during 1949 to 2016, which has not been widely described.
2. Methods
Annual VPD incidence from 1950 to 2016 was obtained from the National Notifiable Disease Reporting System (NNDRS). The NNDRS is a compulsory reporting system established in 1950 for 15 diseases with high epidemic potential including cholera, plague, smallpox, Japanese encephalitis (JE), meningococcal meningitis, poliomyelitis, diphtheria, pertussis, and measles. Data on each case is limited to critical information including name, address, date of birth, date of disease onset, age, sex, and occupation. The NNDRS has since been expanded to 39 diseases, including hepatitis A and hepatitis B, tuberculosis, neonatal tetanus, mumps and rubella but the type of data collected has remained largely unchanged. Hepatitis B burden, using hepatitis B surface antigen (HBsAg) seropositivity as a marker for chronic infection, and hepatitis B vaccination coverage were estimated through national serosurveys conducted in 1992, 2006, and 2014 [4], [5], [6]. All three serosurveys relied on random sampling of persons in the Disease Surveillance Point (DSP) Surveillance System, a nationally representative sample of rural townships and urban neighborhoods representing approximately 1% of the total population.
Vaccination coverage surveys were conducted in selected provinces during 1983–1987 while nationally representative surveys were conducted in 1989, 1991, 1999, 2004, and 2013. All surveys utilized multi-stage probability of selection proportional to population size sampling based on World Health Organization (WHO) guidelines [7]. As a proxy for coverage prior to the 1980s, we searched for references to total numbers of persons vaccinated and total numbers of doses of vaccine produced or administered.
A search was conducted for published and unpublished reports on vaccine development and VPD control in China from 1949 to 2016, focusing on Chinese-language documents not widely available in the published literature, and included manual searches through archived documents at the Ministry of Health (MoH), China Center for Disease Control and Prevention, and provincial health departments [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Data on immunization expenditures was collected as part of national EPI reviews conducted in 1999, 2004, and 2013.
Because few reports on vaccination efforts were found prior to the 1980s, we interviewed senior Chinese experts who were closely involved in spearheading China's early immunization efforts using a structured open-ended questionnaire. Persons interviewed included scientists involved in vaccine research and development; MoH officials responsible for development of national policies and goals; and, provincial health staff responsible for planning and implementing VPD control activities.
3. Results
3.1. Pre-EPI era (1949–1978)
Work on immunizations began in earnest as soon as the People's Republic of China was founded and more than two decades before establishment of the World Health Organization's (WHO) Expanded Programme on Immunizations (EPI). In 1949, only four small vaccine manufacturers existed in China (Beijing, Shanghai, Lanzhou, Changchun) and developing a secure vaccine supply was a high priority. Manufacturing facilities were built in Wuhan (1950) and Chengdu (1953) to create a network of six regional manufacturers under MoH supervision responsible for producing vaccines for the entire country. In 1950, a plan was developed to eradicate smallpox nationwide through free mass compulsory vaccination of the entire population. By the end of 1952, MoH announced that over 200 million people (about 44% of total population) had been vaccinated and smallpox rapidly disappeared from most of the country with the last case occurring in 1960 [Fig. 1 ]. Building on this success, the State Council issued a directive in 1953 requiring establishment of a network of Epidemic Prevention Stations (EPS) at province, prefecture and county levels, with VPD control a main responsibility. Directives on vaccination of children with diphtheria toxoid and Bacillus Calmette Guérin (BCG) were issued in 1953 and 1954, respectively, and research was accelerated to develop vaccines against diseases responsible for high mortality including polio, measles, and JE.
Fig. 1.
Annual incidence of selected vaccine-preventable diseases in China between 1950 and 2016. Data are shown per 100,000 population for (A) smallpox, (B) diphtheria, (C) pertussis, (D) measles, (E) poliomyelitis, (F) meningococcal meningitis, (G) Japanese encephalitis, (H) hepatitis A. Shaded areas are pre-vaccine era.
Oral polio vaccine (OPV) were developed in 1960 and was the first attenuated vaccine developed in China. The Institute of Medical Biology, Academy of Medical Sciences was established in Kunming, to scale-up OPV production. In 1962, MoH issued “Measures on Implementing Preventive Vaccination” requiring that all provinces conduct annual winter campaigns to vaccinate children with smallpox, BCG, diphtheria, pertussis, and polio vaccines. In the early of 1960s, combined diphtheria-tetanus-pertussis toxoid (DTP) was introduced. Three attenuated measles vaccine strains, Beijing-55, Shanghai-191, and Changchun-47, were developed and introduced in 1965.
In contrast to the nationally synchronized smallpox campaigns, campaigns with other antigens were organized at the province-level and conducted during colder months since most vaccines were in liquid formulation with limited thermostability and there was no cold chain system. Annual meetings were held by MoH with provincial health departments and vaccine manufacturers to prioritize limited supplies. To expedite delivery before the vaccines lost potency, a highly coordinated “rush-relay” transport approach was developed; when vaccines were shipped by the manufacturer, usually by train or truck, the provincial EPS was notified by telephone or telegraph when the vaccines would arrive and to mobilize of all forms of transport (motor vehicles, bicycles, pack animals, health staff) to move the vaccines as rapidly as possible to the periphery. Local cold storage solutions included use of refrigerators at food processing plants, wells, cellars, burial under-ground, and wooden boxes built with separate compartments for bottles of frozen water or pieces of ice. Usually vaccines were transported from one administrative level to the next within a day and campaigns were completed in a single day. Limited vaccine supplies precluded province-wide campaigns, so each province was divided into sections targeted on a rotating basis over a five to six-year period, with each campaign targeting all children under 7 years old. As a result, by school entry most children had received only one or two doses each of diphtheria, pertussis and polio vaccines. While killed vaccines and toxoids could remain potent for up to 30 days, OPV and measles were live attenuated vaccines and lost potency within one week, limiting their use to urban areas and impacts. In 1959, a measles epidemic coinciding with rural famine during the Great Leap Forward affected nearly 11 million persons and caused 262,000 deaths; during 1963–1966, epidemic poliomyelitis spread throughout the country with 130,000 cases reported [Fig. 1].
In 1965, Mao Zedong's “June 26 Directive” called for renewed focus on rural health. Civil unrest at the start of the Cultural Revolution, however, disrupted vaccine work. During 1965–1966, outbreaks of poliomyelitis, diphtheria, pertussis and measles started to recur in areas where the diseases had previously been controlled and incidence began to rise, and mass movement of students facilitated transmission of meningococcal meningitis group A resulting in an epidemic in 1965–1966 that affected over 3 million persons and caused 167,000 deaths [Fig. 1]. In the early 1970s, a number of important public health reforms were instituted that had greatly strengthened VPD control efforts. In 1972, the Rural Cooperative Medical System (RCMS) was established with commune-level health stations staffed by a new cadre of part-time peasants (“barefoot doctors”) who received three months of training in delivery of basic medical and preventive services, including vaccinations. These grass-roots health workers were responsible for transporting vaccines from county and commune hospitals to their villages and administering vaccinations, which were essentially free with delivery costs covered by pooled RCMS funds. Vaccine production was increased and the frequency of campaigns were increased with most provinces conducting at least two or three province-wide campaigns each year; live vaccines in the fall and winter and killed vaccines in the spring. The largest absolute reductions in VPD burden in China occurred from 1970 to 1979 when the RCMS was established with incidence per 100,000 population dropping 48% from 3.3 to 1.8 for diphtheria, 50% from 152.2 to 49.4 for pertussis, 77% from 2.5 to 0.6 for polio, 60% from 450.5 to 178.3 for measles, and 72% from 18.0 to 5.1 for JE, averting an average of 4 million VPD cases each year [Fig. 1]. Buoyed by these gains, in 1975, MoH issued the “Ten-Year Plan to Control and Eradicate Poliomyelitis, 1975-1985” setting a goal of zero cases by 1986.
3.2. EPI era (1979–2001)
In 1974, the People's Republic of China was recognized by the United Nations as the sole representative of China allowing for broader international exchange. In 1978, the State Council passed the “Law on Acute Infectious Diseases” required establishment of a national Expanded Programme on Immunizations (EPI) based on WHO guidelines by 1980. During 1980–1985, around one million village and township health staff receiving EPI in-service training using translated WHO materials. An infant schedule for BCG, OPV, DPT and measles vaccines was established and widely popularized through the slogan “Four vaccines, Six diseases (四苗六病)”. Direct technical cooperation with WHO and UNICEF was resumed in 1981 with a pilot vaccine cold chain project in five southern provinces, and later extended to 14 more provinces. In 1982, MoH set EPI coverage targets of 80%–90% for BCG and DPT and 90%–95% for OPV and measles by school-age by 1990, assigned a full-time person in the Department of Disease Control to manage the EPI, and established the National Center for Preventive Medicine (re-structured as the Chinese Academy for Preventive Medicine in 1985 and Chinese Center for Disease Control in 2002) to provide provincial EPSs with technical guidance and support.
In support of the 1985 United Nations resolution on Universal Childhood Immunization (UCI), “85-85” coverage goals were included in China's “7th 5-year Plan for National Social and Economic Development, 1986–1990” setting targets of ≥85% percent coverage at province-level with BCG, DPT, OPV and measles by 12 months of age by 1988, and ≥85% coverage at county-level by 1990. While the UCI goals were lower than China's 1982 coverage targets and excluded JE and meningococcal meningitis, the target age for being fully vaccinated was much younger – 12 months instead of 6 years of age. Administering the entire primary series of BCG, DPT, OPV and measles vaccines during infancy would require at least six vaccination sessions per year nationwide, ability to delivery vaccinations year-round, and huge investments to extend the cold chain to township levels nationwide. The first coverage survey in China was conducted in 11 provinces with assistance from WHO in 1983 highlighted the magnitude of the challenge [Table 1 ].
Table 1.
Results of sub-national and national coverage surveys in China, 1983–2013.
| Year | Number of provinces | Age-group (months) | Number children | Percent coverage by vaccine dose |
|||
|---|---|---|---|---|---|---|---|
| BCG | DTP3 | OPV3 | MV1 | ||||
| 1983 | 11 | 12–23 | 45,686 | 34 | 58 | 79 | 78 |
| 1984 | 19 | 12–23 | 154,839 | 50 | 63 | 78 | 74 |
| 1985 | 11 | 12–23 | 130,482 | 79 | 78 | 87 | 89 |
| 1986 | 25 | 12–23 | 53,292 | 70 | 62 | 68 | 63 |
| 1987 | 30 | 12–23 | 67,878 | 85 | 75 | 78 | 77 |
| 1989 | 31 | 12–23 | 48,346 | 98 | 95 | 95 | 95 |
| 1991 | 31 | 12–23 | 50,411 | 99 | 98 | 97 | 98 |
| 1999 | 31 | 12–23 | 25,878 | 95 | 95 | 95 | 94 |
| 2004 | 31 | 12–35 | 155,954 | 97 | 94 | 93 | 92 |
| 2013 | 31 | 12–35 | 1,536,675 | 98 | 98 | 98 | 95 |
To achieve the UCI goals, a high-level inter-ministerial leading group consisting of MoH, Ministry of Foreign Economic Relations and Trade, Ministry of TV and Broadcasting, State Education Commission, State Ethnic Affairs Commission, and the All China Women’s Federation was formed to oversee planning and progress. April 25 was established as “National Vaccination Day” and huge investments were made in cold chain, training, social mobilization, and vaccines. By the early 1980s most of the EPI vaccines were lyophilized and more thermostable, enabling delivery to more remote areas. By 1985, annual production had increased to 110 million doses each of OPV, DPT, and measles, and 80 million doses of BCG. In 1989, the National People’s Congress passed a law requiring health authorities at all levels implement a system of planned preventive immunizations that included issuing vaccination certificates to all children and establishing registers to monitor vaccination coverage at township levels and above. With tremendous effort, both “85-85” targets were achieved despite large areas of the country still having no cold chain. The first nationwide coverage survey, conducted in 1989 to verify achievement of the UCI goal documented coverage of 92% with all recommended BCG, DPT, OPV and measles doses by 12 months of age [Table 1]. Polysaccharide meningococcal meningitis group A and trivalent OPV were introduced in 1980 and 1985, respectively, and between 1980 and 1989 the incidence of meningococcal meningitis fell 94%, from 23.3 to 1.3 per 100,000 while the incidence of poliomyelitis fell 45%, from 0.76 to 0.42 per 100,000 [Fig. 1].
Incidences of other target diseases also continued to fall during this period. By 1988, <700 cases of poliomyelitis were reported nationwide, although low levels of transmission persisted and the target for national elimination by 1986 was missed. In support of the 1988 World Health Assembly resolution to eradicate poliomyelitis globally by 2000, the MoH issued the “1988–1995 National Plan for Eradication of Poliomyelitis”, setting targets of <1 per 10 million by 1992, and zero cases by 1995, with the main strategy supplementary campaigns with OPV. During 1990–1992, 362 million OPV doses were administered in 44 province-wide campaigns. In 1993, synchronized nationwide campaigns were approved by the State Council targeting all children <4 years of age with two doses of OPV, one dose on December 5 and one dose on January 5 for three consecutive years. The first campaign was conducted in winter 1993/1994 with 148 million OPV doses administered and the last case of polio occurred in September 1994.
Despite EPIs high profile, financing for immunizations became progressively weaker starting in the 1980s as a result of broad market reforms. Farm collectives and the RCMS were dismantled, and while EPI vaccines were still provided for free, funding for vaccine delivery switched to fee-for-service, usually 1–2 RMB paid by parents to the village or township doctor for each dose administered. Public health departments were largely left to generate their own operating expenses. In some regions, innovative financing mechanisms were developed to secure additional funding for immunizations, such as the “EPI Contract”, a lump-sum payment by parents to cover the cost of all EPI vaccinations that was pooled and divided between village, township and county-levels to cover delivery costs, as well as an indemnity payment if a child developed a VPD against which he or she had been vaccinated. By 2003, however, central government funds accounted for only 1% of total immunization expenditures while more than 50% of expenditures were at village and township levels, resulting in falling coverage and large disparities between more and less developed areas. In 2004, coverage in children 12–23 months old with all recommended doses of BCG, DPT, OPV and measles had fallen below 85% in nine (32%) provinces, including seven of the 12 poorer western provinces.
Despite the challenges financing the immunization program, China was one of only two low-income countries (the other was Cuba) to introduce hepatitis B vaccine into their EPI when universal infant immunization with hepatitis B vaccine was endorsed by the World Health Assembly in 1992. At that time, China had the largest disease burden of hepatitis B in the world with approximately 10% of the population chronically infected. Adding hepatitis B vaccine would have tripled government vaccine costs per child from 4.5 to 12.6 RMB, so market strategies were also used to finance introduction of this expensive new vaccine. The government added hepatitis B to the EPI but exceptionally allowed the vaccine cost to be passed to parents. This cost-recovery approach provided a strong incentive to health workers to deliver the vaccine and national coverage with three doses of hepatitis B vaccine in infants increased from 30% in 1992, to 84% in 2001, preventing millions of infections and averting hundreds of thousands of deaths. Coverage, however, was predictably much lower in less developed areas, particularly the western provinces.
3.3. National Immunization Program (2002–present)
In 2002, the EPI was re-organized and renamed the China National Immunization Program (NIP) to reflect work on a growing range of programmatic issues such as vaccine safety, equity, financing, accelerated measles and hepatitis B control initiatives, and new vaccine introductions. China was one of the first countries to apply to the newly created Global Alliance for Vaccines and Immunizations (GAVI) for new vaccine support and the 2002–2006 China-GAVI Project was launched with US$ 38 million from GAVI (at the time the maximum grant that could be awarded to a single country) and matching US$ 38 million from the central government to provide free hepatitis B vaccine to all infants in the 12 western provinces and all national poverty counties. At the same time, governments in the other 19 provinces were required to cover hepatitis B vaccine costs making the vaccine free nationwide, in line with all the other EPI antigens. The removal of price barriers in poor areas had marked effects, and from 1992–2001 to 2002–2005, infant coverage with three doses of hepatitis B vaccine in the western provinces doubled from 34% to 79%.
The 2003 outbreak of Severe Acute Respiratory Syndrome (SARS) highlighted weaknesses in China's public health system and a number of new initiatives were launched to strengthen public health and EPI. In 2003, the New Rural Cooperative Medical Scheme (NRCMS) was launched, a subsidized risk-pooled fund primarily for rural medical care. In 2004, the central government for the first-time allocated funds for vaccine delivery and administration at village and township level. In 2004–2005, the State Council passed the Law on the Prevention and Treatment of Infectious Diseases reaffirming requirements for all children to be fully vaccinated, and the Regulation on Vaccine Circulation and Immunization that included laws specifying that nationally recommended vaccines be fully funded by the government and administered completely free-of-charge. In 2008, the immunization schedule was expanded to include new antigens (measles-mumps-rubella, attenuated hepatitis A), safer products (acelluar pertussis), and older vaccines that had been excluded from the original EPI schedule (JE, meningococcal meningitis) and EPI vaccine procurement was centralized with MoH responsible for procuring all EPI vaccines. In 2009, the government launched the New Essential Public Health Service Package with 11 service categories, including immunizations. Government subsidies of 15 RMB per person were provided to village and township-administrative levels to cover the cost of immunization services, and increased to 25 RMB per person in 2011, 30 RMB in 2014, 35 RMB in 2015, and 40 RMB in 2016. Currently, all village and townships have the full-time health staff that provide vaccination services to all children at 157,000 delivery sites nationwide. There are approximately 20,000 EPI managers at provincial, prefecture and county levels and 550,000 immunization staff at township and village levels. With the majority of China's population now living in urban areas, vaccination has shifted from pulse delivery by village-based health workers to predominantly daily delivery at fixed clinic sites.
Public health reforms during the past decade have strengthen financing for vaccines and vaccination services resulting in increased and more equitable coverage and historically low VPD burden [Table 1, Fig. 1]. In the 2013 national survey, coverage of recommended infant doses of BCG, DPT, OPV, hepatitis B and measles vaccines was ≥85% in 30 of 31 provinces, and ≥90% in 28 province, a major improvement from 2004. From 2002 to 2016, the incidence of pertussis fell 49% from 4.9 to 2.5 per million, measles fell 63% from 4.8 to 1.8 per 100,000, meningococcal meningitis fell 95% from 1.9 to 0.1 per million, JE fell 86% from 6.5 to 0.9 per million, and hepatitis A fell 80% from 8.1 to 1.6 per 100,000. The last case of diphtheria was reported in 2006. In 2012, China was certified as having eliminated maternal and neonatal tetanus and an all-time low of 6183 measles cases were reported nationwide, although numbers of measles cases increased in subsequent years. From 1992 to 2014, the prevalence of chronic hepatitis B infection in children under-5 years old dropped from 9.7% to 0.3%, a 97% decline.
4. Discussion
Sustained political commitment to immunizations at the highest levels, ambitious targets, strong supporting legal frameworks, a vibrant domestic vaccine industry, and innovative financing and delivery strategies to maximize use of available resources have been key to China’s many achievements in VPD control over the past 65 years. Immunizations has been a universal right since the founding of the People's Republic of China, and have been supported at the highest political levels with passage of laws ensuring access and progressively increasing levels of public finance as the country has developed. Goals for universal childhood immunization, smallpox eradication, and poliomyelitis eradication were established in China before comparable UN global resolutions and before there was an established cold chain system. Self-reliance has been a defining characteristic stimulating the development of innovative strategies to maximize use of limited resources, including mass training of village-based lay health workers to deliver vaccinations, cost-sharing strategies to finance introduction of new vaccines, large-scale social mobilization, use of rapid pulse-delivery approaches to leverage existing vaccine thermostability in areas without cold chain, support for domestic vaccine production, and rapid national scale-up of successful pilots. Currently, there are seven state-owned and 38 private vaccine manufacturers in China with annual production capacity of around 1 billion doses, including acellular pertussis, influenza, rabies, yellow fever, Japanese encephalitis, hepatitis A and B, rubella, varicella, typhoid, and live and inactivated polio vaccines (IPV). All vaccines recommended in the national immunization schedule are domestically produced.
Globally, vaccination coverage is stagnating with DPT3 coverage 85% since 2010, and 72% in the African Region [21]. Progress toward the 2020 Global Vaccine Action Plan 90% coverage target is off- track, and in 2017, nearly 20 million infants were not vaccinated [22]. Many of these children are socioeconomically marginalized, live in fragile or remote settings, and have limited access to health care. A delivery model that relies primarily on nurses and clinical officers to administer vaccinations at fixed sites and provide periodic outreach is costly, places a large burden on parents, and likely to be insufficient to achieve and sustain high vaccination coverage levels in resource-poor countries with large dispersed rural populations [23]. China has more than half a century of experience successfully using community-based health workers to periodically come to clinics to collect vaccines in cold boxes to take back to their villages for pulse administration increasing rural access, community buy-in, and sustained high coverage levels. More widespread adoption of alternative service delivery approaches may be needed to close remaining coverage gaps.
Use of vaccine vial monitors that measure cumulative heat exposure and development of pre-filled injection devices further facilitate ease and safety of vaccination by community health workers and could also help address cold-chain challenges in remote areas [24]. In 2017, MenAfriVac® conjugate meningococcal meningitis A vaccine was the first vaccine prequalified by WHO for use outside the cold chain in controlled temperature chain (CTC); use of CTC for a mass vaccination campaign in Chad would have reduced logistics costs by an estimated 50% [25]. In China, in villages where village health workers were provided with hepatitis B vaccine with storage at ambient temperatures at the beginning of hepatitis B vaccine introduction, timely birth dose coverage in infants born at home increased from 3% to 52%, with no difference in antibody response compared to newborns who were vaccinated with hepatitis B kept in the cold chain [26]. Recent economic analyses indicate that CTC delivery of the hepatitis B birth dose would be cost-saving in most low and middle-income countries [27].
Disease control has always been the main goal of China's immunization efforts and disease incidence has always been the main metric used to guide development of immunization strategies and to assess immunization program performance. Close monitoring of temporal, geographic, and demographic trends in VPD incidence has been critical in China for identifying under-immunized populations and for evaluating the effectiveness of delivery strategies, even when the majority of reported cases are primarily clinically confirmed. In contrast, EPIs in most low-income countries primarily rely on administrative coverage despite recognized problems with data quality and reliability [28], [29]. The 2014–2016 Ebola outbreak in West Africa has spurred new initiatives to strengthen communicable disease surveillance and more effective use of surveillance data by immunization program managers, including district and sub-district mapping of disease incidence, could strengthen program monitoring and accountability.
China's experience with hepatitis B vaccine is an interesting case study on use of cost-sharing to finance the introduction of new vaccines that may be of relevance to middle-income countries ineligible for GAVI support. When WHO recommended universal infant immunization with hepatitis B vaccine in 1992, GDP per capita in China was only US$ 365. Adding hepatitis B vaccine to the infant schedule while allowing health workers to recover the vaccine costs from parents enabled early introduction without external or government financing, and provided a delivery incentive that achieved 70% coverage nationwide, preventing millions of infections. China's experience suggests that cost-recovery can be an effective interim option for countries to finance the early introduction of expensive new vaccines, particularly if the government can negotiate lower purchase prices, set caps on allowable charges, and provide subsidies for the poor.
China also has much to learn from experience in other countries. Challenges include strengthening delivery of immunizations within a larger package of integrated health services, balancing policies that make vaccines affordable against those providing incentives for new vaccine research and development, financing new vaccine introductions, and vaccine hesitancy. For many years, vaccination was one of the few preventive services that village and township doctors were required to deliver and China's immunization program is facing the challenges of navigating integration of immunization with delivery of a much wider range of other services. Introduction of new expensive vaccines into the recommended schedule remains a challenge. The current infant schedule requires 11 separate injections and there is urgent need for increased funding to develop and add combined products to the schedule, such as DPT-hepatitis B, DPT-hepatitis B-Haemophilus influenzae type B (Hib), and DPT-hepatitis B-Hib-IPV. Finally, while public trust in immunizations remains high, China has not been immune to problems of vaccine hesitancy. Widespread internet access has facilitated rapid dissemination of often unfounded claims of harmful effects due to vaccination that have had negative effects on vaccination coverage [30]. These and other challenges will require new approaches as China's immunization program continues moving forward.
Funding
This work was supported by United Nations Children's Fund for the literature review and the in-depth interviews of Chinese immunization experts (YH702-2013).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Drs. Yu Jingjin, Director General, Department of Disease Control (DDC), National Health and Family Planning Commission (NHFPC), Lei Zhenglong (Deputy Director General, DDC, NHFPC), Li Qaunle and Lu Ming (DDC, NHFPC), and Yang Baoping (WHO Representative, Fiji and China's first EPI Program Manager, MoH) for providing historical information on China's EPI; senior members of China's Advisory Committee on Immunizations Dr. Wang Zhao, Head, China Hepatitis Foundation and previously, Director General, DDC, MoH, Professor Zhao Kai, Head Scientist, Beijing Biological Product Institute, and Drs. Diao Liandong, Jiangsu CDC, Dai Zhenwei, Anhui CDC, Chu Jingui, Hebei CDC, Xu Aiqiang, Shandong CDC who played key roles in guiding development of China's VPD control efforts since the 1950s and providing in-depth insight into China's immunization work during those formative years; and, Drs. Ma Qianli (Sichuan CDC) and Zhang Siyu (Hunan CDC) for conducting the expert interviews and summarizing the results.
References
- 1.Wang Guoqiang. China Population Publishing House; Beijing: 2015. A 60-year history of disease prevention and control in China; pp. 257–305. [Google Scholar]
- 2.Kung F.C. New China’s achievements in health work. Chin Med J. March 1953:7187–7192. [Google Scholar]
- 3.Chinese Communist Party. Constitutional principles of the Shaanxi-Gansu-Ningxia Border Region. 23 April 1946 [Chinese].
- 4.Dai Z.C., Qi G.M. Beijing; Beijing Science and Technology Press: 1997. Viral hepatitis in China. Seroepidemiological survey in Chinese population (Part one) 1992–1995; pp. 39–58. [Chinese] [Google Scholar]
- 5.Liang X., Bi S., Yang W., Wang L., Cui G., Cui F. Epidemiological serosurvey of hepatitis B in China – declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–6557. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Cui F., Shen L., Wang H., Wang F., Bi S., Liu J. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy. China Emerg Infect Dis. 2017;23:765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. The EPI Coverage Survey. Geneva: World Health Organization, Expanded Programme on Immunization. October 1991. WHO/EPI/MLM/91.10.
- 8.Government Administrative Council of the People's Republic of China. Regarding the launching of the Autumn campaign for smallpox vaccination. 12 October 1950 [Chinese].
- 9.Ministry of Health of the People's Republic of China. Measures for implementing preventive vaccination. March 1962 [Chinese].
- 10.Ministry of Health of the People's Republic of China. Regulations on acute infectious diseases, number 1222. 20 September 1978 [Chinese].
- 11.Ministry of Health of the People's Republic of China. Report of the survey of the third 85% vaccination coverage target of the national Expanded Programme on Immunizations. 1996:1–91 [Chinese].
- 12.Ministry of Health of the People's Republic of China. Compilation of national immunization documents: 1980s National File. Report of the survey of the first 85% vaccination coverage target of the national Expanded Programme on Immunizations. 1989:333–9 [Chinese].
- 13.Ministry of Health of the People's Republic of China. Report of the 1999 national review of the Expanded Programme on Immunizations. May 2000 [Chinese].
- 14.Ministry of Health of the People's Republic of China . People’s Health Publishing House; Beijing: 2005. Report of the 2004 national review of the Expanded Programme on Immunizations; pp. 136–137. [Chinese] [Google Scholar]
- 15.State Council of the Peoples's Republic of China. Regulations of vaccine distribution and vaccination, number 434. 24 March 2005 [Chinese].
- 16.Ministry of Health of the People's Republic of China. Report of the 2013 national review of the Expanded Programme on Immunizations. March 2014 [Chinese].
- 17.National Certification Committee for the Eradication of Poliomyelitis in the People's Republic of China. Documentation for the certification of poliomyelitis eradication. October 2000, p. 114.
- 18.Hubei Province Epidemic Prevention Station Expanded programme on Immunization contract system tested. Wkly Epidem Rec. 1987;20:142–143. [Google Scholar]
- 19.Yu W., Jin S., Cui G., Yu J., Wang J., Tao Z. Study on financing the Expanded Program on Immunizations in selected regions of China. Chin. J Immun. 2005;8:292–297. [Chinese] [Google Scholar]
- 20.Yu W., Lu M., Wang H., Rodewald L., Ji S., Ma C. Routine immunization services costs and financing in China, 2015. Vaccine. 2018;36:3041–3047. doi: 10.1016/j.vaccine.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Progress and challenges with achieving Universal Immunization Coverage. 2017 WHO/UNICEF estimates of national immunization coverage (data as of July 2018) http://www.who.int/immunization/monitoring_surveillance/data/en (accessed September 24, 2018)
- 22.World Health Organization . World Health Organization; Geneva: 2017. 2017 Assessment report of the Global Vaccine Action Plan Strategic Advisory Group of Experts on Immunization; p. 32. [Google Scholar]
- 23.Geng F., Suharlim C., Brenzel L., Resch S.C., Menzies N.A. The cost structure of routine infant immunization services: a systematic analysis of six countries. Health Policy Planning. 2017;32:1174–1184. doi: 10.1093/heapol/czx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen D.D., Lorenson T., Bartholomew K., Villadiego S. Can thermostable vaccines help address cold-chain challenges? Results from stakeholder interviews in six low- and middle-income countries. Vaccine. 2016;34:899–904. doi: 10.1016/j.vaccine.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lydon P., Zipursky S., Tevi-Benissan C., Djingarey M.H., Gbedonou P., Youssoufe B.O. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull World Health Organ. 2014;92:86–92. doi: 10.2471/BLT.13.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L.X., Li J.H., Chen H.P., Li F.J., Armstrong G.L., Nelson C. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold-chain delivery strategy. Bull World Health Organ. 2007;85:688–694. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott N., Palmer A., Morgan C., Lesi O., Spearman C.W., Sonderup M. Cost-effectiveness of the controlled temperature chain for the hepatitis B virus birth dose vaccine in various global settings: a modelling study. Lancet Glob Health. 2018;6:e659–e667. doi: 10.1016/S2214-109X(18)30219-5. [DOI] [PubMed] [Google Scholar]
- 28.Lim S.S., Stein D.B., Charrow A., Murray C.J. Tracking progress towards universal childhood immunisation and the impact of global initiatives: a systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet. 2008;372:2031–2046. doi: 10.1016/S0140-6736(08)61869-3. [DOI] [PubMed] [Google Scholar]
- 29.Ronveaux O., Rickert D., Hadler S., Groom H., Lloyd J., Bchir A. The immunization data quality audit: verifying the quality and consistency of immunization monitoring systems. Bull World Health Organ. 2005;83:503–510. [PMC free article] [PubMed] [Google Scholar]
- 30.Yu W., Liu Dawei, Zheng Jingshan. Loss of confidence in vaccines following media reports of infant deaths after hepatitis B vaccination in China. Int J Epidemiol. 2016;45:441–449. doi: 10.1093/ije/dyv349. [DOI] [PubMed] [Google Scholar]