Highlights
-
•
Protollin-adjuvanted H5N1 vaccine enhanced serum protective antibody responses and mucosal IgA responses.
-
•
Protollin-adjuvanted H5N1 vaccine increased the early B cell response in the lymph nodes and spleen.
-
•
Protollin-adjuvanted H5N1 vaccine increased the frequency of Ag-specific antibody secreting cells and T cells.
-
•
Protollin-adjuvanted H5N1 vaccine conferred enhanced protection against viral challenge.
Keywords: Protollin, H5N1 vaccines, Mucosal immunity, Cell-mediated immunity, Antibody responses, Protective immunity, Mouse model
Abstract
Sporadic, yet frequent human infections with avian H5N1 influenza A viruses continue to pose a potential pandemic threat. Poor immunogenicity of unadjuvanted H5N1 vaccines warrants developing novel adjuvants and formulations as well as alternate delivery systems to improve their immunogenicity and efficacy. Here, we show that Protollin, a nasal adjuvant composed of Neisseria meningitides outer membrane proteins non-covalently linked to Shigella flexneri 2a lipopolysaccharide, is a potent nasal adjuvant for an inactivated split virion H5N1 clade 1 A/Viet Nam1203/2004 (A/VN/1203/04) vaccine in a mouse model. Protollin-adjuvanted vaccines elicited enhanced serum protective hemagglutination inhibition titers, mucosal IgA responses, and H5N1-specific cell-mediated immunity that resulted in complete protection against a lethal challenge with a homologous virus as well as a heterologous clade 2 virus A/Indonesia/05/2005 (A/IN/05/05). Detailed analysis of adaptive immunity revealed that Protollin increased the frequency of lymphoid- as well as local tissue-resident antibody-secreting cells, local germinal center reaction of B cells, broad-spectrum of CD4 T cell response. Our findings suggest that nasal delivery of H5N1 vaccine with Protollin adjuvant can overcome the poor immunogenicity of H5N1 vaccines, induce both cellular and humoral immune responses, enhance protection against challenge with clade 1 and clade 2 H5N1 viruses and achieve significant antigen dose-sparing.
1. Introduction
The world has already experienced three influenza pandemics in the 20th century in addition to the recent 2009 pandemic caused by swine-origin H1N1 virus, which has taught us how newly emerging pathogens can be a risk to global populations [1]. Highly pathogenic H5N1 avian influenza viruses continue to be prime candidates for the next influenza pandemic, as they have steadily caused fatal infections in the human population [2]. So far, direct human-to-human transmission appears to be infrequent; however, the accumulation of mutations may break this genetic barrier and generate an H5N1 virus that is transmissible among humans potentially causing pandemic. The inherently poor immunogenicity of unadjuvanted H5N1 influenza vaccines warranted efforts to explore novel adjuvants and alternate delivery systems to improve immunogenicity and protective efficacy of H5N1 vaccines.
Intradermal (i.d.), oral and nasal delivery of vaccines represent alternative routes to conventional intramuscular (i.m.) delivery [3]. Unlike i.m. and i.d. routes, oral and nasal deliveries are non-invasive and needle-free that are known to induce mucosal and systemic immune responses [4]. Since degradation of antigens by proteolytic enzymes, poor absorption, need for large doses of antigen, and concerns about tolerance still remain as challenges for oral delivery of non-replicating vaccine antigens [5], the nasal route has become an attractive option for vaccination, particularly against respiratory pathogens as the respiratory tract is equipped with pathogen sensing defense mechanisms, has a large surface area for absorption, and is highly vascularized [6], [7]. A live attenuated seasonal influenza vaccine, which is delivered intranasally (LAIV, FluMist®, Fluenz™) (MedImmune AstraZenica) is approved for healthy individuals of 2–49 years of age in the US. However, the absence of correlates of immunity, cold-storage requirement, and potential of genetic recombination with seasonal influenza viruses may limit the use of LAIV as a pre-pandemic influenza vaccine [8]. Therefore, an ideal H5N1 vaccine formulated/adjuvanted for nasal delivery should induce mucosal as well as systemic serological and cellular immune responses that can correlate with protection. Protollin, a nasal adjuvant composed of Neisseria meningitidis outer membrane proteins (OMPs) non-covalently complexed to Shigella flexneri 2a lipopolysaccharide (LPS) activates the innate immune system via activation of TLR2 and TLR4 by OMP and LPS respectively. Protollin has been shown to induce effective systemic as well as mucosal antibody responses in preclinical studies when administered with several viral antigens such as measles, SARS corona virus, RSV, and recombinant plague antigen F1-V [9], [10], [11], [12], [13]. Therefore, Protollin represents an attractive adjuvant suitable for nasal delivery of pre-pandemic H5N1 vaccines. In this study, we evaluated the immunogenicity and protection against challenge conferred by Protollin-adjuvanted H5N1 inactivated split vaccine.
2. Methods
2.1. Reagents and formulations
H5N1 Monovalent Influenza split vaccines (A/VN/1203/04 and A/IN/05/05) were provided by GlaxoSmithKline Vaccines (Ste-Foy, Quebec, Canada). Protollin from GSK Vaccines consists of proteosomes (outer membrane proteins derived from wild-type Neisseria meningitidis group B) noncovalently complexed in approximately a 1:1 ratio with lipopolysaccharide (LPS) isolated from Shigella flexneri serotype 2a. The amounts of Protollin were expressed as μg of LPS and the amount of H5N1 vaccine was expressed as μg of HA.
2.2. Viruses
Two virus strains were made by reverse genetic engineering, HA and NA from clade 1 A/Viet Nam/1203/2004 (A/VN/1203/04) or clade 2 A/Indonesia/05/2005 (A/IN/05/05) and the remaining six gene segments from A/Puerto Rico/8/1934-(PR8). Viruses were propagated in 10-day old embryonated chicken eggs for 48 h. Pooled allantoic fluid was clarified by centrifugation, aliquoted, and stored at −80 °C until use.
2.3. Nasal immunization and challenge of mice
Female BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) 6 weeks of age were anesthetized with intraperitoneal injection (i.p.) of 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma-Aldrich, St. Louis, MO) before nasal inoculation with 3, 1, or 0.3 µg of H5N1 vaccine, either alone or with 2 µg of Protollin [10], [14] in a final volume of 50 µl administered slowly drop by drop at 25 µl each per nostril. Both the vaccine antigens and adjuvants were mixed prior to immunization. Mice nasally administered with adjuvant alone were used as negative controls. Four weeks later, mice received a second dose of the same vaccine preparation. One week after boost, 5 mice were euthanized for collection of spleen and bone marrow tissues to assess adaptive immunity. Bronchoalveolar lavages (BAL) and nasal lavages were also performed at the same time by injecting 1.0 ml PBS with protease inhibitors and re-collecting the fluid to measure mucosal antibody response by ELISA. Mice were bled 3 weeks post-primary and again 3 weeks post-boost to measure hemagglutination inhibition (HI) titers using horse red blood cells. Four weeks after boost, 5 mice from each group were challenged with 5 LD50 of A/VN/1203/04 or A/IN/05/05 viruses. Animal were monitored daily for morbidity by changes in body weight and any mouse that lost >25% of pre-infection body weight was euthanized. Animal research was conducted under the guidance of the CDC’s Institutional Animal Care and Use Committee.
2.4. Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). The Student’s t test was used to analyze differences in antibody isotype between two groups. One-way analysis of variance with Bonferroni post analysis was used to analyze differences among treatments. The Mann-Whitney test was used to determine significance among HI titers. Finally, the Logrank (Mantel-Cox) test was used to compare percent survival among groups of mice. All differences were considered statistically significant when the p-value was ≤0.05.
3. Results
3.1. Protollin enhanced protective efficacy of H5N1 split vaccine
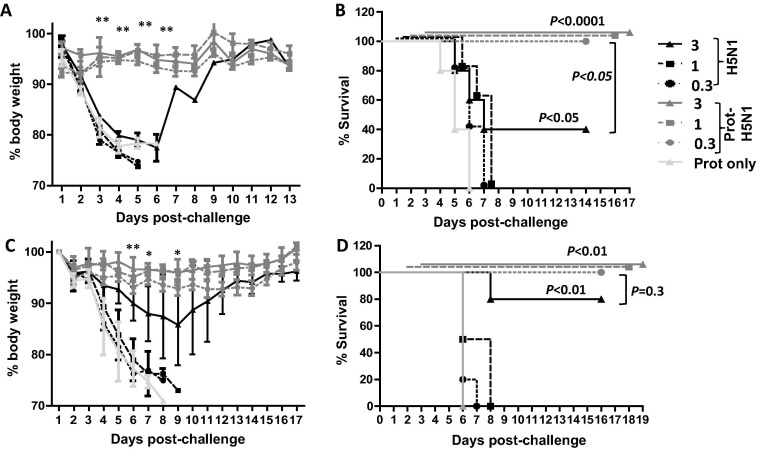
To investigate the adjuvant effect of Protollin, mice were nasally administered with 3, 1, or 0.3 µg of H5N1 vaccine with or without 2 µg of Protollin using a prime-booster regime and then challenged with homologous A/VN/1203/04 viruses. All mice immunized with 1 or 0.3 µg of H5N1 vaccine alone succumbed to challenge between day 5 and 6 post-challenge, while 40% of mice that received the highest dose of vaccine (3 µg) initially showed significant weight loss but eventually survived challenge (Fig.1 A and B). All mice immunized with vaccine plus Protollin including mice that received the lowest vaccine antigen dose (0.3 µg), survived and no significant weight loss was observed (Fig.1A and B). These data demonstrate that Protollin significantly enhanced the protective efficacy of H5N1 vaccine.
Fig. 1.
Protollin enhanced protective efficacy of H5N1 vaccine against homologous as well as heterologous virus challenge. Balb/c mice (10 mice/group) were nasally administered with H5N1 split virion vaccine antigen (0.3, 1, 3 µg based on HA content) with or without 2 µg Protollin (based on LPS content) and a month later, booster immunized with the same vaccine formulations. Control mice received Protollin only at both time points. Four weeks following booster immunization, the mice were lethally infected with 5 × LD50 of A/VN/1203/04 (H5N1) (A and B) or A/IN/05/05 (H5N1) (C and D) virus. Mice were weighed every day to monitor body weight changes (A and C) and mortality (B and D). Mice that lost more than 25% body weight were euthanized and scored as a fatality. One-way analysis of variance with Bonferroni post analysis was used to compare the percentage of body weight changes and the Logrank (Mantel-Cox) test was used to compare percent survival among groups of mice. n = 5 mice for each group from 2 independent experiments and the error bars represent standard error of the mean (SEM).
To test whether the Protollin-mediated protection extended against cross-clade H5N1 virus, H5N1 vaccine-immunized mice were challenged with the clade 2 virus, A/IN/05/05. Mice immunized with either Protollin or vaccine alone (0.3 or 1 µg) succumbed to challenge (Fig.1C and D). Among the mice immunized with 3 µg vaccine alone, 80% of mice showed 15% weight loss and eventually recovered and survived. However, Protollin-adjuvanted vaccine, even at the lowest vaccine dose (0.3 µg) significantly reduced weight loss and completely protected mice against the lethal challenge (Fig.1C and D). Therefore, Protollin also enhanced cross-clade protection with significant antigen dose-sparing.
3.2. Protollin enhanced protective antibody responses
Next, we assessed whether the improved protection conferred by Protollin-adjuvanted H5N1 vaccine was due to enhanced antibody titers. Mice were immunized as described in Fig. 1 and sera were collected at 3 weeks following primary immunization (week 3) and booster immunization (week 7) to measured HI titers against a homologous A/VN/1203/04 virus as well as heterologous A/IN/05/05 virus. Mice immunized with unadjuvanted H5N1 vaccine did not induce detectable antibody titers following primary immunization. However, mice immunized with Protollin –adjuvanted H5N1 vaccine (3 µg HA) developed low level of antibody titers even after a single vaccination (data not shown). At week 7, all mice immunized with vaccine plus Protollin developed significantly higher levels of HI titers against both homologues and heterologous viruses (Fig.2 A and C). However, none of the mice immunized with vaccine alone had detectable HI titers against A/VN/1203/04 virus (Fig.2A) and only a few mice immunized with either 1 or 3 µg HA of unadjuvanted vaccine developed detectable HI titers against A/IN/05/05 virus (Fig.2C). Furthermore, vaccine alone elicited more IgG1 production than IgG2a, whereas Protollin adjuvantation induced a mixed IgG1 and IgG2a response with a clear trend towards a TH1 response (IgG2a-dominant) (Fig.2B and D). These results indicate that Protollin significantly enhanced the immunogenicity of H5N1 vaccine and skewed the immune response towards a TH1 response.
Fig. 2.
Protollin enhanced serum protective antibody responses. Balb/c mice were nasally administered with H5N1 split virion vaccine antigen (0.3, 1, 3 µg HA) with or without 2 µg Protollin and a month later, booster immunized with same vaccine formulations. Control mice received Protollin only at both time points. Sera were collected at the 3rd week following booster immunization (W7) and HI titers were measured against A/VN/1203/04 (A) or A/IN/05/05 (C) virus. Isotypes (IgG1 vs. IgG2a) of serum antibody against A/VN/1203/04 (B) or A/IN/05/05 (D) virus from mice immunized with 3 µg H5N1 split virion vaccine with or without Protollin were measured and its ratio is shown in insert (B and D). The data is representative of 3 independent experiments (5–10 mice per group) and error bars represent standard error of the mean (SEM). One-way analysis of variance with Bonferroni post analysis was used to analyze differences among treatments. The Student’s t test was used to analyze differences in antibody isotype between two groups.
3.3. Protollin increased the frequency of Ag-specific B and T cells
To determine whether Protollin increased the frequency of Ag-specific memory B cells, mice were immunized as described in Fig. 1 and the frequency of H5N1-specific antibody-secreting cells (ASCs) in spleen and bone marrow was examined by ELISPOT assay. As shown in Fig.3 A and B, the frequency of H5N1-specific IgG and IgM ASCs in the spleen increased in mice immunized with unadjuvanted H5N1 vaccine; Protollin significantly increased the frequency of H5N1-specific IgG and IgM ASCs even at the lowest dose (0.3 µg), although Ag-specific IgM ASCs frequency is much lower than that of Ag-specific IgG ASCs. In bone marrow, the frequency of H5N1-specific IgG ASCs was similarly enhanced by the use of Protollin as an adjuvant, while the frequency of IgM ASCs was minimally affected by Protollin (Fig.3C and D). Together, these data suggest that intranasal administration of H5N1 vaccine with Protollin enhanced the overall frequency of ASCs with significant dose-sparing effect.
Fig. 3.
Protollin increased the frequency of Ag-specific ASCs and T cells. Balb/c mice (5 mice/group) were nasally administered with H5N1 split virion vaccine antigen (0.3, 1, 3 µg HA) with or without 2 µg Protollin and a month later, booster immunized with the same vaccine formulations. Control mice received Protollin only at both time points. One week after booster immunization, spleen (A and B) and bone marrow (C and D) were harvested and the frequency of A/VN/1203/04-specific IgG+ ASCs (A and C) or IgM+ ASCs (B and D) were measured by ELISPOT assay. The number of A/VN/1203/04-specific IgG+ (or IgM+) ASCs were normalized against the number of total IgG+ (IgM+) secreting ASCs and presented as % Ag-specific IgG+ (IgM+) B cells. (E) Balb/c mice (5 mice/group) were nasally administered and the frequency of cytokine producing A/VN/1203/04-specific CD4+ T cells in spleen of mice immunized with H5N1 vaccine (3 µg) with or without Protollin were measured by flow cytometry following intracellular cytokine staining after virus infection and culture. Data are presented as fold changes relative to Protollin only group. The data is representative of 2 independent experiments (5 mice per group) and the error bars represent standard error of the mean (SEM). One-way analysis of variance with Bonferroni post analysis was used to analyze differences among treatments.
Next, the activation of vaccine-specific T cells in spleen was assessed in mice immunized with 3 µg H5N1 vaccine with or without Protollin. For CD4 T cells, while the production of IL-10 and IFNγ was detectable in mice immunized with unadjuvanted vaccine as compared to the Protollin control group, Protollin adjuvantation further increased the IL-10 and IFNγ production significantly (Fig.3E). On the other hand, IL-17, IL-21, TNFα, and IL-4 production by activated CD4 T cells was comparable between Protollin alone vs. vaccine group; however, co-administration with Protollin and vaccine significantly increased IL-17, TNFα and IL-4 production from CD4 T cells. Vaccine-specific CD8 T cells responses were slightly increased upon Protollin adjuvantation but this increase was not statistically significant (data not shown). In summary, our data suggest that Protollin-adjuvanted H5N1 vaccine enhanced a broad spectrum of the CD4 T cell activation pathways: TH1 (IFNγ and TNFα), TH2 (IL-4 and IL-10) and TH17 (IL-17).
3.4. Protollin enhanced the mucosal antibody responses
Systemic increase in ASC frequency by Protollin prompted us to assess the extent of homing of ASCs back to the local immune induction site. Thus, we measured the H5N1-binding IgA by ELISA 1 week following booster immunization in the fluids from bronchoalveolar (BAL) and nasal lavage from mice that had been immunized as previously described in Fig. 3. The IgA secretion from unadjuvanted H5N1 vaccine antigen-immunized mice was barely detectable both in nasal lavage (Fig.4 A) and BAL (Fig.4B). In contrast, the mice immunized with H5N1 vaccine antigen plus Protollin produced A/VN/1203/04-specific IgA at a minimum of 25-fold higher than that of mice immunized with the unadjuvanted vaccine. In addition, Protollin significantly enhanced clade 2 A/IN/05/05-specific IgA secretion both in nasal and BAL as compared to vaccine alone (Fig.4C and D); Secretion of IgG antibodies was also greatly enhanced by Protollin and the reactivity was extended against clade 2, A/IN/05/05 virus (Fig.5 A–D). However, the frequency of IgM ASCs was much lower and minimally affected by Protollin (Fig.5E–H). These data indicate that nasal administration of Protollin–adjuvanted H5N1 vaccine enhanced local mucosal immunity throughout the respiratory tract.
Fig. 4.
Protollin increased the mucosal antibody responses. Balb/c mice (5 mice/group) were nasally administered with H5N1 split virion vaccine antigen (0.3, 1, 3 µg HA) with or without 2 µg Protollin and a month later, booster immunized with the same vaccine formulations. Control mice received Protollin only at both time points. One week after booster immunization, Nasal lavage (A) and BAL (B) fluids were collected and A/VN/1203/04-specific IgA antibodies were assessed by ELISA. Cross-reactivity of nasal lavage (C) and BAL (D) fluids were measured against A/IN/05/05 viruses by ELISA. The amount Ag-specific IgA was presented as relative Ag-binding IgA to mouse IgA. The data is representative of 3 independent experiments (4–5 mice per group) and the error bars represent standard error of the mean (SEM). One-way analysis of variance with Bonferroni post analysis was used to analyze differences among treatments.
Fig. 5.
Protollin increased the IgG and IgM production at the mucosal surface. Balb/c mice (5 mice/group) were nasally administered with H5N1 split virion vaccine antigen (0.3, 1, 3 µg HA) with or without 2 µg Protollin and a month later, booster immunized with the same vaccine formulations. Control mice received Protollin only at both time points. One week after booster immunization, nasal lavage (A, C, E and G) and BAL fluids (B, D, F and H) were collected and IgG (A–D) and IgM (E–H) antibodies against A/VN/1203/04 (A–B and E–F) and A/IN/05/05 (C–D and G–H) were assessed by ELISA. The relative amount of virus-specific IgG or IgM antibodies were presented. The data is representative of 3 independent experiments (4–5 mice each group) and error bars represent standard error of the mean (SEM). One-way analysis of variance with Bonferroni post analysis was used to analyze differences among treatments.
3.5. Protollin increased the early B cell response in the draining lymph nodes and spleen
Next, the activation status of B cells at peak of the primary response (day 7) was assessed by flow cytometric analysis. Compared to mice immunized with vaccine alone, mice immunized with vaccine plus Protollin had a higher frequency of B cells participating in GC reaction (Fig.6 A), higher overall mean fluorescence intensity of CD80 among GC-participating B cells, and more GC-B cells expressing high level of CD80 (Fig.6C and D). The plasma cell differentiation was at low level and did not reveal differences among groups (Fig.6B). Interestingly, Protollin alone recruited B cells into GC reaction and induced activation (Fig.6A), emphasizing the significant role of innate signaling through TLR2 and TLR4 in B cell activation. Nonetheless, their recruitment to GC reaction and activation did not result in the production of Ag-specific antibodies (Fig. 2). We also measured the frequency of the splenic Ag-specific IgG and IgM during the primary response and found that Protollin adjuvantation significantly increased the early IgG secretion and marginally IgM secretion (Fig.6E and F). Overall, these data suggest that Protollin recruited more naïve B cells at the early phase of the vaccine response.
Fig. 6.
Protollin increased the early B cell response at the local lymph nodes and spleen. Balb/c mice (2–4 mice/group) were nasally administered with 3 µg HA H5N1 split virion vaccine antigen with or without 2 µg Protollin or Protollin alone as a control. A week later, mediastinal lymph nodes and/or spleens were collected and B cells (B220+CD3−) were stained. The lymph nodes were analyzed for percentage of GC-participating B cells (B220+CD3−GL7+CD38−) (A), plasma cells (B220−CD138+) (B), the mean fluorescence intensity (MFI) of CD80 (C) as well as % B cells expressing high CD80 (D) among GC-participating B cells. Spleen were harvested and the frequency of A/VN/1203/04-specific IgG+ ASCs (E) or IgM+ ASCs (F) were measured by ELISPOT assay. The number of A/VN/1203/04-specific IgG+ (or IgM+) ASCs were normalized against the number of total IgG+ (IgM+) secreting ASCs and presented as % Ag-specific IgG+ (IgM+) B cells. The data is representative of at least 2 independent experiments (4–5 mice each group) and the error bars represent standard error of the mean (SEM). One-way analysis of variance with Bonferroni post analysis was used to analyze differences among treatments.
4. Discussion
The spread and evolution of highly pathogenic influenza H5N1 virus in birds worldwide and the increasing number of cases of direct transmission to humans leading to fatalities, have raised concern about an imminent H5N1 influenza pandemic [15]. Vaccination is unquestionably one of the most cost-effective public health interventions available to protect against such pandemics. However, currently available unadjuvanted H5N1 vaccines administered by i.m. route are poorly immunogenic. New strategies to improve the immunogenicity of vaccines with adjuvants, novel formulations and alternate delivery methodologies to meet the demands of global populations are still urgently needed. In the current study, we evaluated the use of Protollin–adjuvanted H5N1 vaccine as a nasally delivered, pre-pandemic vaccine in a mouse model.
The mucosal immune system in the respiratory track plays an important role in prevention of influenza virus infection. The upper respiratory tract is equipped with not only the physical and dynamic barrier of mucus as well as both soluble and membrane bound pathogen sensors but also contains nasopharyngeal-associated lymphoid tissues (NALT) that are enriched in Ag-specific mucosal effector cells as well as innate immune cells [6]. Hence, nasal delivery of antigens triggers local mucosal as well as a systemic immune response, which is crucial against respiratory pathogens including influenza [16]. Nasal delivery of split, inactivated influenza vaccine generally requires a mucosal adjuvant to induce strong protective immune responses [16]. Some mucosal adjuvants containing bacterial toxin derivatives, including Esherichia coli heat labile enterotoxin (LT) have been clinically evaluated with subunit influenza vaccines and ultimately licensed in Europe, but soon withdrawn from the market due to an increased incidence of Bell’s Palsy post-vaccination [17].
Clinical trials with proteosome-adjuvanted either monovalent H1N1 or trivalent inactivated vaccines have shown that proteosome-adjuvanted vaccines are well-tolerated, while inducing significantly higher serum HI and mucosal secretory IgA titers as compared to unadjuvanted vaccines [18], [19], [20]. Preclinical studies using Protollin-adjuvanted H3N2 vaccines also showed the similar results [14], [21] Consistent with this, our data showed that Protollin–adjuvanted H5N1 vaccine induced significantly higher levels of mucosal IgA antibodies against A/VN/1203/04 virus both in nasal washes and lung, with considerable dose-sparing effect. The breadth of the mucosal IgA response was extended against clade 2 virus, A/IN05/05, As a pre-pandemic vaccine formulation, cross-clade immunity is an important and desirable feature, because the current endemic H5N1 viruses continuously evolve in HA antigenicity through antigenic drift and reassortment, thus making it hard to predict which strain will cause a pandemic. Protollin also induced cross-reactive IgG as well as low, yet detectable levels of IgM antibodies. Lung-resident memory B cells as well as the mucosal, secretory IgA and/or IgG antibodies play a crucial role in conferring protection against influenza virus challenge [22], [23]. The mice immunized with Protollin–adjuvanted H5N1 vaccine were completely protected against lethal challenge with clade 1 and clade 2 viruses. Therefore, the Protollin–adjuvanted H5N1 represents a novel pre-pandemic vaccine candidate that is efficient in inducing protective mucosal and systemic immunity.
The adjuvant properties of Protollin have been documented in conjunction with antigens from various infectious agents including measles [9], recombinant SARS-spike glycoprotein [10], respiratory syncytial virus (RSV) [11], [12], and recombinant plague antigen F1-V [13]. The level of serum IgG response induced by Protollin is generally comparable to that by alum-adjuvanted vaccines and shows a mixed TH1/TH2 response with a TH1 trend (higher IgG2a/IgG1 ratio) as compared to unadjuvanted vaccines [9], [10], [13]. Consistent with these findings, our data demonstrated that Protollin potentiated the immunogenicity of H5N1 vaccine. After a single vaccination, mice immunized with Protollin –adjuvanted H5N1 vaccine (3 µg) developed detectable levels of serum HI titers. Following booster immunization, Protollin –adjuvanted H5N1 vaccine significantly increased serum HI titers compared to unadjuvanted vaccine, which coincided with complete protection against homologous virus challenge. The breadth of antibody response was also broadened by Protollin-adjuvanted H5N1 vaccine, as they significantly increased serum HI titers against A/IN/05/05 virus compared to the vaccine alone group and fully protected mice against A/IN/05/05 virus challenge.
Protection against influenza virus infection includes antibody-mediated neutralization/blocking of virus and cell-mediated clearance of virus-infected cells. Although the antigen-specific CD8 T cell responses was not significantly enhanced by Protollin adjuvantation (data not shown), Protollin-adjuvanted vaccines significantly increased the production of cytokines by antigen-specific CD4 T cells: TH1 (IFN-γ, TNFα), TH2 (IL-4) and TH17 (IL-17). IFNγ, as a representative cytokine of TH1 response promotes overall microbial killing through enhancing phagocytosis and recruiting mononuclear cells into the site of infection, while favoring the production of IgG2a from B cells [24], [25], [26]. On the other hand, IL-4 along with IL-5, shapes the TH2 response and promotes the production of IgG1 isotype [26]. Consistent with the cytokine profiles, the mice immunized with Protollin-adjuvanted H5N1 vaccine showed a mixed IgG1/IgG2a response with higher production of IgG2a than IgG1. IL-17 is typically associated with pathogenesis of inflammatory diseases [27]. However, the protective role of IL-17 for survival against high dose challenge and severe cases has been recently demonstrated [28], [29]. Of interesting note, Protollin-adjuvanted H5N1 vaccine elicited a sharp increase in IL-10 production as compared to vaccine alone. IL-10 is an anti-inflammatory cytokine produced by natural CD4+CD25+Foxp3+ regulatory T cells (Tregs) as well as antigen-specific CD4+ and CD8+ effector T cells [30], [31], [32]. It remains unclear the source of IL-10 in our study, but regardless, the enhanced IL-10 production indicates the fine balance between effector vs. control mechanisms was achieved by Protollin. Using gene knock-out mice, TLR4 has been shown to be critical for antigen-specific antibody responses in Protollin-adjuvanted RSV vaccine, while MyD88 was required to elicit a balanced TH1/TH2 immune responses. Although TLR2 is required for nanoparticle formation, it is not playing a role in the adjuvanticity of Protollin [33].
In summary, Protollin demonstrated a significant antigen dose-sparing effect. With Protollin-adjuvantation, H5N1 vaccine antigen at the 10-fold lower dose elicited higher serum HI titers, mucosal antibody responses than the highest vaccine antigen dose (3 µg HA/mouse) against both clade 1 and clade 2 H5N1 viruses. These enhanced immune responses culminated in enhanced protection against challenge with lethal doses of H5N1 viruses of either clade 1 or clade 2. Therefore, nasal delivery of Protollin-adjuvanted H5N1 vaccines is a promising approach that merits further development to prepare for a H5N1 pandemic.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the funding agencies.
Author contributions
W.C., J.K., S.S. designed the experiments and interpreted the data; W.C., J.K., A.R. and M.H. did the in vivo experiments; J.B. did the challenge experiment; X.L did the HI assay; W.C., J.K., and S.S. wrote the manuscript. S.G., J.K., M.P. and D.B. edited the manuscript.
Conflicts of interest
DSB and MP were employees of the GSK group of companies at the time of the study, own stock options in GSK and are listed as inventors on patents owned by the GSK group of companies. The remaining authors declare no commercial or financial conflict of interest.
Funding information
Work was supported by the Influenza Division, Centers for Disease Control and Prevention.
Acknowledgements
We thank the members of Influenza Division, Centers for Disease Control and Prevention (CDC) for providing reagents and constructive comments during the course of this investigation.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.05.004.
Appendix A. Supplementary material
References
- 1.Cheng V.C., To K.K., Tse H., Hung I.F., Yuen K.Y. Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin Microbiol Rev. 2012;25:223–263. doi: 10.1128/CMR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claas E.C., Osterhaus A.D., van Beek R., De Jong J.C., Rimmelzwaan G.F., Senne D.A. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 3.Kang S.M., Song J.M., Kim Y.C. Microneedle and mucosal delivery of influenza vaccines. Expert Rev Vaccines. 2012;11:547–560. doi: 10.1586/erv.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose M.A., Zielen S., Baumann U. Mucosal immunity and nasal influenza vaccination. Expert Rev Vaccines. 2012;11:595–607. doi: 10.1586/erv.12.31. [DOI] [PubMed] [Google Scholar]
- 5.Shalaby W.S. Development of oral vaccines to stimulate mucosal and systemic immunity: barriers and novel strategies. Clin Immunol Immunopathol. 1995;74:127–134. doi: 10.1006/clin.1995.1019. [DOI] [PubMed] [Google Scholar]
- 6.McGhee J.R., Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis S.S. Nasal vaccines. Adv Drug Deliv Rev. 2001;51:21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 8.Jin H., Subbarao K. Live attenuated influenza vaccine. In: Oldstone M.B.A., Compans R.W., editors. vol. II. Springer; 2014. pp. 181–204. (Influenza Pathogenesis and Control). [Google Scholar]
- 9.Chabot S., Brewer A., Lowell G., Plante M., Cyr S., Burt D.S. A novel intranasal Protollin-based measles vaccine induces mucosal and systemic neutralizing antibody responses and cell-mediated immunity in mice. Vaccine. 2005;23:1374–1383. doi: 10.1016/j.vaccine.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Hu M.C., Jones T., Kenney R.T., Barnard D.L., Burt D.S., Lowell G.H. Intranasal Protollin-formulated recombinant SARS S-protein elicits respiratory and serum neutralizing antibodies and protection in mice. Vaccine. 2007;25:6334–6340. doi: 10.1016/j.vaccine.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cyr S.L., Jones T., Stoica-Popescu I., Burt D., Ward B.J. C57Bl/6 mice are protected from respiratory syncytial virus (RSV) challenge and IL-5 associated pulmonary eosinophilic infiltrates following intranasal immunization with Protollin-eRSV vaccine. Vaccine. 2007;25:3228–3232. doi: 10.1016/j.vaccine.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y., Cyr S.L., Burt D.S., Anderson R. Murine host responses to respiratory syncytial virus (RSV) following intranasal administration of a Protollin-adjuvanted, epitope-enhanced recombinant G protein vaccine. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2009;44:287–291. doi: 10.1016/j.jcv.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Jones T., Adamovicz J.J., Cyr S.L., Bolt C.R., Bellerose N., Pitt L.M. Intranasal Protollin/F1-V vaccine elicits respiratory and serum antibody responses and protects mice against lethal aerosolized plague infection. Vaccine. 2006;24:1625–1632. doi: 10.1016/j.vaccine.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Baz M., Samant M., Zekki H., Tribout-Jover P., Plante M., Lanteigne A.M. Effects of different adjuvants in the context of intramuscular and intranasal routes on humoral and cellular immune responses induced by detergent-split A/H3N2 influenza vaccines in mice. Clin Vaccine Immunol. 2012;19:209–218. doi: 10.1128/CVI.05441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson A.P., Tabak L.A., Fauci A.S., Collins F.S., Howard S. Research funding. A framework for decisions about research with HPAI H5N1 viruses. Science. 2013;339:1036–1037. doi: 10.1126/science.1236194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burt D., Mallett C., Plante M., Zimmermann J., Torossian K., Fries L. Proteosome-adjuvanted intranasal influenza vaccines: advantages, progress and future considerations. Expert Rev Vaccines. 2011;10:365–375. doi: 10.1586/erv.10.172. [DOI] [PubMed] [Google Scholar]
- 17.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. New Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 18.Treanor J., Nolan C., O'Brien D., Burt D., Lowell G., Linden J. Intranasal administration of a proteosome-influenza vaccine is well-tolerated and induces serum and nasal secretion influenza antibodies in healthy human subjects. Vaccine. 2006;24:254–262. doi: 10.1016/j.vaccine.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 19.Langley J.M., Halperin S.A., McNeil S., Smith B., Jones T., Burt D. Safety and immunogenicity of a Proteosome -trivalent inactivated influenza vaccine, given nasally to healthy adults. Vaccine. 2006;24:1601–1608. doi: 10.1016/j.vaccine.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 20.Langley J.M., Aoki F., Ward B.J., McGeer A., Angel J.B., Stiver G. A nasally administered trivalent inactivated influenza vaccine is well tolerated, stimulates both mucosal and systemic immunity, and potentially protects against influenza illness. Vaccine. 2011;29:1921–1928. doi: 10.1016/j.vaccine.2010.12.100. [DOI] [PubMed] [Google Scholar]
- 21.Ann J., Samant M., Rheaume C., Dumas C., Beaulieu E., Morasse A. Adjuvanted inactivated influenza A(H3N2) vaccines induce stronger immunogenicity in mice and confer higher protection in ferrets than unadjuvanted inactivated vaccines. Vaccine. 2014;32:5730–5739. doi: 10.1016/j.vaccine.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Onodera T., Takahashi Y., Yokoi Y., Ato M., Kodama Y., Hachimura S. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci USA. 2012;109:2485–2490. doi: 10.1073/pnas.1115369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew F.Y., Russell S.M., Appleyard G., Brand C.M., Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14:350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 24.Paludan S.R. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand J Immunol. 1998;48:459–468. doi: 10.1046/j.1365-3083.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 25.Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 26.Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 27.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramirez P. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinstry K.K., Strutt T.M., Buck A., Curtis J.D., Dibble J.P., Huston G. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almansa R., Socias L., Ramirez P., Martin-Loeches I., Valles J., Loza A. Imbalanced pro- and anti-Th17 responses (IL-17/granulocyte colony-stimulating factor) predict fatal outcome in 2009 pandemic influenza. Crit Care. 2011;15:448. doi: 10.1186/cc10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez A.M., Zhu J., Huang X., Yang Y. The development and function of memory regulatory T cells after acute viral infections. J Immunol. 2012;189:2805–2814. doi: 10.4049/jimmunol.1200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S.M., Tsai M.H., Lei H.Y., Wang J.R., Liu C.C. The regulatory T cells in anti-influenza antibody response post influenza vaccination. Hum Vaccines Immunother. 2012;8:1243–1249. doi: 10.4161/hv.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J., Madan R., Karp C.L., Braciale T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cyr S.L., Angers I., Guillot L., Stoica-Popescu I., Lussier M., Qureshi S. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine. 2009;27:421–430. doi: 10.1016/j.vaccine.2008.10.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.