Abstract
We studied the adjuvanticity of recombinant Onchocerca volvulus activation associated protein-1 (rOv-ASP-1) for ovalbumin (OVA) in mice. After a single immunization and one boost, rOv-ASP-1 exceeded the efficacy of alum or MPL + TDM adjuvants in terms of end-point total IgG or IgG1 and IgG2a anti-OVA titres. Using the helminth-derived adjuvant, IgG isotype responses to OVA were of a mixed Th1/Th2 profile and spleen cell cytokines exclusively Th1-type. The potent adjuvanticity of rOv-ASP-1 was confirmed in mice vaccinated with a 37-mer peptide from the S protein of SARS-CoV and an HIV-1 gp120-CD4 chimeric polypeptide antigen. Unusually for a helminth product, the rOv-ASP-1 adjuvant augmented not only Th2 but also Th1 responses, the latter property being of potential utility in stimulating anti-viral immune responses.
Keywords: Onchocerca volvulus activation associated protein-1 (rOv-ASP-1), Adjuvant, Th1/Th2, Nematode
1. Introduction
Filarial nematodes are long-lived parasites in the human host, for example, Onchocerca volvulus worms live up to 15 years. They use a large armoury of immunoregulatory molecules to subvert the protective immune responses of the host and minimize severe pathology [1], [2]. Some of these helminth-derived modulators of human immune responses have great potential as new therapeutics. For example, ES-62, a secreted glycoprotein product of the rodent filarial nematode Acanthocheilonema viteae, has broadly anti-inflammatory properties that inhibit Th1 cytokine production in experimentally induced arthritis in mice [3]. ES-62 is currently being developed as a novel anti-inflammatory therapeutic [4]. In another nematode, body fluid from the pig roundworm, Ascaris suum, contained potent activity that stimulated IL-10, which is characteristic of Th2 and regulatory T cells. The parasite products reduced the inflammation caused by experimentally induced delayed type hypersensitivity in mice [5].
Two helminth products have also been reported to act as adjuvants. Both are strong inducers of Th2 responses to bystander proteins in a vaccine. In particular, proteins secreted by adult Nippostrongylus brasiliensis (a parasite of rodents) were found to be strong inducers of Th2 responses in mice immunized with an unrelated protein, hen egg lysozyme [6]. Similarly, lacto-N-fucopentaose III, a carbohydrate found on the surface of the eggs of a human parasite, Schistosoma mansoni, acted as a Th2 adjuvant for a bystander protein (human serum albumin) when injected intranasally, subcutaneously or intraperitonealy into mice [7].
Activation-associated secreted proteins (ASP) of parasitic nematodes are highly immunogenic and have been studied as potential vaccine components, particularly hookworm ASPs [8], [9], [10]. While conducting experiments designed to evaluate recombinant Onchocerca volvulus ASP-1 (rOv-ASP-1) as a possible vaccine candidate against onchocerciasis in humans, we vaccinated mice with the recombinant protein alone or with alum or Freund's adjuvants and measured IgG1 and IgG2a isotypes that are broadly associated with Th2 and Th1 T cell responses, respectively. rOv-ASP-1 stimulated both IgG1 and IgG2a antibody responses to the protein itself, with a slight Th1 dominance [11]. Since these effects occurred in vaccinated mice in the absence of adjuvant, we asked the question whether the protein could act as an adjuvant to assist unrelated proteins in inducing antibody responses. Until the present study, purified products from helminths have been found to be strong inducers of exclusively Th2 responses.
2. Materials and methods
2.1. Preparation of rOv-ASP-1
The recombinant Ov-ASP-1 protein was expressed as a histidine-tagged protein in E. coli (DH5α) using the pTrcHis expression vector (Invitrogen, Carlsbad, CA). Inclusion bodies containing insoluble rOv-ASP-1 were treated with 6 M urea at 4 °C overnight, and the urea-soluble rOv-ASP-1 was then further purified by preparative SDS–PAGE on a PrepCell (Bio-Rad, Hercules, CA). The protein-containing fractions were eluted in Laemmli buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3) and analyzed by SDS–PAGE. Fractions containing the purified rOv-ASP-1 were identified by INDIA™ HisProbe-HRP (Pierce, Rockford, IL) chemiluminescent Western blotting, and protein concentrations were determined using detergent-compatible protein assay reagents (Bio-Rad). Although the rOv-ASP-1 protein at working dilutions tested negative for bacterial endotoxin using a Limulus amoebocyte lysate assay (Sigma, St. Louis, MO), we sent a sample for quantitative LPS testing following FDA guidelines to Cambrex Bio Science (Baltimore, MD). Testing revealed that there was a residue of 0.44 Endotoxin Units (EU)/μg rOv-ASP-1 protein. At a dose of 25 μg/mouse, therefore, the animals would receive 11 EU per injection which is well below the FDA accepted limit of 50 EU/mL for biologicals. However, to definitively exclude any possibility of residual LPS in rOv-ASP-1 contributing to any adjuvant effects, we batch-treated the concentrated stock solution (2.5 mg/mL) of rOv-ASP-1 three times with LPS-removing gel (Detoxi-gel™ system, Pierce Biotechnology, Rockford, IL), and adjusted the protein concentration accordingly. Repeat testing by Cambrex showed no detectable LPS in the treated rOv-ASP-1 preparation and a single batch was used throughout the experiments presented here.
2.2. Antigens
We first used ovalbumin (OVA, Sigma, Grade V) as a model antigen that does not stimulate appreciable antibody responses when injected into mice without adjuvants. The peptide derived from the spike (S) protein of SARS-associated coronavirus (SARS-CoV), designated CP-1 [12], was synthesized by a standard solid-phase FMOC method in the Microchemistry Laboratory of the New York Blood Center. CP-1 spans amino acids 1153–1189 of the heptad repeat 2 (HR2) region of the SARS-CoV S protein, which plays an important role in fusion between the virus and target cell membranes [12]. The FLSC polypeptide is a chimera of the full-length HIV-1 BaL gp120 and the first and second extracellular domains (D1D2) of soluble CD4 joined by a 20-amino acid linker. This polypeptide faithfully duplicates the structural, functional and antigenic properties of the native gp120-CD4 complex intermediate that arises during HIV replication and enables binding to CCR5 on target cells [13]. FLSC inhibited binding of HIV-1 in vitro to target cells expressing CCR5 [13]. Therefore, the antibodies directed against FLSC may block HIV-1 binding to CCR5. A plasmid expressing the FLSC protein was kindly provided by Dr. A. Pinter at the Public Health Research Institute with the permission from Dr. A.L. DeVico at the University of Maryland. A stable cell line expressing recombinant FLSC was established by transfecting the plasmid into 293T cells using FuGene 6 (Boehringer Mannheim, Indianapolis, IN) according to the manufacture's protocol. Soluble FLSC was purified from cell culture medium by lectin chromatography with Galanthus nivalis snowdrop agglutinin (Sigma-Aldrich, St. Louis, MO) as described [14].
2.3. Immunization studies
The procedures dealing with mice were approved by the Institutional Animal Care and Use Committee at The New York Blood Center. We used 6–8-week-old male BALBC/cByJ mice (Charles River Laboratories Inc., Wilmington, MA) for immunization. The commercial adjuvants, alum (Sigma) or MPL + TDM (Sigma), were used as controls of our test adjuvant, rOv-ASP-1. MPL + TDM (equivalent to RIBI™ adjuvant) is composed of equal amounts of monophosphoryl lipid A (detoxified endotoxin) from S. Minnesota (MPL) and synthetic trehalose dicorynomycolate (TDM) in 2% oil (squalene)-Tween 80-water. Per immunization, each animal received 50 μg of antigen (OVA, SC-1 or FLSC) in 0.1 mL sterile, LPS-free phosphate-buffered saline (PBS) mixed with one of the commercial adjuvants following the manufacturer's instructions, or with rOv-ASP-1 (25 μg/0.1 mL PBS). Each of the antigen/adjuvant mixtures or adjuvants (as controls) was injected into a group of five mice subcutaneously in the nape of the neck. Mice received one boost (for OVA) or two boosts (for CP-1 and FLSC) 14 days post-immunization. All experiments were performed twice or more and representative data are shown.
2.4. Measurement of antibodies
Mice were bled retro-orbitally prior to vaccination to establish antibody baselines. Ten days after the final vaccination, the mice were euthanized and bled. Total IgG and IgG isotype antibody responses to each of the antigens were measured by ELISA. Ninety-six-well vinyl ELISA plates (Costar #2595, Bio-Rad, Hercules, CA) were coated overnight at 4 °C with 50 μL/well of OVA at 5 μg/mL, CP-1 at 10 μg/mL or FLSC at 1 μg/mL in carbonate coating buffer (pH 9.6). After washing five times, plates were blocked with 200 μL 2% non-fat milk powder in PBS for 1.5 h at 37 °C. Mouse sera serially diluted in PBS were added (50 μL/well), followed by incubation for 1.5 h at 37 °C. After extensive washes, goat anti-mouse IgG, IgG1, IgG2a, IgG2b or IgG3 antibodies (Sigma) were added at 1:1000 dilution (50 μL/well for 1 h at 37 °C) for IgG isotyping. Then, the biotinylated rabbit anti-goat IgG and extravidin peroxidase conjugate (both from Sigma, 1:2000 dilution for 1 h at 37 °C) were added sequentially. After the final washing step, 50 μL of TMB (Sigma) was added and the reaction was stopped with an equal volume of 1 M H2SO4. Absorbance at 450 nm was measured on a SpectraMax 190 ELISA reader (Molecular Devices, Sunnyvale, CA). Serum antibody titres were determined by measuring the last dilution in the ELISA that resulted in 2 × S.D. above the appropriate control treatment optical density (OD). Serum from each mouse was titrated and the mean titres for each treatment group are presented here.
2.5. Spleen cell stimulation and measurement of cytokines
After exsanguination of the mice under anaesthesia, spleens were removed, cut into two pieces with sterile scissors and made into single cell suspensions using sterile glass Potter–Elvehjem Tissue Grinders (Fisher Scientific International, Pittsburgh, PA). The cell suspensions were then passed through sterile disposable 70 μm cell strainers (Falcon brand, BD Biosciences, Bedford, MA). Cells were washed three times in RPMI supplemented with 2% heat-inactivated foetal bovine serum (FBS), 10 mM HEPES buffer, 0.2 mM l-glutamine, 50 μm 2-mercaptoethanol, 100 U/mL penicillin and 100 μg/mL streptomycin (all from Sigma). Cells were counted for viability (always ≥95%) and cultured in RPMI + 10% FBS in quadruplicate wells at 4 × 105 per well in round-bottomed 96-well culture plates (Nunc brand, Fisher Scientific) for 72 h at 37 °C in a humidified 5% CO2 incubator. Spleen cells were cultured with medium alone, the optimal concentration of OVA (5.0 μg/mL) or PHA or PMA + anti-mouse CD3 (BD Biosciences) as positive control stimuli. Supernatants were collected after 72 h and assayed for mouse IFN-γ, IL-4, IL-5 and IL-10 using ELISA assays (Quantikine brand, R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
3. Results
3.1. Evaluation of the adjuvanticity of rOv-ASP-1
In order to evaluate any possible contribution of the small amount of residual LPS in the rOv-ASP-1 to any adjuvant effect, we compared the adjuvanticity of working dilutions of batches of rOv-ASP-1 that were either untreated (rOv-ASP-1B) or batch treated three times (rOv-ASP-1A) with LPS-removing gel. In Fig. 1 , the uppermost lines show that the treated recombinant protein (rOv-ASP-1A) performed better than the untreated protein in augmenting antibody responses to OVA in immunized mice. The mean anti-OVA IgG1 titre obtained using treated rOv-ASP-1 as an adjuvant was 426,000 compared with 64,000 using the untreated batch and for IgG2a, 128,000 and 64,000, respectively. We could, therefore, rule out LPS as a contributing factor to the adjuvant properties of our recombinant Ov-ASP-1 protein and, since it was a better adjuvant, we used the LPS removing gel-treated batch of rOv-ASP-1 in all further experiments.
Fig. 1.

Mean amounts of anti-OVA IgG1 and IgG2a in mouse sera (n = 5 per group) after immunization with OVA formulated with rOv-ASP-1 that was either treated (rOv-ASP-1A) or untreated (rOv-ASP-1B) with LPS-removing gel. Antibody amounts are expressed as optical densities (OD) in the ELISA assays. The reciprocal dilutions of serum are indicated on the x axis. For control purposes, mice were also immunized with the test adjuvant (rOv-ASP-1A or rOv-ASP-1B) without OVA. The same symbols apply in both graphs.
3.2. Comparison of rOv-ASP-1 with other adjuvants
When rOv-ASP-1 was used at 25 μg/mouse, this protein was much more potent in enhancing antibody production than the commercial adjuvants alum and MPL + TDM (Fig. 2 ). Even though the end-point titres using rOv-ASP-1 were lower in the experiment shown in Fig. 2 than the data in Fig. 1, they were still higher than those achieved using the other adjuvants. The IgG1 anti-OVA reciprocal end-point titre using rOv-ASP-1 as the adjuvant was 102,400, while the titres obtained using MPL + TDM or alum adjuvants were 18,000 and 15,000, respectively. The IgG2a titres were considerably lower than those of IgG1 and only the rOv-ASP-1 adjuvant induced an appreciable anti-OVA IgG2a titre (25,600). There were no detectable IgG2b, IgG3 or IgE antibodies to OVA.
Fig. 2.
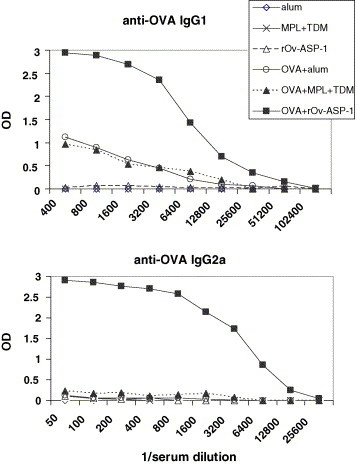
Mean amounts of anti-OVA IgG1 and IgG2a in mouse sera (n = 5 per group) after immunization with OVA formulated with alum, MPL + TDM or the test rOv-ASP-1 adjuvant. Antibody amounts are expressed as optical densities (OD) in the ELISA assays. The reciprocal dilutions of serum are indicated on the x axis. For control purposes, mice were also immunized with the adjuvants alone. The same symbols apply in both graphs.
3.3. Spleen cell cytokine responses in immunized mice
Spleen cells from mice receiving OVA in rOv-ASP-1 showed an exclusively Th1 cytokine profile (IFN-γ+++, IL-5−, IL-10−) when re-stimulated with OVA in vitro (Fig. 3 ). In contrast, the response using alum adjuvant was solely Th2-type (IFN-γ−, IL-5+, IL-10+) in nature. MPL + TDM adjuvant resulted in a mixed, but Th1-dominated response (IFN-γ+++, IL-10+) to the immunizing antigen. We were unable to measure appreciable amounts of IL-4 in any of the supernatants from mouse spleen cell cultures with the exception of the positive control-treated (PMA + anti-CD3) cultures.
Fig. 3.

Cytokines in supernatants of mouse spleen cells after stimulation with OVA in vitro (5 μg/mL). Spleen cells were pooled from five mice in each treatment group. The test adjuvant, rOv-ASP-1 was used at 25 μg per immunization. Data represent the mean amounts of cytokines in quadruplicate cultures of spleen cells. All spleen cell cultures responded normally to PHA or PMA + anti-CD3 positive control stimuli (data not shown).
3.4. Evaluation of the adjuvanticity of rOv-ASP-1 for pathogen antigens
Having shown that rOv-ASP-1 acted as a better adjuvant than alum or MPL + TDM in stimulating production of antibodies to OVA, we then tested if the protein had similar adjuvant potency for antigens derived from human pathogens, namely SARS-CoV and HIV-1. We immunized BALBC/cByJ mice as before using the same batch of LPS-negative rOv-ASP-1 mixed with 50 μg of SARS-CoV CP-1 peptide or HIV-1-CD4 FLSC polypeptide, instead of OVA. All immunized mice were given two boosts this time to optimize the response to the small 37-mer CP-1 peptide, i.e. a total of three injections of CP-1 or FLSC with rOv-ASP-1 as the test adjuvant or MPL + TDM as a control. Using OVA as the control antigen, the end-point titres were about 2,096,000 and 1,024,000 when r-Ov-ASP-1 and MPL + TDM were used as adjuvants, respectively. These total IgG titres were approximately 10 times higher than in the previous experiments, suggesting that an additional boost significantly enhances antibody production. The adjuvanticity of rOv-ASP-1 for the CP-1 peptide exceeded that of MPL + TDM judging by end-point IgG titres of 256,000 versus 64,000, respectively (Fig. 4 A). The anti-FLSC end-point IgG titres achieved using both adjuvants were equivalent (approximately 1,024,000; Fig. 4B).
Fig. 4.

Mean amounts of anti-CP-1 (A) and anti-FLSC (B) total IgG in mouse sera (n = 5 per group) after immunization with control treatments (antigens or adjuvants alone) or antigens formulated with MPL + TDM or the test rOv-ASP-1 adjuvant. Antibody amounts are expressed as optical densities (OD) in the ELISA assays. The reciprocal dilutions of serum are indicated on the x axis.
The IgG isotype responses to the CP-1 peptide and the FLSC polypeptide are summarized in Table 1 . The rOv-ASP-1 protein stimulated higher IgG1, IgG2a and IgG2b titres than MPL + TDM. IgG3 titres were equally low using both adjuvants. IgG1 titres to the HIV-1 polypeptide were considerably lower than those to the CP-1 peptide. MPL + TDM induced a higher IgG2b titre to FLSC than rOv-ASP-1, whereas IgG1 and IgG3 titres were the same using both adjuvants. The most striking differences between the rOv-ASP-1 and MPL + TDM-induced responses were: (1) the lack of an IgG2a (Th1) response to CP-1 using MPL + TDM; (2) an eight-fold higher IgG1 (Th2) response to CP-1 using rOv-ASP-1 rather than MPL + TDM as the adjuvant; (3) a four-fold higher IgG2a response to FLSC adjuvanted by rOv-ASP-1 compared with MPL + TDM. In contrast to CP-1 and FLSC antigens, IgG2b and IgG3 antibodies to OVA were not detectable using either rOv-ASP-1 or MPL + TDM adjuvants (data not shown). Each adjuvant/antigen model performed differently depending on the antigen—Th2 dominant antibodies with CP-1 and Th1 with FLSC formulated with either of the adjuvants. However, with either antigen, the Th1 (IgG2a) response was always higher when rOv-ASP-1 was used as the adjuvant. With FLSC as the immunogen, there was a switch in IgG2a and IgG2b antibodies between ASP-1 and Ribi adjuvants. ASP-1 favoured IgG2a and Ribi enhanced IgG2b. No IgE was detectable using rOv-ASP-1 as an adjuvant. IgM and IgA were not tested.
Table 1.
Reciprocal end-point titres of mouse IgG isotypes to FLSC or CP-1 antigens formulated with either the rOv-ASP-1 test adjuvant or the MPL + TDM adjuvant
| IgG isotypes | Anti-FLSC |
Anti-CP-1 |
||
|---|---|---|---|---|
| Adjuvants |
Adjuvants |
|||
| rOv-ASP-1 | MPL + TDM | rOv-ASP-1 | MPL + TDM | |
| IgG1 | 3600a | 3600 | 115200 | 14400 |
| IgG2a | 28800 | 7200 | 7200 | 0 |
| IgG2b | 3200 | 25600 | 1067 | 334 |
| IgG3 | 6400 | 6400 | 320 | 320 |
End-point titres are the mean of five mice per group.
4. Discussion
Ov-ASP-1 is a member of a family of proteins found in both free-living and parasitic nematodes. The native protein is located in secretory granules of the glandular oesophagus of the infective third-stage larvae of O. volvulus [11]. The recombinant protein has a predicted molecular weight of 24.9 kD and also has angiogenic activity in mice [15]. A hookworm homologue of Ov-ASP-1 has been shown to be a promising vaccine candidate when the yeast-expressed recombinant protein was formulated in Quil A adjuvant (Brenntag Biosector, Frederikssund, Denmark) in hamsters [8].
Our studies have clearly shown that we have discovered a new helminth-derived adjuvant that was highly effective in eliciting antibody responses in mice immunized with unrelated protein, polypeptide and peptide antigens. Furthermore, the parasite protein, rOv-ASP-1 exceeded the adjuvanticity of alum or MPL + TDM adjuvants, especially in the induction of the Th-1 associated IgG2a isotype.
In these studies, we used the subcutaneous route of immunization in BALBC/cByJ mice—a strain which tends to Th2 responses more than Th1 [16]. The fact that we can induce Th1 responses in this strain with rOv-ASP-1 but not MPL + TDM encouraged us to investigate whether we could obtain similar results in other mouse strains that are not as Th2-biased. In recent preliminary studies using OVA-immunized C57BL/6 mice which favour Th1 responses [16], rOv-ASP-1 adjuvant was associated with a Th1-dominated (IgG2a) antibody response to OVA but also with considerable IgG1 titres (IgG1:IgG2a = 0.5). The antibody response to OVA in Balb/c mice was Th2-skewed (IgG1) but, as in the data presented here, there was also a significant IgG2a component (IgG1:IgG2a = 3.0). Thus, the rOv-ASP-1 adjuvant augments both Th2 and Th1 antibody responses in mice with the overall balance dependent on the genetic background of the immunized animal. We have used a range of doses of rOv-ASP-1 adjuvant; 2.5 μg/mouse was not significantly active for antibody responses and 10 μg/mouse gave adjuvanticity intermediate to the final dose that we chose (25 μg/mouse). Mice tolerated the rOv-ASP-1 immunization and there were no overt signs of toxicity.
To investigate T cell responses that might be associated with the high antibody titres, we looked at cytokines secreted by spleen cells from mice immunized with OVA with adjuvant. Recall cytokine responses to OVA in mice immunized with OVA in rOv-ASP-1 adjuvant were strongly Th1-dominated (Fig. 3). It is unclear why rOv-ASP-1 adjuvant resulted in an exclusively Th1 cytokine (IFN-γ-positive, IL-5, IL-10-negative) response from splenocytes of OVA-vaccinated mice (Fig. 3), but mixed Th1/Th2 serum antibody responses (Fig. 1, Fig. 2). It is possible that the cytokine profile from the draining lymph node cells might more closely reflect the Th1/Th2 antibody phenotype or that the antibody and cytokine responses were temporally dissociated.
An ability to stimulate a Th1 cellular response is unusual for a helminth protein. Ov-ASP-1 is highly expressed in the infective O. volvulus third-stage larva (L3) [15], but its precise role in the invasion process is not known. The early human response to first exposure to live L3 of Brugia malayi, another filarial nematode, has recently been shown to be Th1-dominated. Numbers of CD4+ and CD8+ T cells expressing IFN-γ, TNF-α and GM-CSF were increased on exposure to L3 in vitro but not T cells expressing Th2 cytokines [17]. It is possible that L3-secreted ASPs are involved in the stimulation of the Th1 cytokines. In support of this, we found that rOv-ASP-1 is a potent stimulator of Th1 cytokines (IFN-γ, TNF-α and GM-CSF) from O. volvulus-naïve human PBMCs (unpublished data). The mechanism responsible for the adjuvanticity of rOv-ASP-1 is not yet clear, however, from our studies using human PBMC we know that the protein binds to a subpopulation of monocytes. It is possible that rOv-ASP-1 exerts a direct activating effect on antigen-presenting cells and we are currently investigating this and the potential role of toll-like receptors in binding rOv-ASP-1.
We were not able to detect HIV-1 neutralizing activity in serum of mice vaccinated with FLSC in either rOv-ASP-1 or MPL + TDM adjuvants. In a separate study, mice immunized with purified FLSC, all developed high antibody titres for the immunogen, but none of the sera possessed neutralizing activities against HIV virus in vitro [18]. The authors suggested that a major portion of the antibody response against the FLSC protein may be directed against immunodominant conformational epitopes unique to the fusion polypeptide that do not mediate viral neutralization. In the present study, serum from FLSC + adjuvant-immunized mice also recognized HIV-1 gp120 protein in ELISA. The mean total IgG anti-gp120 titre obtained with rOv-ASP-1 was 1,250,000 and with MPL + TDM adjuvant, 670,000 (data not shown). Titres to gp120 were lower than to FLSC since all of the gp120 epitopes may not be accessible in the FLSC fusion polypeptide. For safety and regulatory reasons, we are not currently able to assess neutralizing activity to SARS-CoV.
In conclusion, we have shown that a secreted protein from O. volvulus infective larvae acts as a very potent adjuvant for bystander protein, polypeptide and peptide antigens, exceeding the responses induced by commercially produced alum and MPL + TDM adjuvants. Unusually for a helminth protein, the antibody and cellular responses induced had a strong Th1 component. Depending on the immunogen, adjuvanting with rOv-ASP-1 resulted in a Th2-type (IgG1)-dominant antibody response that lacked IgE. This immunostimulatory helminth protein may have utility as a human therapeutic.
Acknowledgements
We would like to thank Drs. James Farmer and Jinkui Niu at the MicroChemistry Laboratory for peptide synthesis. This work was supported in part by a grant from the National Institutes of Health (R01 AI04328) and by the New York Blood Center.
References
- 1.Maizels R.M., Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3(9):733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 2.Maizels R.M., Gomez-Escobar N., Gregory W.F., Murray J., Zang X. Immune evasion genes from filarial nematodes. Int J Parasitol. 2001;31(9):889–898. doi: 10.1016/s0020-7519(01)00213-2. [DOI] [PubMed] [Google Scholar]
- 3.McInnes I.B., Leung B.P., Harnett M., Gracie J.A., Liew F.Y., Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171(4):2127–2133. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 4.Harnett W., McInnes I.B., Harnett M.M. ES-62, a filarial nematode-derived immunomodulator with anti-inflammatory potential. Immunol Lett. 2004;94(1–2):27–33. doi: 10.1016/j.imlet.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Paterson J.C., Garside P., Kennedy M.W., Lawrence C.E. Modulation of a heterologous immune response by the products of Ascaris suum. Infect Immun. 2002;70(11):6058–6067. doi: 10.1128/IAI.70.11.6058-6067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland M.J., Harcus Y.M., Riches P.L., Maizels R.M. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30(7):1977–1987. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Okano M., Satoskar A.R., Nishizaki K., Harn D.A., Jr. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167(1):442–450. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- 8.Goud G.N., Zhan B., Ghosh K., Loukas A., Hawdon J., Dobardzic A. Cloning, yeast expression, isolation, and vaccine testing of recombinant Ancylostoma-secreted protein (ASP)-1 and ASP-2 from Ancylostoma ceylanicum. J Infect Dis. 2004;189(5):919–929. doi: 10.1086/381901. [DOI] [PubMed] [Google Scholar]
- 9.Hotez P.J., Zhan B., Bethony J.M., Loukas A., Williamson A., Goud G.N. Progress in the development of a recombinant vaccine for human hookworm disease: the human hookworm vaccine initiative. Int J Parasitol. 2003;33(11):1245–1258. doi: 10.1016/s0020-7519(03)00158-9. [DOI] [PubMed] [Google Scholar]
- 10.Yatsuda A.P., Eysker M., Vieira-Bressan M.C., De Vries E. A family of activation associated secreted protein (ASP) homologues of Cooperia punctata. Res Vet Sci. 2002;73(3):297–306. doi: 10.1016/s0034-5288(02)00125-x. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald A.J., Tawe W., Leon O., Cao L., Liu J., Oksov Y. Ov-ASP-1, the Onchocerca volvulus homologue of the activation associated secreted protein family is immunostimulatory and can induce protective anti-larval immunity. Parasite Immunol. 2004;26(1):53–62. doi: 10.1111/j.0141-9838.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363(9413):938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouts T.R., Tuskan R., Godfrey K., Reitz M., Hone D., Lewis G.K. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J Virol. 2000;74(24):11427–11436. doi: 10.1128/jvi.74.24.11427-11436.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilljam G. Envelope glycoproteins of HIV-1, HIV-2, and SIV purified with Galanthus nivalis agglutinin induce strong immune responses. AIDS Res Hum Retroviruses. 1993;9(5):431–438. doi: 10.1089/aid.1993.9.431. [DOI] [PubMed] [Google Scholar]
- 15.Tawe W., Pearlman E., Unnasch T.R., Lustigman S. Angiogenic activity of Onchocerca volvulus recombinant proteins similar to vespid venom antigen 5. Mol Biochem Parasitol. 2000;109(2):91–99. doi: 10.1016/s0166-6851(00)00231-0. [DOI] [PubMed] [Google Scholar]
- 16.Reiner S.L., Locksley R.M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 17.Babu S., Nutman T.B. Proinflammatory cytokines dominate the early immune response to filarial parasites. J Immunol. 2003;171(12):6723–6732. doi: 10.4049/jimmunol.171.12.6723. [DOI] [PubMed] [Google Scholar]
- 18.He Y., D’Agostino P., Pinter A. Analysis of the immunogenic properties of a single-chain polypeptide analogue of the HIV-1 gp120-CD4 complex in transgenic mice that produce human immunoglobulins. Vaccine. 2003;21(27–30):4421–4429. doi: 10.1016/s0264-410x(03)00451-1. [DOI] [PubMed] [Google Scholar]


