Abstract
A new type of coronavirus has been identified as the causative agent underlying Middle East Respiratory Syndrome (MERS). The MERS coronavirus (MERS-CoV) has spread in the Middle East, but cases originating in the Middle East have also occurred in the European Union and the USA. Eight hundred and thirty-seven cases of MERS-CoV infection have been confirmed to date, including 291 deaths. MERS-CoV has infected dromedary camel populations in the Middle East at high rates, representing an immediate source of human infection. The MERS-CoV spike (S) protein, a characteristic structural component of the viral envelope, is considered as a key target of vaccines against coronavirus infection. In an initial attempt to develop a MERS-CoV vaccine to ultimately target dromedary camels, we constructed two recombinant adenoviral vectors encoding the full-length MERS-CoV S protein (Ad5.MERS-S) and the S1 extracellular domain of S protein (Ad5.MERS-S1). BALB/c mice were immunized with both candidate vaccines intramuscularly and boosted three weeks later intranasally. All the vaccinated animals had antibody responses against spike protein, which neutralized MERS-CoV in vitro. These results show that an adenoviral-based vaccine can induce MERS-CoV-specific immune responses in mice and hold promise for the development of a preventive vaccine that targets the animal reservoir, which might be an effective measure to eliminate transmission of MERS-CoV to humans.
Keywords: Adenoviral vaccines, Codon-optimized, MERS-CoV S
1. Introduction
A new type of coronavirus has been identified as the causative agent underlying a respiratory syndrome that recently emerged in the Middle East [1], [2]. The Coronavirus Study Group of the International Committee on Taxonomy of Viruses proposed a new name for this novel betacoronavirus: the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Several Middle Eastern countries have been affected by the emerging MERS-CoV epidemic, including Jordan, Qatar, Oman, Saudi Arabia, and the United Arab Emirates. Tunisia has reported three confirmed cases of human infection. France, Italy, Germany, the United Kingdom, Greece, the Netherlands, and the USA have also reported cases directly or indirectly connected to the Middle East. Eight hundred and thirty-seven cases of MERS-CoV infection have been confirmed to date, including 291 deaths [3]. The rapid accumulation of information about the sequences [2] of MERS-CoV, its genome structure, and its proteins open exciting possibilities for the development of candidate vaccines.
We and others recently provided evidence that dromedary camels are a reservoir of MERS-CoV virus [4], [5], [6], [7]. Both MERS-CoV spike protein-binding antibodies and virus-neutralizing antibodies have been reported in dromedary camels from different regions, including Qatar, Saudi Arabia, Oman, and Egypt. Moreover, MERS-CoV in dromedary camels from a farm in Qatar were linked to two virologically confirmed human cases of the infection in October 2013 [4] and about one in five human cases have been reported to be caused by exposure to animals [8], [9]. The full MERS-CoV genome isolated from a Qatari dromedary camel is highly similar to the human England/Qatar 1 virus isolated in 2012 and has efficiently been replicated in human cells using human DPP4 as entry receptor, providing further evidence for the zoonotic potential of dromedary MERS-CoV [10]. Although, we cannot conclude whether the people were infected by camels or vice versa or if yet another source was responsible, increasing evidence indicates that camels represent an important link in human infections with MERS-CoV. Intensive vaccine control and risk-reduction targeting dromedary camels might be effective in eliminating the virus from the human population.
The coronavirus spike protein (S) is a class I fusion protein. Cellular entry of the virus has been demonstrated to be mediated by the S protein through the receptor binding domain (RBD) in the N-terminal subunit (S1) and the fusion peptide in the C-terminal subunit (S2) [11], [12]. For betacoronaviruses, the S protein has been shown to be the main antigenic component responsible for inducing high titers of neutralizing antibodies and/or protective immunity against infection in patients who had recovered from SARS [13], [14] and response levels correlated well with disease outcomes [15], [16]. The S protein has therefore been selected as an important target for vaccine development [17], [18], [19], [20], [21]. Recent work shows that modified vaccinia virus Ankara expressing the S protein of MERS-CoV elicits high titers of S-specific neutralizing antibodies in mice [22].
Adenovirus 5 (Ad5)-vectored candidate vaccines induce potent and protective immune responses against several pathogens in humans and a variety of animals [18], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]. Although a trial of a candidate DNA/rAd5 HIV-1 preventive vaccine showed lack of efficacy [37] and the high prevalence of pre-existing anti-Ad5 immunity may have been a major limitation [38] in humans, replication-defective adenovirus vaccines are among the most attractive vectors for veterinary vaccine development, given the relative speed and low cost of development and production. Most adenoviruses infect their host through the airway epithelium and replicate in the mucosal tissues of the respiratory tracts [39]. Because of their ability of to elicit mucosal immune responses, adenoviruses could be an attractive vector for inducing MERS-CoV-specific immunity in dromedary camels, the putative animal reservoir. Interestingly, sera antibodies against adenovirus type 3 were detected in 1.3% of dromedaries in Nigeria [34] and in 43 of 120 camels in Egypt [35]. The occurrence of adenovirus type 3 respiratory infections in camels was studied in Sudan and a 90% seroprevalence was detected [36].
Here, we describe the development of recombinant type 5 adenoviral vector expressing, codon-optimized MERS-S and MERS-S1 (Ad5.MERS-S and Ad5.MERS-S1) as vaccine candidates and investigate their ability to induce neutralizing immune responses in mice. Moreover, to demonstrate the feasibility of using of a human adenovirus 5 based vaccine in dromedary camels, we have evaluated the infectivity and the presence of anti-adenovirus 5-neutralizing antibodies in this animal species.
2. Materials and methods
2.1. Construction of recombinant adenovirus vectors
The MERS-S (GenBank JX869059) gene was codon-optimized for optimal expression in mammalian cells using the UpGene codon optimization algorithm [40] and synthesized (GenScript). pAd/MERS-S was generated by subcloning the codon-optimized MERS-S gene into the shuttle vector, pAdlox (GenBank U62024), at SalI/NotI sites. The coding sequence for MERS-S1 (amino acids 1 to 725 of full-length MERS S, according to the GeneBank database) was amplified by polymerase chain reaction and inserted into the shuttle vector (Fig. 1A). Subsequently, replication-defective human adenovirus serotype 5, designated as Ad5.MERS-S and Ad5.MERS-S1, were generated by loxP homologous recombination and purified and stored as described previously [26], [41], [42].
Fig. 1.

Construction of adenoviral vector vaccines. (A) A shuttle vector carrying the codon-optimized MERS-S gene encoding full-length protein (1-1353) or MERS-S1 gene encoding the soluble form (1-725) was designated, as shown in the diagram. The positions of the RBD (small dots) and transmembrane domain (stripes) are indicated and S is divided into two subdomains, S1 and S2, at position 751 [46]. The vector was used to generate recombinant replication-deficient adenoviruses by homologous recombination with the adenoviral genomic DNA. (B) Detection of the MERS-S protein by immunocytochemical staining of A549 cells infected with Ad5.MERS-S or Ad5.MERS-S1 (5 MOI) using sera from mice obtained at three weeks after the second Ad5.MERS-S1 boost. As a negative control, mock or AdΨ5-infected cells were treated the same.
2.2. Immunocytochemistry
For detection of MERS-S protein expression in A549 cells (human lung adenocarcinoma epithelial cell line) infected with five multiplicity of infection (MOI) of AdΨ5, Ad5.MERS-S, or Ad5.MERS-S1, cells were fixed with cold methanol 36 h following infection and were incubated with pooled mouse sera against adenoviral vaccines. After washing, the cells were incubated with horseradish peroxidase-coupled anti-mouse secondary antibody (Invitrogen) and the MERS-S protein was visualized by Avidin/Biotin Complex solution (Vector).
2.3. Animals and Immunization
BABL/c mice were inoculated intramuscularly (i.m.) with 1 × 1011 viral particles (v.p.) of Ad5.MERS-S, Ad5.MERS-S1, or AdΨ5 control, respectively. Three weeks after the primary immunization, mice were boosted intranasally (i.n.) with the same dose of the respective immunogens. For the immunization study, a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee was followed.
2.4. Analysis of antibody response
Three weeks after prime immunization, pooled sera were obtained from all mice and screened for MERS-S-specific antibodies using fluorescence-activated cell sorter (FACS) analysis of Human Embryonic Kidney (HEK) 293 cells transfected with either pAd/MERS-S or pAd control using Lipofectamine 2000 (Invitrogen). After 24 h at 37 °C, cells were harvested, trypsinized, washed with phosphate buffered saline (PBS), and stained with mouse antiserum against Ad5.MERS-S, Ad5.MERS-S1, or AdΨ5 followed by a PE-conjugated anti-mouse secondary antibody (Jackson Immuno Research). Data acquisition and analysis were performed using LSRII (BD) and FlowJo (Tree Star) software.
Sera from the animals were collected every week and tested for S protein-specific IgG1 and IgG2a by conventional enzyme-linked immunosorbent assay (ELISA). Briefly, A549 cells were infected with 10 MOI of Ad5.MERS-S1. At six hours after infection, cells were washed three times with PBS, serum-free media was added, and the cells were incubated for 48 h. ELISA plates were coated with this supernatant from A549 cells infected with Ad5.MERS-S1 overnight at 4 °C in carbonate coating buffer (pH 9.5) and then blocked with PBS containing 0.05% Tween 20 (PBS-T) and 2% bovine serum albumin (BSA) for 1 h. Mouse sera were diluted 1:50 for IgG2a and 1:100 for IgG1 ELISA in PBS-T with 1% BSA and incubated for 2 h. After the plates were washed, biotin-conjugated IgG1 and IgG2a (1:1000, eBioscience) and avidin-horseradish peroxidase (HRP) (1:500, PharMingen) were added to each well and incubated for 1 h. The plates were washed three times and developed with 3,3′5,5′-tetramethylbenzidine, and the reaction was stopped with 1 M H2SO4 and absorbance at 450 nm was determined using an ELISA reader (BIO-TEK instruments).
2.5. Virus preparation
Stocks of MERS-CoV were produced by preparing a sixth passage of the MERS-CoV EMC isolate on Vero cells. Cells were inoculated with virus in Dulbecco's Modified Eagle Medium (BioWhittaker) supplemented with 1% serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM glutamine. After inoculation, the cultures were incubated at 37 °C in a CO2 incubator and three days after inoculation, supernatant from Vero cells was collected.
2.6. MERS-CoV neutralization assay
We tested the MERS-CoV neutralization activity of sera derived from mice immunized with Ad5.MERS-S, Ad5.MERS-S1, or AdΨ5 vaccines. Mouse sera were obtained from the retro-orbital plexus weekly for six weeks and tested for their ability to neutralize MERS-CoV (EMC isolate). Briefly, virus (200 PFU) was premixed 1:1 with serial dilutions of sera from animal groups prior to inoculation onto Vero cells, and viral infection was monitored by the occurrence of a cytopathic effect at 72 h post-infection. Virus neutralization titers (VNTs) were determined as the highest serum dilutions that showed full protection against the cytopathic effect of MERS-CoV.
2.7. Human adenovirus type 5 neutralization assay
We tested the adenovirus neutralization activity of sera from camels [4] and humans from Qatar (healthy individuals). All procedures were performed in compliance with relevant laws and institutional guidelines. Briefly, adenovirus expressing green fluorescent protein (GFP) (400 PFU) was premixed 1:1 with serial dilutions of sera prior to inoculation onto A549 cells, and viral infection was monitored by the detection of GFP-positive cells after 48 h. VNTs were determined as the highest serum dilution that showed a 50% reduction in the number of adenovirus-infected cells.
2.8. Human adenovirus type 5 infection of dromedary camel cells
Freshly isolated camel or human peripheral blood mononuclear cells (PBMCs) were seeded at 1–2 × 106 cells/ml in a 24-well plate and incubated for 2 h at 37 °C. Next, cells were infected with 109 v.p. of Ad5.EGFP/ml in complete medium and incubated for 24 h at 37 °C and 5% CO2. Adenovirus-infected cells were examined for enhanced GFP expression using an inverted fluorescent microscope (Olympus) and the percentage of Ad5.EGFP-infected cells was analyzed by measurement of mean fluorescence signal on a FACS (BD Biosciences). EGFP-expressing cells in the monocyte populations were analyzed by gating using FlowJo software.
The dromedary camel fibroblast cell line Dubca (ATCC® CRL-2276™) cells were seeded at 3 × 105 cells/well in a 24-well plate and infected with 10 MOI of Ad5.EGFP. At 24 h after infection, flow cytometry of cells was analyzed using LSRII and FlowJo software.
2.9. Statistical analysis
For statistical analysis, the one-way analysis of variance and Tukey's test were performed using Prism software (San Diego, California, USA). Results were considered statistically significant when the p value was <0.05. Symbols *, **, ***, and **** are used to indicated the P values <0.05, <0.005, <0.001, <0.0001, respectively.
3. Results
3.1. Construction and characterization of recombinant adenoviruses
E1/E3 deleted human type 5 adenoviral vector was used to insert the full-length S and extracellular domain S1 of the codon-optimized MERS-S open reading frames to generate Ad5.MERS-S and Ad5.MERS-S1 adenoviral vectors (Fig. 1A).
To detect MERS S protein expression of recombinant adenoviral candidate vaccines, A549 cells were infected with AdΨ5, Ad5.MERS-S, or Ad5.MERS-S1 and incubated with pooled day 28 sera from Ad.MERS or control immunized mice. Immunocytochemical analysis showed expression of MERS S protein in A549 cells infected with either Ad5.MERS-S or Ad5.MERS-S1, while no expression was detected in the mock and AdΨ5-infected cells. These same sets of infected cells were not stained with pooled sera from mice immunized with AdΨ5 (data not shown). Furthermore, cells transduced with Ad5-encoding full-length MERS-S showed a plaque-like structure, which may have resulted from syncytium formation due to MERS full length S protein expression, while the soluble form of MERS S1 protein, which was detected intracellularly (presumably before secretion), showed no syncytium formation (Fig. 1B).
3.2. Detection of MERS-S-specific antibodies
Both the Ad5.MERS-S- and Ad5.MERS-S1-immunized mice developed MERS-S-specific antibodies, measured as reactivity on A549 cells transfected with pAd using flow cytometry, while no specific antibody response was detected in serum samples from control animals inoculated with AdΨ5 or with pre-immunized naïve mouse sera (Fig. 2 ). Specific response was slightly higher in mice immunized with Ad5.MERS-S than in mice immunized with Ad5.MERS-S1 (76.9% vs. 65.9% positive cells). These data suggest that adenoviral vaccines expressing MERS-S and MERS-S1 were able to induce S-specific antibodies.
Fig. 2.

Antibodies in sera of mice immunized with Ad5.MERS-S or Ad5.MERS-S1 bind to MERS-S-expressing cells. HEK 293 cells were transfected with control pAd (black line) or with pAd/MERS-S (gray shaded area). At 36 h post-transfection, MERS-S expressions at the cell surface were analyzed with pooled murine sera obtained after the first intramuscular immunization with Ad5.MERS-S, Ad5.MERS-S1, AdΨ5, or pre-immunized naïve mouse sera as a control followed by staining with PE-conjugated anti-mouse IgG and flow cytometric analysis. Numbers represent the percentage of cells positive for spike proteins.
Sera from mice collected every week after i.n. boosting with 1 × 1011 v.p. of Ad5.MERS-S, Ad5.MERS-S1, or control AdΨ5 respectively, were tested for S protein-specific IgG2a and IgG1 immunoglobulin isotypes, indicating a Th1- or Th2-like response, respectively, by ELISA. Both IgG1 and IgG2a were detected as soon as one week after the first immunization. The induction of MERS-S-specific IgG1 and IgG2a antibodies were comparable between immunized groups. As shown in Fig. 3A, more significantly different IgG1 responses (Th-2) were observed in the sera of mice vaccinated with Ad5.MERS-S1 (**P < 0.005 at week 2; ***P < 0.001 at weeks 3, 4, 5, and 6) than Ad5.MERS-S when compared with the sera of mice vaccinated with AdΨ5. In fact, IgG1 levels in the sera of mice vaccinated with Ad5.MERS-S showed a less significant difference (*P < 0.05 at weeks 2, 3, and 4; **P < 0.005 at week 5 and 6). In contrast, a highly significant difference in IgG2a response (Th-1) was observed in the sera of mice vaccinated with both Ad5.MERS-S and Ad5.MERS-S1 (****P < 0.0001 at weeks 2, 3, 4, 5, and 6) (Fig. 3B). Interestingly, MERS-S induced an earlier IgG2a response than MERS-S1 (*P < 0.05 vs. no significance at week 1), with IgG2a titers significantly higher at week 2 (P = 0.0005), but not after week 3. No MERS-S or -S1 specific serum antibody responses could be detected within the seven week period in mice immunized with the control adenovirus, AdΨ5. These data indicate that Ad5.MERS-S and Ad5.MERS-S1 can induce both Th1 and Th2 immune responses.
Fig. 3.

Characterization of MERS-S1-specific immune responses induced by adenoviral vector vaccines. BALB/c mice were immunized intramuscularly with 1 × 1011 viral particles of AdΨ5 (C), Ad5.MERS-S (S), or Ad5.MERS-S1 (S1), respectively, and boosted intranasally with the same amount of each virus three weeks later. MERS-S1-specific IgG1 (A) and IgG2a (B) antibody levels were measured at the indicated time points by ELISA. Statically significant differences (Tukey's test) are marked by bars and asterisks. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001.
3.3. Induction of neutralizing antibodies to Ad5.MERS-S and Ad5.MERS-S1
Mouse sera were also tested for their ability to neutralize MERS-CoV (EMC isolate). Even a single immunization with adenoviral-based MERS vaccines induced detectable levels of MERS-CoV-neutralizing antibodies in all animals tested. After week 3 of booster immunization, animals developed robust levels of neutralizing antibodies, while control animals inoculated with AdΨ5 did not (Fig. 4 ). In some mice immunized with Ad5.MERS-S1, the highest neutralizing titers were observed as compared to mice immunized with Ad5.MERS-S, although no significant differences between the groups were noted. This result might suggest that Ad5.MERS-S1 expressing secreted S proteins induced a stronger Th2-polarized response, which led to a better antibody-mediated neutralizing activity when compared with Ad5.MERS-S (Fig. 3A).
Fig. 4.
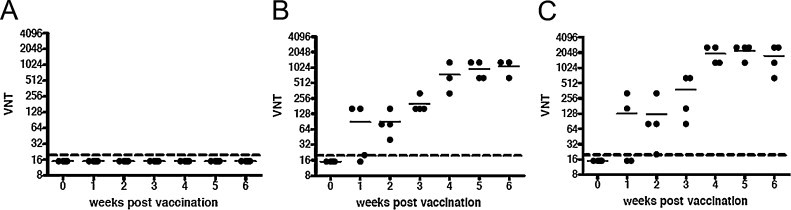
Induction of neutralizing antibodies in mice vaccinated with AdΨ5 (A), Ad5.MERS-S (B), or Ad5.MERS-S1 (C), respectively, as described in Fig. 3. MERS-CoV-neutralizing titers (VNTs) were measured every week after primary immunization using Vero cells by determining the highest dilution inhibiting MERS-CoV infection by 100%.
3.4. Anti-adenovirus type 5 neutralizing immunity in dromedary camel sera
Notably, one of the main limitations for the use of adenoviral-based vaccine in humans would be the presence of anti-adenoviral neutralizing immunity in a large percentage of camel populations. Thus, to demonstrate the potential of the proposed use of the Ad5.MERS candidate vaccines to be deployed as a veterinary vaccine in dromedary camels, we evaluated the presence of anti-human adenovirus type 5 neutralizing antibodies in this species. As shown in Fig. 5 , no neutralization was detected in 12 sera from dromedary camels, which is an encouraging first indication of the potential of this candidate vaccine for dromedary camels.
Fig. 5.
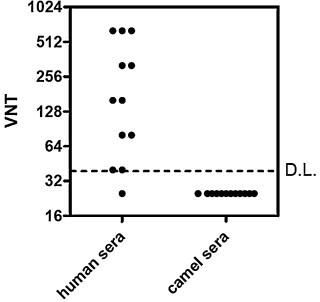
Presence of anti-adenovirus type 5-neutralizing immunity in dromedary camel sera. Twelve human and 12 dromedary camel sera were tested for the presence of anti-adenovirus neutralizing antibodies using standard adenoviral neutralization assay.
3.5. In vitro infectivity of recombinant adenovirus type 5 of dromedary camel PBMCs and fibroblasts
To provide further evidence for the potential use of Ad5.MERS-S1 as a vaccine in dromedary camels, we determined the susceptibility of dromedary camel cells to be infected by the human adenovirus serotype 5. Human or dromedary camel PBMC cells were transduced with recombinant adenovirus expressing EGFP and evaluated by flow cytometry analysis for EGFP expression. As shown in Fig. 6 , both human as well as dromedary camel PBMCs were successfully infected with Ad5.EGFP. Moreover, a large percentage of the dromedary camel fibroblast cell line, Dubca, were infected by Ad5.EGFP, indicating that the human adenovirus type 5 can indeed efficiently infect camel cells (Fig. 6E and F).
Fig. 6.

FACS analysis of recombinant adenovirus serotype 5 expressing EGFP (Ad5.EGFP) infection of human (A, B) and camel PBMCs (C, D) and camel fibroblasts (E, F). PBMCs and camel fibroblast Dubca cells were mock infected (A, C, and E) or infected with Ad5.EGFP (B, D, and F). After 24 h of infection, cells were washed with PBS and analyzed by FACS. Monocyte PBMC populations are shown and the percentage of GFP positive cells is represented in the rectangular box.
4. Discussion
Our results show that an adenoviral-based vaccine that expresses full-length or the S1 subunits of the S protein can induce MERS-CoV-specific neutralizing antibody responses in mice. It will be important to demonstrate whether dromedary camels vaccinated with these candidate vaccines or convalescing from MERS-CoV infection have similar responses and will be protected from MERS-CoV challenge, since this may indicate whether such vaccine-induced responses are indeed protective and future use of the Ad5.MERS-S vaccine as a veterinary vaccine in dromedary camels would be possible.
Previous studies have shown that RBDs of SARS-CoV presenting in the S1 subunit strongly react with antisera from SARS patients in the convalescent phase, and depletion of RBD-specific antibodies from SARS patients results in significant elimination of the neutralizing activity [43]. The RBD is the main domain that induces neutralizing antibody and T-cell immune responses against SARS-CoV infection [44]. A truncated RBD of MERS-CoV S protein was recently reported to potently inhibit viral infection and induce strong neutralizing antibody responses [45], [46]. SARS-CoV S and S2, but neither S1 nor other structural proteins, can induce apoptosis in Vero E6 cells [47], [48] and no histopathological changes were observed in various tissues of rats immunized with a recombinant adenovirus containing a truncated S1 fragment of the SARS-CoV [49]. In contrast, vaccination with recombinant modified MVA expressing SARS-CoV S protein is associated with enhanced hepatitis after challenge with SARS-CoV [50], [51] and SARS-CoV has been shown to infect hepatocytes and cause hepatitis in some human cases [51], [52], [53], raising concerns about the safety of a vaccine that contains the full-length SARS-CoV S protein. A causal relationship between the induction of hepatitis and the full-length nature of the S protein could not be conclusively demonstrated; it can be presumed that the S1 gene has less risk for spontaneous recombination with wild type virus following the generation of new virus types. Thus, we believe that an S1-expressing MERS-CoV vaccine would be a preferable vaccine candidate format. However, an alternative S antigen format such as the entire S-ectodomain or the S RBD domain could be evaluated for comparison. Since the capacity of our immunization strategy to protect from infection will require challenge tests in clinically relevant MERS-CoV disease animal models such as dromedary camels, establishment of such a model will also be important to exclude the potential for vaccine-induced immunopathology, as seen in the feline infectious peritonitis virus model [54], [55]. To this end, a mouse model for MERS-CoV infection that was generated by prior transduction of the animals with an adenoviral vector expressing the human host-cell receptor dipeptidyl peptidase 4 (hDPP4) was recently reported [56]. This mouse model will also be considered in the future for the evaluation of the protective efficacy of the adenovirus-based vaccines. While further investigations are necessary to evaluate the mucosal immunity and the ultimate protective efficacy of Ad5.MERS-S and Ad5.MERS-S1 in dromedary camels or the proper animal models, our results demonstrate that recombinant adenoviruses encoding MERS-S antigens may be protective vaccine candidates with a safe profile. Moreover, we have also investigated in the present study the infectivity of adenovirus type 5 of dromedary camel cells and the presence of anti-adenovirus type 5 neutralizing antibodies in a limited set of dromedary camel sera. Altogether, the presented studies support further exploration of Ad5.MERS vaccines to target the animal reservoir, reducing the risk of human exposure to MERS-CoV.
Acknowledgements
This project utilized the University of Pittsburgh Cancer Institute Vector Core Facilities supported by the University of Pittsburgh's National Institutes of Health Cancer Center Support Grant, award P30 CA047904. A.D.M.E.O., V.S.R., and B.L.H. are inventors on a patent application related to this work.
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3(6) doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haagmans B.L., Al Dhahiry S.H., Reusken C.B., Raj V.S., Galiano M., Myers R. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer B., Muller M.A., Corman V.M., Reusken C.B., Ritz D., Godeke G.J. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20(4):552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reusken C.B., Ababneh M., Raj V.S., Meyer B., Eljarah A., Abutarbush S. Middle East Respiratory Syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill. 2013;18(50):20662. doi: 10.2807/1560-7917.es2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- 7.Reusken C.B., Haagmans B.L., Muller M.A., Gutierrez C., Godeke G.J., Meyer B. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Who Mers-Cov Research Group State of Knowledge and Data Gaps of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Humans. PLoS Curr. 2013:5. doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson N.M., Van Kerkhove M.D. Identification of MERS-CoV in dromedary camels. Lancet Infect Dis. 2014;14(2):93–94. doi: 10.1016/S1473-3099(13)70691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raj S.V., Farag E.A., Reusken C.B., Lamers M.M., Pas S.D., Voermans J. Isolation of MERS coronavirus from a Dromedary Camel, Qatar, 2014. Emerg Infect Dis. 2014;20(8) doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrov D.S. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol. 2004;2(2):109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann H., Pohlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12(10):466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X., Gao X. Immunological responses against SARS-coronavirus infection in humans. Cell Mol Immunol. 2004;1(2):119–122. [PubMed] [Google Scholar]
- 14.Nie Y., Wang G., Shi X., Zhang H., Qiu Y., He Z. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis. 2004;190(6):1119–1126. doi: 10.1086/423286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV – a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T., Xie J., He Y., Fan H., Baril L., Qiu Z. Long-term persistence of robust antibody and cytotoxic T cell responses in recovered patients infected with SARS coronavirus. PLoS ONE. 2006;1:e24. doi: 10.1371/journal.pone.0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R., St Claire M. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363(9427):2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W., Tamin A., Soloff A., D‘Aiuto L., Nwanegbo E., Robbins P.D. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362(9399):1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci U S A. 2004;101(17):6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Zhang L., Qin C., Ba L., Yi C.E., Zhang F. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J Virol. 2005;79(5):2678–2688. doi: 10.1128/JVI.79.5.2678-2688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song F., Fux R., Provacia L.B., Volz A., Eickmann M., Becker S. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87(21):11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barratt-Boyes S.M., Soloff A.C., Gao W., Nwanegbo E., Liu X., Rajakumar P.A. Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J Gen Virol. 2006;87(Pt 1):139–149. doi: 10.1099/vir.0.81445-0. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan N.J., Geisbert T.W., Geisbert J.B., Xu L., Yang Z.Y., Roederer M. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424(6949):681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiver J.W., Fu T.M., Chen L., Casimiro D.R., Davies M.E., Evans R.K. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 26.Gao W., Soloff A.C., Lu X., Montecalvo A., Nguyen D.C., Matsuoka Y. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol. 2006;80(4):1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E., Okada K., Beeler J.A., Crim R.L., Piedra P.A., Gilbert B.E. Development of an adenovirus-based respiratory syncytial virus vaccine: preclinical evaluation of efficacy, immunogenicity, and enhanced disease in a cotton rat model. J Virol. 2014;88(9):5100–5108. doi: 10.1128/JVI.03194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smaill F., Jeyanathan M., Smieja M., Medina M.F., Thanthrige-Don N., Zganiacz A. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med. 2013;5(205):205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 29.Ouedraogo A., Tiono A.B., Kargougou D., Yaro J.B., Ouedraogo E., Kabore Y. A phase 1b randomized, controlled, double-blinded dosage-escalation trial to evaluate the safety, reactogenicity and immunogenicity of an adenovirus type 35 based circumsporozoite malaria vaccine in Burkinabe healthy adults 18 to 45 years of age. PLOS ONE. 2013;8(11):e78679. doi: 10.1371/journal.pone.0078679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braucher D.R., Henningson J.N., Loving C.L., Vincent A.L., Kim E., Steitz J. Intranasal vaccination with replication-defective adenovirus type 5 encoding influenza virus hemagglutinin elicits protective immunity to homologous challenge and partial protection to heterologous challenge in pigs. Clin Vaccine Immunol. 2012;19(11):1722–1729. doi: 10.1128/CVI.00315-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemula S.V., Mittal S.K. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther. 2010;10(10):1469–1487. doi: 10.1517/14712598.2010.519332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira T.B., Alves P.M., Aunins J.G., Carrondo M.J. Use of adenoviral vectors as veterinary vaccines. Gene Ther. 2005;12(Suppl. 1):S73–S83. doi: 10.1038/sj.gt.3302618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steitz J., Wagner R.A., Bristol T., Gao W., Donis R.O., Gambotto A. Assessment of route of administration and dose escalation for an adenovirus-based influenza A Virus (H5N1) vaccine in chickens. Clin Vaccine Immunol. 2010;17(9):1467–1472. doi: 10.1128/CVI.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qlaleye O.D., Baba S.S., Omolabu S.A. Preliminary survey for antibodies against respiratory viruses among slaughter camels (Camelus dromedarius) in North Eastern Nigeria. Rev Sci Technol Off Int Epiz. 1989;8(3):779–783. doi: 10.20506/rst.8.3.422. [DOI] [PubMed] [Google Scholar]
- 35.Hadia A.A.M., Lamia A.A., Shahain M.A. Estimation of adenovirus, bovine virus diarrhea and corona virus antibodies in camel serum. J Egypt Vet Med Assoc. 2001;60(6A):169–174. [Google Scholar]
- 36.Intisar K.S., Ali Y.H., Khalafalla A.I., Taha K.M., Rahman M.E.A. Adenovirus type 3 infection in camels in Sudan. Afr J Microbiol Res. 2010;4(13):1356–1358. [Google Scholar]
- 37.Hammer S.M., Sobieszczyk M.E., Janes H., Karuna S.T., Mulligan M.J., Grove D. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369(22):2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiver J.W., Emini E.A. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 39.Dietzschold B., Faber M., Schnell M.J. New approaches to the prevention and eradication of rabies. Expert Rev Vaccines. 2003;2(3):399–406. doi: 10.1586/14760584.2.3.399. [DOI] [PubMed] [Google Scholar]
- 40.Gao W., Rzewski A., Sun H., Robbins P.D., Gambotto A. UpGene. Application of a web-based DNA codon optimization algorithm. Biotechnol Prog. 2004;20(2):443–448. doi: 10.1021/bp0300467. [DOI] [PubMed] [Google Scholar]
- 41.Steitz J., Barlow P.G., Hossain J., Kim E., Okada K., Kenniston T. A candidate H1N1 pandemic influenza vaccine elicits protective immunity in mice. PLoS ONE. 2010;5(5):e10492. doi: 10.1371/journal.pone.0010492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M.L. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71(3):1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Y., Zhu Q., Liu S., Zhou Y., Yang B., Li J. Identification of a critical neutralization determinant of severe acute respiratory syndrome (SARS)-associated coronavirus: importance for designing SARS vaccines. Virology. 2005;334(1):74–82. doi: 10.1016/j.virol.2005.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Y., Li J., Du L., Yan X., Hu G., Zhou Y. Identification and characterization of novel neutralizing epitopes in the receptor-binding domain of SARS-CoV spike protein: revealing the critical antigenic determinants in inactivated SARS-CoV vaccine. Vaccine. 2006;24(26):5498–5508. doi: 10.1016/j.vaccine.2006.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du L., Kou Z., Ma C., Tao X., Wang L., Zhao G. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLOS ONE. 2013;8(12):e81587. doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mou H., Raj V.S., van Kuppeveld F.J., Rottier P.J., Haagmans B.L., Bosch B.J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013;87(16):9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chow K.Y., Yeung Y.S., Hon C.C., Zeng F., Law K.M., Leung F.C. Adenovirus-mediated expression of the C-terminal domain of SARS-CoV spike protein is sufficient to induce apoptosis in Vero E6 cells. FEBS Lett. 2005;579(30):6699–6704. doi: 10.1016/j.febslet.2005.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeung Y.S., Yip C.W., Hon C.C., Chow K.Y., Ma I.C., Zeng F. Transcriptional profiling of Vero E6 cells over-expressing SARS-CoV S2 subunit: insights on viral regulation of apoptosis and proliferation. Virology. 2008;371(1):32–43. doi: 10.1016/j.virol.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R.Y., Wu L.Z., Huang B.J., Huang J.L., Zhang Y.L., Ke M.L. Adenoviral expression of a truncated S1 subunit of SARS-CoV spike protein results in specific humoral immune responses against SARS-CoV in rats. Virus Res. 2005;112(1–2):24–31. doi: 10.1016/j.virusres.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78(22):12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czub M., Weingartl H., Czub S., He R., Cao J. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005;23(17–18):2273–2279. doi: 10.1016/j.vaccine.2005.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vennema H., de Groot R.J., Harbour D.A., Dalderup M., Gruffydd-Jones T., Horzinek M.C. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J Virol. 1990;64(3):1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss R.C., Scott F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis. 1981;4(2):175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Zhao J. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111(13):4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]


