Highlights
-
•
Efficient laboratories are key to the success of any disease programme.
-
•
PEI resources have contributed efforts in strengthening laboratory capacity in the region.
-
•
These contributions have not been systematically documented as lesson learnt.
-
•
With PEI programme on the ramp down, this paper documents it contributions for posterity.
Keywords: Laboratories, Polio, Network, Africa
Abstract
Background
The laboratory has always played a very critical role in diagnosis of the diseases. The success of any disease programme is based on a functional laboratory network. Health laboratory services are an integral component of the health system. Efficiency and effectiveness of both clinical and public health functions including surveillance, diagnosis, prevention, treatment, research and health promotion are influenced by reliable laboratory services. The establishment of the African Regional polio laboratory for the Polio Eradication Initiative (PEI) has contributed in supporting countries in their efforts to strengthen laboratory capacity. On the eve of the closing of the program, we have shown through this article, examples of this contribution in two countries of the African region: Côte d’Ivoire and the Democratic Republic of Congo.
Methods
Descriptive studies were carried out in Côte d’Ivoire (RCI) and Democratic Republic of Congo (DRC) from October to December 2014. Questionnaires and self-administered and in-depth interviews and group discussions as well as records and observation were used to collect information during laboratory visits and assessments.
Results
The PEI financial support allows to maintain the majority of the 14 (DRC) and 12 (RCI) staff involved in the polio laboratory as full or in part time members. Through laboratory technical staff training supported by the PEI, skills and knowledge were gained to reinforce laboratories capacity and performance in quality laboratory functioning, processes and techniques such as cell culture. In the same way, infrastructure was improved and equipment provided. General laboratory quality standards, including the entire laboratory key elements was improved through the PEI accreditation process.
Conclusion
The Polio Eradication Initiative (PEI) is a good example of contribution in strengthening public health laboratories systems in the African region. It has established strong Polio Laboratory network that contributed to the strengthening of capacities and its expansion to surveillance of other viral priority diseases such as measles, yellow fever, Influenza, MERS-CoV and Ebola.
This could serve as lesson and good example of laboratory based surveillance to improving diseases prevention, detection and control in our middle and low income countries as WHO and partners are heading to polio eradication in the world.
1. Introduction
The World Health Organization (WHO) African Region bears the highest burden of communicable diseases including vaccine preventable diseases and non-communicable diseases [1]. Since 1994, several laboratory networks have been established in the African Region as part of immunization programmes. These include polio, measles, and yellow fever. In some countries, these networks utilize the same infrastructure and human resources.
The laboratory network established in the African Region for the Polio Eradication Initiative (PEI) is composed of national and regional reference laboratories (Fig. 1 ). This network has supported PEI by effectively confirming outbreaks of the different types of strains of polioviruses. The quality of results correlates with the availability of resources, such as laboratory quality standards and the availability of skilled staff.
Fig. 1.
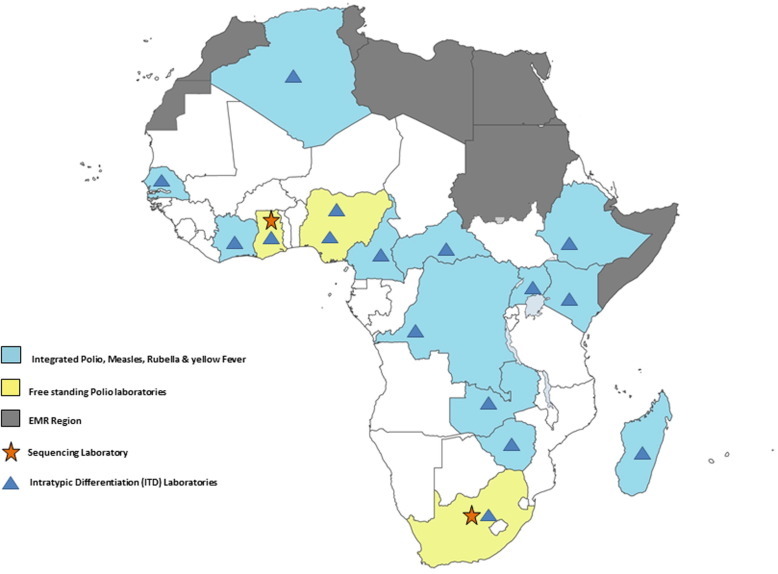
Map of laboratories in Africa.
The WHO global polio laboratory network is the largest public health laboratory network ever created. The African Regional Polio Laboratory Network forms part of this network and consists of three Regional Reference Laboratories (RRLs) based in Central African Republic, Ghana, and South Africa. In addition, 13 Intratypic Differentiation (ITD) laboratories are responsible for isolation of polioviruses from faecal samples using standardized procedures and reagents, molecular characterization of isolates and referral of critical isolates to a sequencing laboratory. In the African region, the RRLs in South Africa and in Ghana also provide genetic sequencing services to the African polio lab network.
The polio laboratory network is also in charge of reporting results to stakeholders in coordination with the PEI programme for further case investigations and poliovirus containment activities. As of January 2016, the most recent result to note is the eradication of the poliovirus type 2 in September 2015 [2]. The performance of these laboratories are regularly monitored and documented through WHO annual accreditation exercises and showed several successes in the current achievement.
The measles and yellow fever laboratory network has been built on a similar platform as the polio lab network and is currently made up of 37 Laboratories including RRLs based at Institut Pasteur de Côte d’Ivoire in Côte d’Ivoire, Uganda Virus Research Institute in Uganda, Institut Pasteur de Dakar in Senegal, and National Institute of Communicable Diseases (NICD) in South Africa. Cote d’Ivoire, Uganda and NICD laboratories cover regional functions for measles while Senegal laboratory covers regional functions for yellow fever. Because most of the measles elimination programs in the WHO African Region have moved to case based surveillance (which requires laboratory confirmation of all cases), laboratory support has become critical in assessing program progress.
The laboratory is indispensable for infectious diseases diagnosis, required for confirmation and is an important component of surveillance and response in line with existing and new frameworks such as the Integrated Disease Surveillance and Response (IDSR), the International Health Regulations (IHR 2005), the One Health approach and the Global Health Security Agenda (GHSA).
In the WHO African Region, despite the progress made during the recent years for investigation and response to epidemic prone diseases, laboratory services and systems are in general not fully efficient in supporting disease surveillance and control. Laboratories show weaknesses regarding the overall organization, qualified human resources, sustained financial support and required infrastructures and materials as well as compliance with quality assurance management and biosafety and security measures [3].
Safety in the laboratory is a concern for laboratory staff and is still a responsibility of the network to address such issues through various training and education to promote risk assessment and good laboratory safe practices. This will involve the reasonable safety handling of the laboratory equipment [4]. In such a context of weak national laboratory systems, the Poliomyelitis eradication programme which has established strong Polio Laboratory network contributed by the same way to the strengthening of capacities and its expansion to surveillance of other viral priority diseases such as measles, yellow fever, influenza, MERS-CoV and Ebola. These disease control programmes and other initiatives or programmes have partially benefited from PEI in term of infrastructure, staff capacity building, equipment, logistics, and financing. However, this statement is not yet fully documented by the WHO Regional Office for Africa (AFRO).
The purpose of this publication is to give a feedback of the laboratory best practice that was developed as part of PEI in two countries namely, Côte d’Ivoire and DRC. The overview demonstrates how the platform of the polio laboratory network was used to strengthen the laboratory based-surveillance of other diseases such as measles and yellow fever in these two countries through infrastructure and trainings. These best practices are to improve laboratory system and integration of laboratory services as well as networking of regional public health laboratories as a part of PEI legacy planning.
2. Methods
As part of documentation of PEI best practices in the African region in selected countries descriptive studies were carried out in Côte d’Ivoire and Democratic Republic of Congo (DRC) from October to December 2014. The studies used two standardized electronic questionnaires and self-administered and in-depth interviews and group discussions as well as on examination of documents and observation during laboratory visits and assessments. The first was to collect detailed information on best practices and the second questionnaire to collect information on the contribution or impact of the resources acquired for the eradication of polio on the laboratory infrastructures, functioning, services, equipment and staff.
Both laboratories have undergone trainings organized by the regional office on different methods to determine poliovirus (PV) serotypes and whether the virus was wild or related to vaccine strains (known as intratypic differentiation or ITD) [5], [6], [7], [8], [9], [10], [11]. Any wild poliovirus is further characterized to determine if it is an importation or indigenous virus [12], [13].
2.1. Trainings
African Polio Lab network has made changes to the laboratory diagnostics protocols and this has improved laboratory performance and reduced the result turnaround time dramatically. These changes have necessitated the need to strengthen the laboratory quality control and quality assurance systems through the implementation of quality cell culture techniques including cell sensitivity testing, cell validation and calibration trainings. In 2014, a refresher course was organized to strengthen and sustain the necessary support to the Polio Eradication Programme and to provide practical orientation on how to maintain cells and monitor their sensitivity. The objectives was to provide practical orientation to participants on the mechanisms of cell culture propagation, train participants on making their own house reference standards and train them on their technical orientation on monitoring cell sensitivity (Fig. 2 ).
Fig. 2.
Training of polio laboratories.
2.2. Technical support and accreditation visits
The purpose is to conduct an on-site review of the performance of the polio laboratory at the Institut for the last 12 months to check if the laboratory still meets the required WHO recommended standards.
Accreditation process is used to measure if the laboratory has the capability and the capacity to detect, identify, and promptly report wild polioviruses, vaccine derived polioviruses (VDPV) and Sabin viruses that may be present in clinical and environmental specimens. This process further provides a mechanism for identifying resource and training needs of the laboratory staff. It also a measure of progress made by laboratories in order to meet WHO set criteria for all WHO Global Polio Laboratory Network (GPLN). The 11 criteria set by WHO are as stated in Table 1 for different assays as stated in the checklists [14].
Table 1.
Accreditation criteria for virus isolation and intratypic differentiation (ITD).
| 1 | Tests are performed on at least 150 stool specimens annually: | |
| 2 | Results are reported on at least 80% of all AFP specimens within 14 days: | |
| 3 | At least 80% of suspected poliovirus isolates from AFP cases and contacts are forwarded for ITD within 7 days, if applicable: | |
| 4 | Accuracy of identification of polioviruses among all isolates obtained in L20B cells is at least 90%: | |
| 5 | Score on most recent virus isolation PT is at least 90%: | |
| 6 | Score on annual on-site review is at least 80%: | |
| 7 | ITD test results on ⩾80% of poliovirus isolates are reported within 7 days: | |
| 8 | Non-Sabin-like poliovirus and ITD-discordant isolates from ⩾80% of AFP cases, contacts and other sources are referred for sequencing within 7 days of detection, if applicable: | |
| 9 | Score on most recent intratypic differentiation PT is at least 90%: Score for rRT-PCR ITD: Score for rRT-PCR VDPV screening: |
|
| 10 | Accuracy of poliovirus serotype and intratype is at least 90%: | |
| 11 | Score on annual on-site review is at least 90%: | |
| 12 | Annual NPEV isolation rate is: |
3. Results
The DRC is one of the largest (2.35 million square kilometers) and most populous (approximately 75 million inhabitants) countries in sub-Saharan Africa. It shares borders with nine countries, two borders (with Angola in the south and the Republic of Congo [Congo] in the west) extend >2000 km. The DRC is administratively divided in 11 provinces (including the city-province of Kinshasa); each province is further divided into districts. The public health system is organized in 515 health districts (Zones de Santé). The national polio laboratory of the Democratic Republic of the Congo is hosted since its operation by the National Institute of Biomedical Research (INRB).
Côte d’Ivoire is a West African country on the Atlantic coast, is the country’s major urban center. The estimated total population is approximately 20.3 million. The country is divided into 31 regions with Abidjan having the highest population.
The poliomyelitis eradication programme has contributed to the establishment and strengthening of capacities of the Polio Laboratory in several domains of laboratory, as well as key elements as quality standards or process aspects. That allows its expansion to surveillance of other diseases. The most important contribution to laboratory capacity and system strengthening are as follows.
3.1. Human resources
Like in all programmes, human resource crisis is a limiting laboratory services at all levels of the health systems.
The INRB has 14 staff members comprising of a Director, 3 biologists, 8 technicians and 1 logistician. All members are fully involved will the Polio laboratory activities, however there is a cross cutting activities such as measles and yellow fever. These staff members are fully supported financially by the polio programme.
The Reference Laboratory for Polio, located in Pasteur Institute Côte d’Ivoire has 12 staff composed 03 doctors and 6 technicians, 2 engineers and 1 logistician. The majority of the staff is involved in the polio laboratory as full or in part time members. The polio work is fully financial supported by WHO and some staff are paid by WHO.
The funding support that the regional office implemented to these two laboratories has decreased a staff turnover by closed 100% which previously had caused a serious paralysed system within the network as staff members had to leave the polio work and look for better paying positions elsewhere.
3.2. Trainings
The National laboratories are responsible for isolation of polioviruses from faecal samples using standardized procedures and reagents, referral of suspected poliovirus isolates to the Regional Reference Laboratories, reporting results to stake holders, coordination with the Programme in case investigations and poliovirus containment activities. The Regional Reference Laboratories and ITD laboratories serve as Polio National Laboratories to their own countries and to other specified countries that do not have National Laboratories. They also perform intratypic differentiation on suspected poliovirus isolates to classify them into wild or Sabin viruses. In the case of the South African laboratory it also provides genetic sequencing services to the African laboratory network. Based on the annual trainings organized by the regional office, both laboratories have maintained a stable laboratory performance that is above target of all laboratory performance indicators.
The eradication of poliomyelitis will be accomplished only when these laboratories provide convincing diagnostic evidence of the absence of wild poliovirus infections in humans and circulation in the environment. In addition, because these laboratories store specimens from acute flaccid paralysis (AFP) cases and wild polioviruses isolates, containment of these materials and viruses remains one of the laboratories responsibilities that are a pre-requisite for certifying Africa free of polio.
The above functions require that the network laboratories are efficiently coordinated in order to ensure the desired quality performance. This quality performance is made possible through the implementation of quality control and quality assurance systems.
3.3. Infrastructure
A new procedure adopted by the polio laboratory for poliovirus isolation and identification as per the new standard WHO algorithm of testing provides results of primary isolation within 14 days of receipt of sample in the laboratory. Also results of characterization as a wild type, VPDV or vaccine strain of polioviruses by an intratypic differentiation (ITD) testing procedure should be available within seven days.
In August 2008, the Global Polio Working Group has approved the RT-PCR technique developed by the United States Centers for Disease Control and Prevention (CDC) for implementation in the Global Polio Laboratory Network (GPLN) to differentiate the wild, VDPV and vaccine poliovirus strains.
In 2010, the new technique was adopted and implemented in the African region. Based on the agreement to adopt real time PCR for ITD laboratories, there was a need of procuring the real time machine as the majority of the laboratories were not molecular laboratories. The GPLN procure 16 machines for each laboratory in the African region, and DRC and Côte d’Ivoire laboratories were part of the beneficiaries for this new change.
3.4. Technical support
A hands-on training was planned to introduce the newly developed real time PCR technique for rapid detection of wild type, VDPV and vaccine poliovirus strains. Participants were trained for laboratory procedures and work practices in confirmation of poliovirus using the new real time PCR techniques for ITD and VDPV screening. With the introduction of this technique, the time and cost in performing sequencing to detect VDPV was reduced.
In 2014 a technical visit was conducted by WHO in the Institut Pasteur de Cote d’Ivoire Polio Laboratory to assess the laboratory performance based on the low indicators of non-polio enterovirus isolation rate. The findings revealed that some existing standard operation procedures need to be developed and some to be updated. It was noted that some equipment need to be procured based on the amount of work handled by the laboratory. There was a need of follow-up regarding the maintenance of some equipment as they were no records available during the time of the visit.
3.5. Accreditation visits
The National Polio Laboratory (NPL) of Côte d’Ivoire is hosted by the Institut Pasteur de Côte d’Ivoire in Abidjan. Its activity includes the acute flaccid paralysis (AFP) surveillance of five countries namely; Côte d’Ivoire, Burkina-Faso, Mali, Liberia and Sierra Leone. The NPL performs viral isolation and ITD and uses the ITD methodology of real-time polymerase chain reaction technique (rRT-PCR).
The number of stool specimens received during the recent accreditation visit was 1853. Following the Ebola Epidemics, for biosafety reasons, stool specimen of Liberia and Sierra-Leone were not received from July 2014.
Staffs are competent and the number is adequate for the current workload. NPL activities are perfectly traceable and documentation of records and archives is available. Job descriptions mentioning tasks and responsibilities of each staff are available. Work assignments in a weekly work plan are however recommended. Training courses for two technical staffs are also recommended.
The laboratory met all the criteria for full accreditation with regard to viral isolation and ITD activities. Scores of proficiency testing for virus isolation and ITD are satisfactory. Score on on-site review of laboratory procedures and methodologies are met. Timeliness of reporting virus isolation and ITD results, timeliness of referral of isolates for sequencing, accuracy of L20B positives serotype and intratypic identification are adequate.
The National Polio Laboratory (NPL) of the Democratic Republic of the Congo is hosted since its operation by the National Institute of Biomedical Research (INRB). During last accreditation visit, the laboratory has received and tested 3977 faecal samples from AFP cases; no wild poliovirus identified of these samples. Performance indicators showed (i) a good timeliness of rendering the results of virus isolation and intratypic differentiation (ITD), (ii) a rapid transfer of the isolates with results of said discordant to the laboratory in charge of sequencing, (iii) to excellent scores on the Proficiency Test virus isolation and ITD, and (iv) a good concordance of the results with those received from the sequencing laboratory.
The main weaknesses of the laboratory reside in - infrastructure: problem of supply electricity dimensions of the rooms and corridors by non-functional equipment - safety/security: wiring and operation of the network (mains vs generator) pose a serious safety concern for the staff and the laboratory. Biosecurity: the hood biological safety used for the treatment of samples is more functional and the viral isolation room does not autoclave. The lack of maintenance and calibration of equipment.
3.6. Contribution to the improvement of Laboratory key elements
The poliomyelitis reference laboratory (Institut Pasteur of Côte d’Ivoire (IPCI) in Côte d’Ivoire and Institut National de Recherché Biologique (INRB) in DRC) were partially used for diagnosis activities of other diseases, mainly vaccine preventable diseases such Yellow fever, Measles, Influenza, Rubella, Ebola, seroprevalence studies (Hemorrhagic fevers - Rabies - Herpes - Measles, Influenza). The two reference laboratories are also sub-regional reference laboratories for other countries. They offer biological diagnosis using methods such as cell culture and pathogens characterization and equipment which are used for the implementation of other public health programmes (e.g. Measles, Yellow Fever, and Influenza).
Infrastructure was improved, making the laboratory space and commodities adequate for the work conditions and concordant with biosafety and security requirements. This also improved the workload and thus allowed satisfactory conditions for staff. A virology laboratory was installed at the IPCI (to be extended), and new and adapted equipment was acquired (to be detailed in a table together with equipment gained in DRC). Those facilities were used by the units implementing other public health programmes. PEI dedicated staff also perform diagnosis activities of other priority diseases under surveillance as indicated in Table 1.
3.7. Contribution to the improvement of Laboratory quality standards
Accreditation provides documentation that the laboratory has the capability and the capacity to detect, identify, and promptly report wild polioviruses, vaccine derived polioviruses (VDPV) and Sabin viruses that may be present in clinical and environmental specimens. The accreditation process further provides a learning opportunity, a mechanism for identifying resource and training needs, a measure of progress, and a link to the WHO Global Polio Laboratory Network (GPLN).
Accreditation of laboratories is reviewed annually by the WHO Regional Office and is based on laboratory performance during the immediately preceding 12 months with complete data and accreditation is given for the upcoming period of 12 months.
Institut National de Recherché Biologique (INRB) was accredited as a WHO national reference laboratory in 2000 and has facilitated disease surveillance and control in the DRC and other countries such as Republic of Congo. In the same note Institut Pasteur of Côte d’Ivoire (IPCI) in Côte d’Ivoire is a fully WHO accredited national reference laboratory servicing Burkina Faso, Côte d’Ivoire, Mali, Liberia and Sierra Leone. For the past years both laboratories have been fully accredited to serve as the national laboratories for the countries mentioned above.
In addition to accreditation, laboratories are expected to participate in the external proficiency scheme provided by Centers for Disease Control and Prevention (CDC), Atlanta and Rijksinstituut voor Volksgezondheid en Milieu (RIVM), Netherlands. Both laboratories have performing very well in the past exercises by reaching the above passing marks and were deemed competent to remain in the regional polio network.
3.8. Contribution to the improvement of Laboratory functioning and processes
Polio networks used to support safely collection and timely transportation of specimens (personnel, costs, transportation means)
Equipment maintenance costs and other financial contributions from the Polio laboratory help to reduce the financial burden of the institutions hosting the laboratory.
Full time and half time staff paid by the PEI also work on other diseases. Incentive allowance paid to “polio staff” and the incentives granted to “polio staff” also go to some measles-yellow fever laboratory workers.
Surveillance and monitoring and evaluation of Yellow Fever, Measles and Influenza control programmes were strengthened as they were partially taken into account by the PEI laboratory network. Using the same polio network platform, introduction of new vaccines such Rota, meningitis, Hepatitis benefited as the same molecular techniques implemented for polio is in used of the other programmes such the ones mentioned before (Table 2 ).
Table 2.
Time polio laboratory staff spent on other programmes in Côte d’Ivoire and Democratic Republic of Congo.
| Côte d’Ivoire |
Democratic Republic of Congo |
||||
|---|---|---|---|---|---|
| Function | Number of staff | Mean percentage of time spent on managing other pathogens (%) | Function | Number of staff | Mean percentage of time spent on managing other pathogens (%) |
| Lab head | 1 | 20 | Director | 1 | 70 |
| Biologist | 1 | 50 | Biologist | 4 | 25 |
| Data manager | 1 | 50 | Data manager | 1 | 25 |
| Technicians | 6 | 48.8 | Technicians | 8 | 25 |
4. Discussion
The laboratory staff members in the African region of whom many are working in insecure or very challenging have shown commitment and dedication. They also had been at the center of strengthening immunization. The polio network has been used to strengthen integrated disease surveillance and response (IDSR) in most African countries. This network has supported the elimination and control of other vaccine preventable diseases such as particularly yellow fever and measles. The same staff members have been involved with the epidemiological and virological surveillance of influenza as well as response to other major public health threats including the Ebola virus and Marburg virus outbreaks. The valuable technical support that they are providing to the national authorities and other stake-holders is a major contributory factor to the progress that the polio network continues to register.
Both laboratories have contributed tremendously to the polio eradication programme in providing reliable and timely results for the PEI programme to act on outbreak response should a wild poliovirus or vaccine derived poliovirus is reported.
However, apart from the excellent contribution of the polio network to immunization, there is still a big gap in meeting the needs of other vaccine preventable diseases programmes. Funding still creates a separation between polio and other VPD programme and causes some divisions in other laboratories.
The lesson learnt by other programme from polio surveillance had helped to maintain a stable surveillance system for other programmes. Collection, storage and transportation of stool samples within a set turnaround time, has taught other programmes to maintain similar process in adequate shipment of infectious substances within and outside the country.
The polio laboratory network is the system that manages the processes and standards required to produce accurate reliable and timely results. The implementation of new techniques as it was the case needed dedicated and motivated staff, good quality management systems, safe environment, adequate infrastructure and a good supervisory role. The WHO funds spent on PEI has helped in investing in people, infrastructure and system successfully.
Using the same infrastructure, this can improve and strengthened other disease surveillance systems, laboratory networks and monitoring and evaluation other diseases. The support will be in the form of technical guidance, effective quality assurance, systems and capacity building are all-attainable. After the eradication of poliovirus, a new focus on other diseases in terms of strengthening laboratory systems for quality assurance as it was learned from polio programme will help achieve the target for surveillance and response to priority diseases including any disease elimination or eradication.
Conflict of interest
The authors have no conflict to report.
Acknowledgements
We would like to thank the staff from the National Polio Laboratory at Institut Pasteur de Côte d’Ivoire, the staff from Institut National de Recherché Biologique (INRB) and EPI focal persons from WHO offices in DRC and Côte d’Ivoire. We are indebted with support received from the World Health Organization laboratory coordinators from the African region. World Health Organization supported this work.
References
- 1.WHO/AFRO In the African Region making people healthier [Internet]. Brazzaville, Congo. 2014. www.afro.who.int/index.php?option=com_docman&task
- 2.WHO Global eradication of wild poliovirus type 2 declared. [Internet]. Global Polio Eradication Initiative. 2015. www.polioeradication.org›Media room›News stories
- 3.Ridderhof J.C., Deun A.V., Kam K. Roles of laboratories and laboratory systems in effective tuberculosis programmes. Bull World Health Org. 2007;85(5) doi: 10.2471/06.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz M.A., Rysweska K., Laszlo A. Strategic approach for the strengthening of laboratory services for tuberculosis control, 2006–2009 [Internet]. Geneva, Switzerland. 2006. http://whqlibdoc.who.int/hq/2006/WHP_HTM_2006.364_eng
- 5.WHO Progress towards global polio eradication – status of wild poliovirus circulation in Africa. Wkly Epidemiol Rec [Internet] 2012;12(87):109–116. http://www.who.int/wer/2012/wer8712.pdf [PubMed] [Google Scholar]
- 6.van der Aoort H.G., Hull B.P., Hovi T., Pallansch M.A., Kew O.M., Crainic R. Comparative study of five methods for intratypic differentiation of polioviruses. J Clin Microbiol. 1995;33(10):2562–2566. doi: 10.1128/jcm.33.10.2562-2566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Vaccines and biologicals: supplement to the WHO Polio Laboratory Manual [Internet]. Geneva, Switzerland. 2004. http://www.who.int/immunization_monitoring/Supplement_polio_lab_manual.pdf
- 8.Kilpatrick D.R., Nottay B., Yang C.F., Yang S.J., Da Silva E., Penaranda S. Serotype-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J Clin Microbiol. 1998;36(2):352–357. doi: 10.1128/jcm.36.2.352-357.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C.F., De L., Holloway B.P., Pallansch M.A.K.O. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 1991;20(2):159–179. doi: 10.1016/0168-1702(91)90107-7. [DOI] [PubMed] [Google Scholar]
- 10.Chezzi C. Rapid diagnosis of poliovirus infection by PCR amplification. J Clin Microbiol. 1996;34(7):1722–1725. doi: 10.1128/jcm.34.7.1722-1725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick D.R., Nottay B., Yang C.F., Yang S.J., Mulders M.N., Holloway B.P. Group-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosineresidue at positions of codon degeneracy. J Clin Microbiol. 1996;34(12):2990–2996. doi: 10.1128/jcm.34.12.2990-2996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 13.Gascuel O., Steel M. Neighbor-joining revealed. Mol Biol Evol. 2006;23(11):1997–2000. doi: 10.1093/molbev/msl072. [DOI] [PubMed] [Google Scholar]
- 14.WHO . 4th ed. 2004. Polio laboratory manual. 166 p. [Google Scholar]


