Abstract
Infectious bronchitis virus (IBV) poses a major threat to the chicken industry worldwide. In this study, we developed a recombinant fowlpox virus (rFPV) vaccine expressing the IBV S1 gene and chicken interleukin-18 gene (IL-18), rFPV-S1/IL18. Recombinant plasmid pSY-S1/IL18 was constructed by cloning chicken IL-18 into fowlpox virus transfer plasmid containing S1 gene and transfected into the chicken embryo fibroblasts cell pre-infected with S-FPV-017 to generate rFPV-S1/IL18. Expression of the recombinant proteins was confirmed by RT-PCR and IFA. We also constructed the recombinant fowlpox virus rFPV-S1 without IL-18. One-day-old chickens were vaccinated by wing-web puncture with the two rFPVs, and the induced humoral and cellular responses were evaluated. There was a significant difference in ELISA antibody levels (P < 0.05) elicited by either rFPV-S1 or rFPV-S1/IL18. The ratios of CD4+ to CD8+ in chickens immunized with rFPV-S1/IL18 were significantly higher (P < 0.05) than in those immunized with rFPV-S1. All chickens immunized with rFPV-S1/IL18 were completely protected (20/20) after challenge with the virulent IBV HN99 strain 43 days after immunization, while only 15 out of 20 of the chickens immunized with the rFPV-S1 were protected. Our results show that the protective efficacy of the rFPV-S1 vaccine could be enhanced significantly by simultaneous expression of IL-18.
Keywords: Infectious bronchitis virus, S1 gene, Chicken interleukin-18 gene, Recombinant fowlpox virus
1. Introduction
Infectious bronchitis (IB) is an acute, highly contagious respiratory, renal, and urogenital disease of chickens caused by the coronavirus, infectious bronchitis virus (IBV). It is still a major health problem in the chicken industry worldwide. Vaccination to control IB has been practiced for over half a century [1], [2], [3]. Such conventional vaccines, although generally effective, do have some disadvantages. Attenuated vaccines, which generally induce long-lasting immunity, have a risk of insufficient attenuation and/or genetic instability [4]. The limitations of inactivated vaccines include high manufacturing costs and lack of long-term immunity. Thus, developing a vaccine to control this disease with higher efficacy and fewer side effects is highly desirable.
Since IBV was first described by Schalk and Hawn in the 1930s [5], numerous serotypes or variants have been identified worldwide, against which little or no cross-protection exists [6], [7]. IBV was first detected in China in 1972, and numerous nephropathogenic strains have been isolated since 1982 [8]. In spite of extensive vaccine use, IBV outbreaks remain frequent in China. Infected broilers show clinical signs of depression, dehydration, and polyuria, with swelling of the kidneys and severe urate deposition, which results in death. Infected breeders or layers have decreased egg production. The HN99 nephropathogenic strain of IBV was isolated from one of a group of approximately 3-week-old broilers in Henan Province suffering from depression, dehydration, and polyuria. Because it is the most prevalent strain in China, we developed a recombinant anti-IBV vaccine based on this virus.
The IBV gene encoding the virus surface glycoprotein spike protein 1 (S1) was a logical choice for inclusion in a recombinant candidate vaccine vector. The S1 protein is considered to be a primary inducer of protective immunity [9]. For example, four intramuscular administrations of immuno-affinity purified S1 induced 78% protection against IBV challenge [10]. Likewise, recombinant baculovirus expressing the S1 gene of a Korean nephropathogenic strain of IBV protected 50% of inoculated chickens against IBV challenge, as assessed by examination of their kidneys [11]. Overall, the results of these studies indicate that the S1 glycoprotein would be a useful candidate for inclusion in alternative IBV vaccines.
Fowlpox virus (FPV) has a large double stranded DNA genome and a host range limited to avian species [12]. FPV has been developed as an effective live viral vector, successfully expressing protective foreign genes from various poultry pathogens, including Newcastle disease virus, avian influenza virus, IBV, infectious laryngotracheitis virus, and Marek's disease virus. Fowlpox virus has a number of advantages as a vector, but its side effects cannot be ignored: its effects on weight gain and on immune function [13]. It has been confirmed that some cytokines can relieve these side effects, and some of these cytokines are effective immunomodulators in animal models or clinical trials. Cytokine adjuvants have been widely used to promote the induction of immune responses and enhance the immunoprotective effects of vaccines against bacteria, viruses, or parasites [14]. IL-18 is one possible option, and is known as interferon-gamma (IFN-γ)-inducing factor because of its ability to stimulate T helper 1 (Th1) cells to secrete IFN-γ [15]. Therefore, the purpose of the present study was to construct two recombinant fowlpox viruses (rFPVs) expressing the S1 gene of IBV and co-expressing the S1 gene of IBV and the chicken IL-18 gene, which was included to overcome the FPV-induced inhibition of weight gain, and increase the efficacy of immunization.
2. Materials and methods
2.1. Virus, experimental animals and plasmids
Fertilised White Leghorn specific-pathogen-free (SPF) eggs were purchased from Shangdong Institute of Poultry Science, Shandong, PR China. Chickens were hatched and housed in a SPF environment at the Laboratory Animal and Resources Facility, Henan Agricultural University. The nephropathogenic strain HN99 of IBV was propagated in the allantoic cavities of 10-day-old SPF embryonated chicken eggs, and the allantoic fluid was harvested 48 h after inoculation. The median embryo infective dose (EID50) was determined by inoculating a 10-fold dilution series of the virus into 10-day-old SPF embryonated chicken eggs.
The parental fowlpox virus, S-FPV-017, was a less attenuated FPV strain (a kind gift from Dr. Hua-Lan Chen, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences).
Recombinant plasmids pGEM-T-S1 and pGEM-T-IL18 were used in this study. A cDNA fragment encoding the full-length S1 gene was amplified from the RNA of IBV HN99 strain and cloned into the pGEM T-Easy vector. The resulting plasmid, pGEM-T-S1 was sequenced (GenBank accession no. AY775551). The chicken IL-18 gene was obtained from chicken splenocytes by reverse transcriptase-polymerase chain reaction (RT-PCR) and cloned into pGEM T-Easy, and the resultant recombinant plasmid, pGEM-T-IL18, was sequenced (GenBank accession no. AY775780).
2.2. Homologous recombination and screening of the recombinant virus
The plasmid pSY-S1/IL18 was constructed as described previously [16]. Briefly, cDNA encoding the whole S1 gene of IBV was amplified by PCR from the plasmid pGEM-T-S1 using the forward primer 5′-ATGAGGATCCAATGTTGGTGAAG TCACT-3′ and the reverse primer 5′-ATGCG GATCCATA ACTAACATAAGGGCA-3′ (BamH I restriction enzyme site is shown by an underline on the sense and antisense primers). The PCR product was digested with BamH I and cloned into similarly digested plasmid pSY538 under the control of the early-late LP2EP2 promoter of FPV. The LacZ gene fragment with the P11 late promoter of vaccinia virus from the plasmid pSC11 was digested with Pst I and Xba I, and also cloned into the Sma I site of the pSY538 containing the S1 gene. The fragment containing the S1 and LacZ genes was cloned into a Not I site between the homologous arms of the poxvirus gene in the FPV transfer vector pSY681, resulting in the plasmid pSY-S1. For the construction of plasmid pSY-S1/IL18, the chicken IL-18 gene was amplified by PCR from pGEM-T-IL18 using the primers 5′-CCCGAATTCATGAG CTGTGAAG AGATC-3′ and 5′-CGGGGAATTCTCATAGGTTGTGCCTTT-3′ (EcoR I restriction enzyme site is shown by an underline on the sense and antisense primers). The PCR product was digested with EcoR I and cloned into similarly digested pSY538 under the control of the early-late LP2EP2 promoter of FPV. Finally, pSY-S1/IL18 was constructed by the insertion of the fragment containing the LP2EP2 promoter and the IL-18 gene into pSY-S1.
The rFPVs were generated by homologous recombination using published procedures [17]. Briefly, two rFPVs (rFPV-S1 and rFPV-S1/IL18) were generated by transfecting into chicken embryo fibroblasts (CEF) with the corresponding recombinant plasmids in six-well plates which had been infected with the parental fowlpox virus S-FPV-017 at multiplicity of infection (m.o.i.) of 0.01 two hours before transfection. Parental fowlpox virus S-FPV-017 infected CEF cells were used as an infection control. The viruses were collected after cytopathic effect (CPE) appeared, and rFPVs were screened for beta-galactosidase activity in the presence of 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal) (TaKaRa, Dalian, China). After eight rounds of blue plaque purification, the two rFPVs were obtained and cultured in CEF cells. Insertion of the recombinant gene into the FPV genome was confirmed by PCR, and expression of S1 and IL-18 confirmed by RT-PCR and indirect immunofluorescence assay (IFA) as described below.
2.3. PCR analysis of the rFPVs
The genomic DNA of rFPVs, extracted using SDS-proteinase K-phenol, was used as PCR template and amplifications were performed with TaKaRa Ex Taq DNA polymerase and the primers described above.
2.4. RT-PCR analysis
After infection for 48 h, the cells were harvested and total cellular RNA was prepared from the cells using Trizol reagent (Gibco BRL, USA). The reverse transcription (RT) reaction was performed using 20-μl volumes; the reaction mixture contained 5× Strand buffer, 25 mM of each deoxynucleoside triphosphate (dNTP; Amersham Biosciences Corp., Piscataway, NJ, USA), 2.5 U of RNase inhibitor (Promega Corporation, Madison, WI, USA), 50 pmol/ml random hexamers, Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA, USA), and 5 μl of total cellular RNA and diethyl pyrocarbonate (DEPC)–water. RT was performed at 42 °C for 60 min and at 75 °C for 10 min. Polymerase chain reaction (PCR) was then amplified with specific primer sets for the S1 gene and IL-18 as described above.
2.5. Indirect immunofluorescence assay of rFPV infected cells
After infection for 48–72 h, cells were washed with phosphate-buffered-saline (PBS) and fixed with cold methanol for 10 min. Cells were blocked with 1% bovine serum albumin (BSA) in PBS for 30 min at 37 °C. The fixed cells were incubated at 37 °C for 1 h with an IBV specific-chicken antiserum at a dilution of 1: 50. After three washes for 5 min each with PBS, the cells were incubated for 45 min at 37 °C with secondary fluorescein isothiocyanate (FITC)-labeled rabbit anti-chicken antibody (Sigma Chemical Co., St. Louis, USA) at a dilution 1:800. Cells were washed three times with PBS, and then examined with a fluorescent microscope (Model AX70, Olympus).
2.6. Immunization of chickens with the rFPVs
Eighty one-day-old White Leghorn SPF chickens were randomly allocated into four groups of 20. Chickens in groups 1 and 2 were immunized with rFPV-S1/IL18 or rFPV-S1, respectively. Chickens in group 3, which served as negative controls, were immunized with the same amount of S-FPV-017. Chickens in group 4 were inoculated with sterile PBS. All immunizations were done by wing-web puncture with a double needle used for commercial vaccination of poultry with FPV. Approximately 50 μl of inoculum containing 106 plaque-forming units (PFU) of FPV were given to each chicken.
2.7. Detection of anti-IBV specific antibodies
Pre-vaccination sera were collected from all vaccinated chickens. Five chickens were sampled randomly from each group at 1, 2, 3, 4, 5, and 6 weeks after immunization and blood samples were collected via wing vein puncture. Sera at a 1:20 dilution were tested for development of specific antibodies.
Total serum immunoglobulin G (IgG) specific for IBV was measured by ELISA as described previously [17], with modifications. Briefly, ELISA plates were coated with IBV lysate at 6 μg/ml in carbonate buffer, pH 9.6, overnight at 4 °C and blocked with 5% skimmed milk in PBS at 37 °C for 2 h. Serum samples were tested at a 1:20 dilution in 5% skimmed milk in PBS containing 0.25% Tween-20 (PBST). IgG against IBV was detected with horseradish peroxidase (HRP)-labeled rabbit-anti-chicken conjugate diluted 1:2000 in PBST. After 20 min incubation in the dark with TMB microwell peroxidase substrate solution, the reaction was stopped by the addition of 100 μl of 2 M H2SO4, and the optical density at 450 nm was measured in an ELISA microplate reader. Tests were run in duplicate. Negative and positive control sera were included in each assay. Total serum IgG specific for IBV was represented by the optical density.
2.8. Analysis of CD4+, CD8+ and CD3+ T-lymphocytes
Five chickens were sampled randomly from each group at 1, 2, 3, 4, 5, and 6 weeks after immunization and peripheral blood samples were collected from the jugular vein in 2.5 ml syringes containing 0.2 ml of sodium heparin to prevent clotting. Peripheral blood mononuclear cells (PBMC) were isolated from each blood sample by Ficoll-Hypaque density gradient centrifugation. PBMC were adjusted to 1 × 107 cells/ml, and 100 μl of the resuspended cells (1 × 106 cells) were incubated for 1 h at room temperature with the following antibodies (double labeled): mouse anti-chicken CD3-Spectral Red (SPRD) and mouse anti-chicken CD4-R-phycoerythrin (R-PE) or mouse anti-chicken CD8a-R-PE (BD Biosciences Pharmingen). The samples were analyzed on a fluorescence activated cell sorter.
2.9. Virus challenge experiment
All of the chickens were challenged with 100 EID50 of IBV strain HN99 in 0.1 ml by the oculonasal route on day 43 after immunization. The challenged chickens were examined daily for signs of clinical illness, including coughing, sneezing, ataxia or dyspnea, for 2 weeks. Dead chickens were necropsied to confirm that death was caused by IBV. The challenged chickens generally began to show clinical signs between 4 and 10 days after challenge. Chickens in each group were euthanized at 14 days post-infection. Necropsies were performed immediately and kidney tissues were collected for further detection of virus.
2.10. Detection of virus in kidney tissues by RT-PCR
Virus in the kidney tissues of the challenged chicken was detected by RT-PCR. Total RNA was extracted using Trizol reagent (Gibco) and subjected to RT-PCR using primers directed at the 3′ untranslated region (forward primer: 5′-GATGAGGAGAGGAACAATGC-3′; reverse primer: 5′-TGGG CGTCCTAGTGCTGT-3′). Total protection was defined as the absence of detectable virus in the kidney.
2.11. Effect of rFPV on body weight
To investigate the effect of infection with rFPV on body weight, 80 one-day-old White Leghorn SPF chickens were immunized in the same manner. At 1 and 2 weeks after immunization, all chickens were weighed.
2.12. Statistical analysis
Serological responses, T-lymphocytes, and weight gain of vaccinated chickens were compared with those of control animals by analysis of variance (ANOVA) and Student's t-test using SPSS11.5 biostatistics software. P values less than 0.05 were regarded as significant and those less than 0.01 were regarded as highly significant.
3. Results
3.1. Construction and confirmation of the recombinant plasmids
To prepare recombinant fowlpox viruses expressing S1 and co-expressing S1 and IL-18, two recombinant plasmids were constructed (Fig. 1 ). Plasmid pSY538 (A) had the early-late LP2EP2 promoters of FPV, while plasmid pSC11 had a LacZ gene fragment with the P11 late promoter of vaccinia virus and pSY681 had two FPV DNA regions. These FPV DNA regions were the recombinant arms of FPV that allowed crossing over to occur when the plasmids were co-infected with FPV in CEF cells. Plasmid pSY-S1 (B) was used to produce recombinant virus rFPV-S1. Plasmid pSY-S1/IL18 (C) was used to generate the recombinant virus rFPV-S1/IL18 expressing IBV S1 and IL-18.
Fig. 1.
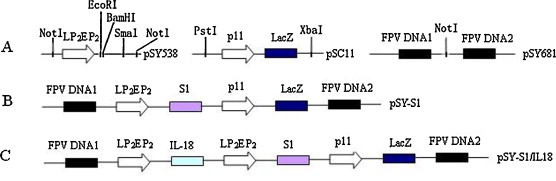
Schematic representations of fowlpox virus expression plasmids (pSY538, P11 and pSY681) and recombinant plasmids pSY-S1 and pSY-S1/IL18.
The presence of all genes in the plasmids was confirmed by restriction endonuclease digestion and the bands seen exactly matched the expected sizes (data not shown). And the results were further confirmed by DNA sequencing done on the recombinant plasmids. Both restriction endonuclease digestion and sequencing results showed that recombinant plasmids were successfully constructed.
3.2. Screening of recombinant virus by PCR analysis
To confirm that the genes of interest were successfully inserted into the fowlpox genome via homologous recombination, the genomic DNA of rFPVs was extracted, and PCR was performed with the two pairs of specific primers shown. As shown in Fig. 2 , the expected products of 1.74 kb (S1) and 0.6 kb (IL-18) were amplified from cells infected with rFPV-S1/IL18, no product was amplified from cells infected with the parental fowlpox virus S-FPV-017 (data not shown). The expected product of the 1.74 kb (S1) was amplified from cells infected with rFPV-S1 (Fig. 2). The sequencing results showed the nucleotide sequence of S1 and chicken IL-18 was just the same as S1 (AY775551) and chicken IL-18 (AY775780) published, respectively. Both PCR and sequencing results proved that the target genes had been successfully recombined into the rFPVs.
Fig. 2.
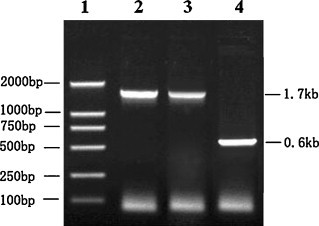
PCR confirmation of presence of S1 and chicken IL-18 genes in rFPVs. 1, DNA molecular Marker (DL2000); 2, S1 gene in rFPV-S1; 3, S1 gene in rFPV-S1/IL18; 4, chicken IL-18 in rFPV-S1/IL18. Also, the results were double checked by DNA sequencing done on the PCR products. DNA sequencing results showed that S1 and chicken IL-18 genes are 1 675 bp and 597 bp, respectively, and consistent with predicted results.
3.3. Expression of S1 and IL-18 proteins in rFPVs
To confirm the expression of S1 and IL-18 in CEF cells, total RNA was extracted from the cells infected with rFPV-S1/IL18 after 48 h and analyzed by RT-PCR for the presence of each corresponding mRNA. The predicted RT-PCR products were 1.7 kb in size for the S1 gene and 0.6 kb for the chicken IL-18 gene, all of which were confirmed by gel electrophoresis. No specific band of a similar size was seen in any of the mRNA samples in the absence of reverse transcription (data not shown). The sequencing results showed the nucleotide sequence of S1 and chicken IL-18 was just the same as S1 (AY775551) and chicken IL-18 (AY775780) published, respectively. Both RT-PCR and sequencing results showed that S1 and IL-18 can be successfully expressed in CEF cells infected with rFPV-S1/IL18.
IFA was performed to confirm the expression of S1 in CEF cells. All the cells that were infected with different rFPVs generated CPE and bound detectable levels of FITC-labeled rabbit anti-chicken antibody after they were incubated with IBV specific-chicken antibody. No any fluorescence was detected in negative control cells infected with S-FPV-017 (Fig. 3 ). The IFA results were demonstrated that IBV S1 protein could be expressed correctly in two rFPVs. And all these rFPVs can grow and produce CPE on CEF cells.
Fig. 3.
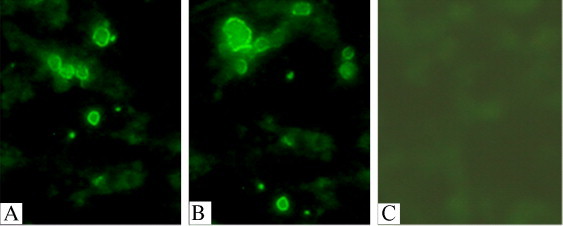
Detection of S1 expression by IFA. CEF cells were infected with (A) rFPV-S1 and (B) rFPV-S1/IL18 at a m.o.i. of 2.0. (C) Negative control cells were infected with S-FPV-017.
3.4. Antibody responses to IBV in chickens immunized with rFPVs
The rFPV-S1 and rFPV-S1/IL18 induced detectable antibodies to IBV Ag in chickens 1 week after vaccination and the levels further increased during the following weeks. Antibody titers of the rFPV-vaccinated groups were significantly greater than those of the groups inoculated with PBS or S-FPV-017 (P < 0.05). There was a significant difference in ELISA antibody levels (P < 0.05) elicited by either rFPV-S1 or rFPV-S1/IL18 from the first week after vaccination (Fig. 4 ). The results suggested that rFPV-S1/IL18 could induce enhanced humoral responses.
Fig. 4.
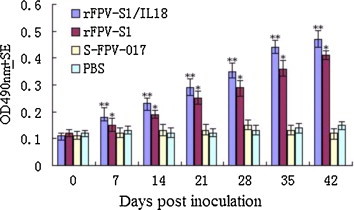
Detection of antibodies in different vaccine inoculated groups by ELISA (n = 5, i.e. number of times the test was repeated). Values are expressed as mean optical density ± standard error. Statistically significant differences (P < 0.05) are indicated by * (compared with S-FPV-017 or PBS) or ** (compared with rFPV-S1 alone).
3.5. Cellular immune responses induced by rFPVs vaccination
As CD4+ and CD8+ T-lymphocytes are among the most crucial components of antiviral effectors, these lymphocytes were assessed in vaccinated chickens. Flow cytometric analysis of unstimulated cells was used to standardize the background responses. As shown in Fig. 5 , vaccination with rFPV-S1/IL18 or rFPV-S1 significantly increased the percentages of CD3+, CD4+CD3+and CD8+CD3+ T-lymphocytes compared with the percentages in the groups inoculated with S-FPV-017 or PBS (P < 0.05). There was a significant difference in the percentages of CD3+, CD4+CD3+and CD8+CD3+ T-lymphocytes between the group immunized with rFPV-S1/IL18 and the group immunized with rFPV-S1 (P < 0.05). The ratios of CD4+ to CD8+ lymphocytes in rFPV-vaccinated groups were significantly higher (P < 0.01) than in groups inoculated with S-FPV-017 or PBS from the first week after vaccination. The ratios of CD4+ to CD8+ lymphocytes in chickens immunized with rFPV-S1/IL18 were significantly higher (P < 0.05) than in those immunized with rFPV-S1 (Table 1 ). These results showed that the rFPV-S1 can elicit a cellular immune response in chickens and that the rFPV-S1/IL18 can significantly enhance cellular immune response induced by the rFPV-S1.
Fig. 5.
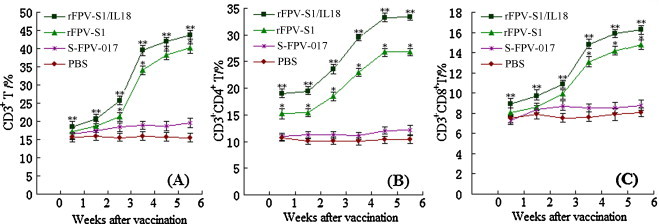
The percentage of CD3+, CD4+CD3+ and CD8+CD3+ T-lymphocytes of different vaccine inoculated groups (n = 4, i.e. number of times the test was repeated). Values are expressed as mean counts ± standard error. Statistically significant differences (P < 0.05) are indicated by * (compared with S-FPV-017 or PBS) or ** (compared with rFPV-S1 alone).
Table 1.
Ratio of CD4+:CD8+ T-lymphocytes after vaccination.a
| Group | Week post vaccination |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| rFPV-S1/IL18 | 2.14 ± 0.09a | 2.01 ± 0.29a | 2.16 ± 0.35a | 1.99 ± 0.37a | 2.09 ± 0.20a | 2.05 ± 0.30a |
| rFPV-S1 | 1.89 ± 0.33b | 1.79 ± 0.30b | 1.88 ± 0.23b | 1.76 ± 0.18b | 1.89 ± 0.08b | 1.80 ± 0.05b |
| S-FPV-017 | 1.51 ± 0.15c | 1.35 ± 0.13c | 1.29 ± 0.12c | 1.30 ± 0.13c | 1.42 ± 0.11c | 1.39 ± 0.08c |
| PBS | 1.42 ± 0.14c | 1.26 ± 0.05c | 1.35 ± 0.11c | 1.33 ± 0.09c | 1.31 ± 0.09c | 1.29 ± 0.07c |
Number of times the test was repeated is 5. Data are expressed as means ± SD.
Data with the same letter (a–c) are not significantly different (P > 0.05).
3.6. Protection induced by immunization with rFPV-S1 and rFPV-S1/IL18
Chickens immunized with rFPV-S1 and rFPV-S1/IL18 were challenged with IBV HN99. Morbidity, mortalities, renal infection and the proportion protected after challenge are summarized in Fig. 6 and Table 2 . Clinical signs were first seen on day 4 after challenge. Chickens inoculated with either S-FPV-017 or PBS were not protected and developed coughing, nasal discharge, and dyspnea. The mortality rates in chicken inoculated with S-FPV-017 or PBS were 65% or 75% at 14 days after challenge, respectively. Chickens immunized with 106 PFU rFPV-S1/IL18 were completely protected (20/20). None of these chickens had clinical signs of IBV infection or died after challenge with IBV, whereas 15 of 20 of the chickens immunized with rFPV-S1 were protected (P < 0.05).
Fig. 6.
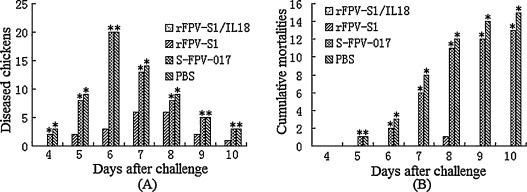
Diseased and dead chickens of different groups challenged with IBV HN99 strain. (A) Diseased chickens of different groups. Presence of one of the following can be used to judge morbidity: (1) coughing; (2) nasal discharge; (3) wheezing or dyspnea; (4) swinging of the head; (5) feathers erected, dullness. (B) The cumulative mortalities of different groups. Statistically significant differences (P < 0.05) are indicated by * (compared with rFPV-S1 or rFPV-S1/IL18).
Table 2.
Mortality and protection rate after challenge with the virulent HN99 strain of IBV.
| Groups |
||||
|---|---|---|---|---|
| rFPV-S1/IL18 | rFPV-S1 | S-FPV-017 | PBS | |
| Mortality (%)a | 0 (0/20)* | 5 (1/20) | 65 (13/20) | 75 (15/20) |
| Detectable IBV in kidneyb | 0/20* | 5/20 | 20/20 | 20/20 |
| Protection rate (%)c | 100* | 75 | 0 | 0 |
Mortality was recorded for each day after challenge and is presented as total number of dead chickens in each group.
Detectable IBV in kidney as determined by RT-PCR.
Percent protection was determined by the number of unaffected chickens/total number of chickens.
Statistically significant differences (P < 0.05) (compared with rFPV-S1 alone).
To evaluate the level of protective responses after challenge, the kidney samples were analyzed by RT-PCR. These studies indicated that 25% of birds vaccinated with rFPV-S1 had detectable virus in their kidneys. All chickens inoculated with either S-FPV-017 or PBS had detectable virus in their kidneys. None of chickens vaccinated with rFPV-S1/IL18 had detectable virus in their kidneys.
3.7. Effect of rFPV on body weight
Chickens vaccinated with rFPV-S1/IL18 or unvaccinated controls had greater body weights (P < 0.05) than chickens vaccinated with rFPV-S1 and FPV (Table 3 ), suggesting that IL-18 can reduce the effects of the FPV vector on weight gain.
Table 3.
Effects of rFPV on weight gain of 1-day-old SPF chickens.a
| Groups | Dose of inoculation | Body weight (g) 1 week post-inoculationb | Body weight (g) 2 weeks post-inoculationb | Body weights at post mortemc |
|---|---|---|---|---|
| rFPV-S1/IL18 | 106 PFU | 66.3 ± 5.6a | 119.9 ± 11.4a | 756.3 ± 21.7a |
| rFPV-S1 | 106 PFU | 61.9 ± 5.4b | 101.5 ± 11.2b | 667.1 ± 19.3b |
| S-FPV-017 | 106 PFU | 61.3 ± 6.9b | 99.3 ± 13.2b | 654.6 ± 19.6b |
| PBS | 0.2 ml PBS | 67.8 ± 6.9a | 121.7 ± 17.8a | 769.7 ± 20.8a |
Data are expressed as means ± SD.
Number of times the test was repeated is 20.
Only those remaining alive at the conclusion of the experiment.
Data with the same letter (a and b) are not significantly different (P > 0.05).
4. Discussion
Although FPV vectors have many advantages, there is a need to enhance immunization-induced protection by recombinant fowlpox virus vaccines. Many studies have shown that the immunogenicity of an antigen could be enhanced by various cytokines, including IL-2, IFN-γ, IL-6, and IL-18. Among the large array of cytokines, IL-18 was initially identified as a potent IFN-γ-inducing factor, and it is an important cytokine with multiple functions in innate and acquired immunity [18], [19]. As a vaccine adjuvant and an immunomodulatory molecule, IL-18 has been recently shown to regulate both Th1 and Th2 immune responses [20], and enhances the immune responses in vaccines [21].
In our study we chose to test IL-18 as an adjuvant for the S1 antigen expressed from a FPV vector vaccine. rFPV-S1/IL18 and rFPV-S1 were constructed, inoculated into chickens and tested in a protection-challenge experiment. The result showed that vaccination with the rFPV-S1/IL18 can induce stronger immune responses than vaccination with rFPV-S1. Compared to some earlier descriptions of FPV recombinants expressing IBV S1 with and without cytokines [17], [22], different cytokines, vector, and IBV strains were used. Wang et al. [22] reported that their constructed recombinant fowlpox virus expressing the S1 protein was indicated by the manifested, relatively mild clinical signs of disease, decreased titers of recovered challenge virus, and less severe histologic changes of the tracheas in virulent IBV Mass 41-challenged chickens previously receiving rFPV-S1 as compared with parental fowl poxvirus (FPV)-vaccinated control birds. A fowlpox virus (rFPV-S1-ChIFNγ) co-expressing the S1 gene and the chicken type II interferon gene provided the strongest protection against an IBV LX4 virus challenge, and chickens in the rFPV-S1-ChIFNγ group eliminated virus more quickly and decreased the presence of viral antigen more significantly in renal tissue when compared to those of the rFPV-S1 [17]. Our results show that the rFPV-S1/IL18 vaccinated group displayed significantly increased weight gain relative to the rFPV-S1 group, and the protective efficacy of the rFPV-S1 vaccine could be enhanced significantly by simultaneous expression of IL-18. Compared to Wang et al. [22] reported, Wang et al. [17] and our results showed the protective efficacy of the rFPV-S1 vaccine could be enhanced significantly by simultaneous expression of a cytokine and normal weight gain in vaccinated chickens. In our study, all chickens immunized with rFPV-S1/IL18 were completely protected (20/20) after challenge with the virulent IBV HN99 strain, 15 of 16 of the chickens immunized with rFPV-S1-ChIFNγ constructed by Wang et al. [17] were protected, whether these different survival proportions are due to different cytokines and challenge with different IBV strains is further studying.
The level of specific antibodies induced in the rFPV-S1/IL18 group was higher than that induced in the rFPV-S1 group, and this virus induced better protection. However, the precise role of antibodies in control of IBV infection remains controversial. Some reports have shown that the circulating antibody titer did not correlate with protection from IBV infection [23], [24]. Other studies have demonstrated that humoral immunity plays an important role in disease recovery and virus clearance [25], [26]. It is possible that the T-cell response may play an important role in protection.
CD4+ T-lymphocytes (T-helper cells) can induce and enhance the immune response by secreting cytokines. CD8+ T-lymphocytes (cytotoxic T cells) can mediate cytotoxic killing of target cells [27]. Hence, CD4+ and CD8+ lymphocytes represent key functional subsets of adaptive cell-mediated immunity. The ratio of CD4+ to CD8+ cells has been shown to be indicative of the general state of immune function (e.g., a high CD4+:CD8+ ratio may be indicative of improved immune activity). Our evaluation by flow cytometry of the T-cell concentrations in peripheral blood revealed that the ratios of CD4+ to CD8+ cells in chickens immunized with rFPV-S1/IL18 were significantly higher (P < 0.05) than in those immunized with rFPV-S1, and vaccination with rFPV-S1/IL18 significantly increased the percentages of CD3+, CD4+CD3+and CD8+CD3+ T-lymphocytes compared with the percentage in the groups inoculated with rFPV-S1 (P < 0.05). This indicated that chicken IL-18 may enhance cellular immunity by promoting the differentiation or proliferation of CD4+ T-lymphocytes.
Cell-mediated immunity is believed to provide protection against IBV infection. CD8+ CTL are critical in the control of infectious bronchitis in poultry [28], [29]. CD4+ T-cell responses may increase the proliferation, maturation and functional activity of CD8+ CTL, provide increased help for B-cells and directly produce anti-viral cytokines. In this study the number of the CD8+ T-lymphocytes in peripheral blood of chickens in rFPV-S1/IL18 group was significantly higher than that of chickens in rFPV-S1 group (data not shown), which may explain the better clinical protection of rFPV-S1/IL18 as CD8+ T-cells is cytotoxic T-lymphocytes that play a major role in clearing or controlling viral infections [30], this is consistent with those found by Wang et al. [17]. The increased numbers of T-cells would have a limiting effect on viral replication and lead to better protection [31]. In addition, the percentages of CD3+, CD4+CD3+and CD8+CD3+ T-lymphocyte subgroups in chickens immunized with rFPV-S1 were significantly higher than those in the S-FPV-017-immunized chickens (P < 0.05), similar results were also reported by Shen et al. [21]. This may be target exogenous proteins expressed in rFPVs inhibit a T-cell response induced by normal FPV, this is worth further studying. The ratios of CD4+ to CD8+ cells in chickens immunized with rFPV-S1 were significantly higher (P < 0.05) than in those immunized with S-FPV-017. The effect of S1 expression on the T-cell ratios may be FPV expressing S1 protein replicates in the epidermal layer of the skin. At day 42 after immunization, the percentages of CD4+CD3+ and CD8+CD3+ T-lymphocytes in three vaccinated groups were all higher than that at day 7 after immunization (Fig. 5). This demonstrated that rFPVs have the ability to stimulate both Th1-mediated immune response and Th2-mediated immune response.
In our study, vaccinated chickens were challenged with a nephropathogenic strain of IBV to evaluate the level of protection elicited by vaccines expressing S1 with or without IL-18. Chickens that received the rFPV-S1/IL18 were better protected than those administered with rFPV-S1. With the highest protection rate among all the vaccination groups, our findings indicate that the rFPV-S1/IL18 vaccine can induce a potent protective immune response and inhibit viral replication.
We found that all rFPV-vaccinated groups produced ELISA-specific antibodies in addition to the significantly increased ratios of CD4+ to CD8+ cells in chickens immunized with rFPV-S1/IL18 over in those immunized with rFPV-S1. The rFPV-S1/IL18 vaccinated group displayed significantly increased weight gain relative to the rFPV-S1 group. The comparison of peripheral blood T-lymphocyte subgroups and the effect of rFPV on body weight showed that chicken IL-18 may enhance cell-mediated immune responses to some extent, consistent with a previous report [32]. Thus rFPV-S1/IL18 may be a valuable candidate vaccine for the control of IBV and its effectiveness in the field applications should be determined in the future.
Acknowledgement
This work was supported by a grant from the National Key Project of Scientific and Technical Supporting Programs of China (2008BADB2B01).
References
- 1.Bijlenga G., Cook J.K., Gelb J., Jr., de Wit J.J. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review. Avian Pathol. 2004;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh D., Naqi S.A. Infectious bronchitis. In: Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E., editors. Diseases of poultry. 11th ed. Iowa State University Press; Ames, Iowa: 2003. pp. 101–119. [Google Scholar]
- 4.Cook J.K., Smith H.W., Huggins M.B. Infectious bronchitis immunity: its study in chickens experimentally infected with mixtures of infectious bronchitis virus and Escherichia coli. J Gen Virol. 1986;67:1427–1434. doi: 10.1099/0022-1317-67-7-1427. [DOI] [PubMed] [Google Scholar]
- 5.Schalk A.F., Hawn M.C. An apparently new respiratory disease of baby chicks. J Am Vet Med Assoc. 1931;78:413–422. [Google Scholar]
- 6.Cowen B.S., Hitchner S.B. Serotyping of avian infectious bronchitis viruses by the virus-neutralization test. Avian Dis. 1975;19:583–595. [PubMed] [Google Scholar]
- 7.Hofstad M.S. Cross-immunity in chickens using seven isolates of avian infectious bronchitis virus. Avian Dis. 1981;25:650–654. [PubMed] [Google Scholar]
- 8.Gan M.H. Chinese Agriculture Press; Beijing: 2003. Diseases of Chinese poultry. p. 43–50. [Google Scholar]
- 9.Cavanagh D., Davis P.J., Darbyshire J.H., Peters R.W. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination inhibiting antibody, or induce chicken tracheal protection. J Gen Virol. 1986;67:1435–1442. doi: 10.1099/0022-1317-67-7-1435. [DOI] [PubMed] [Google Scholar]
- 10.Ignjatovic J., Gallo L. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song C.S., Lee Y.J., Lee C.W., Sung H.W., Kim J.H., Mo I.P. Induction of protective immunity in chickens vaccinated with infectious bronchitis virus S1 glycoprotein expressed by a recombinant baculovirus. J Gen Virol. 1998;79:719–723. doi: 10.1099/0022-1317-79-4-719. [DOI] [PubMed] [Google Scholar]
- 12.Bolte L., Meurer J., Kaleta E.F. Avian host spectrum of avipoxviruses. Avian Pathol. 1999;28:415–432. doi: 10.1080/03079459994434. [DOI] [PubMed] [Google Scholar]
- 13.Leong K.H., Ramsay A.J., Boyle D.B., Ramshaw I.A. Selective induction of immune responses by cytokines coexpressed in recombinant fowlpox virus. J Virol. 1994;68(12):8125–8130. doi: 10.1128/jvi.68.12.8125-8130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noll A., Autenrieth I.B. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or Yersinia HSP60 plus interleukin-12. Infect Immun. 1996;64(8):2955–2961. doi: 10.1128/iai.64.8.2955-2961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider K., Puehler F., Baeuerle D., Elvers S., Staeheli P., Kaspers B. cDNA cloning of biologically active chicken interleukin-18. J Interferon Cytokine Res. 2000;20:879–883. doi: 10.1089/10799900050163244. [DOI] [PubMed] [Google Scholar]
- 16.Chen H.Y., Huang Q.Y., Cui B.A., Li X.S., Guan Q. Construction of recombinant fowlpox virus coexpressing HA from subtype H5 of avian influenza virus and chicken interleukin-18. Acta Microbiol Sin. 2008;48(8):1025–1030. [PubMed] [Google Scholar]
- 17.Wang Y.F., Sun Y.K., Tian Z.C., Shi X.M., Tong G.Z., Liu S.W. Protection of chickens against infectious bronchitis by a recombinant fowlpox virus co-expressing IBV-S1 and chicken IFNgamma. Vaccine. 2009;27(50):7046–7052. doi: 10.1016/j.vaccine.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello C.A., Fantuzzi G. Interleukin-18 and host defense against infection. J Infect Dis. 2003;187:S370–S384. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- 19.Marshall D.J., Rudnick K.A., McCarthy S.G., Mateo L.R., Harris M.C., McCauley C. Interleukin-18 enhances Th1 immunity and tumor protection of a DNA vaccine. Vaccine. 2006;24:244–253. doi: 10.1016/j.vaccine.2005.07.087. [DOI] [PubMed] [Google Scholar]
- 20.Nakanishi K., Yoshimoto T., Tsutsui H., Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 21.Shen G., Jin N., Ma M., Jin K., Zheng M., Zhuang T. Immune responses of pigs inoculated with a recombinant fowlpox virus coexpressing GP5/GP3 of porcine reproductive and respiratory syndrome virus and swine IL-18. Vaccine. 2007;25(21):4193–4202. doi: 10.1016/j.vaccine.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Schnitzlein W.M., Tripathy D.N., Girshick T., Khan M.I. Construction and immunogenicity studies of recombinant fowl poxvirus containing the S1 gene of Massachusetts 41 strain of infectious bronchitis virus. Avian Dis. 2002;46(4):831–838. doi: 10.1637/0005-2086(2002)046[0831:CAISOR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Gough R.E., Alexander D.J. Comparison of duration of immunity in chickens infected with a live infectious bronchitis vaccine by three different routes. Res Vet Sci. 1979;26:329–332. [PubMed] [Google Scholar]
- 24.Gelb J., Jr., Nix W.A., Gellman S.D. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis. 1998;42:364–374. [PubMed] [Google Scholar]
- 25.Cook J.K., Davison T.F., Huggins M.B., McLauthlan P. Effect of in vivo bursectomy on the course of an infectious bronchitis virus infection in line C White Leghoen chickens. Arch Virol. 1991;118:225–234. doi: 10.1007/BF01314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson G., Mohammed H., Bauman B., Naqi S. Lack of correlation between infectivity, serologic antibody responses to infectious bronchitis virus in chickens inoculated with response and challenge results in immunization with an avian infectious bursal disease virus and control chickens. Avian Dis. 1997;41:519–527. [PubMed] [Google Scholar]
- 27.Summerfield A., Rziha H.J., Saalmüller A. Functional characterization of porcine CD4+CD8+ extrathymic T lymphocytes. Cell Immunol. 1996;168:291–296. doi: 10.1006/cimm.1996.0078. [DOI] [PubMed] [Google Scholar]
- 28.Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis in poultry. Dev Comp Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 29.Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed alpha beta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harty J.T., Tvinnereim A.R., White D.W. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 31.Tang M., Wang H., Zhou S., Tian G. Enhancement of the immunogenicity of an infectious bronchitis virus DNA vaccine by a bicistronic plasmid encoding nucleocapsid protein and interleukin-2. J Virol Methods. 2008;149(1):42–48. doi: 10.1016/j.jviromet.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gobel T.W., Schneider K., Schaerer B., Mejri I., Puehler F., Weigend S. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: conservation of a Th1-like system in a nonmammalian species. J Immunol. 2003;171:1809–1815. doi: 10.4049/jimmunol.171.4.1809. [DOI] [PubMed] [Google Scholar]


