Abstract
We have investigated novel vaccine strategies against severe acute respiratory syndrome (SARS) CoV using cDNA constructs encoding the structural antigens: (S), (M), (E), or (N) protein, derived from SARS CoV. PBL from healthy human volunteers were administered i.p. into IL-2 receptor γ-chain disrupted SCID mice, and SCID-PBL/hu mice were constructed. These mice can be used to analyze the human immune response in vivo. SARS M DNA vaccine and N DNA vaccine induced human CTL specific for SARS CoV antigens. Alternatively, SARS M DNA vaccines inducing human neutralizing antibodies and human monoclonal antibodies against SARS CoV are now being developed. These results show that these vaccines can induce virus-specific immune responses and should provide a useful tool for development of protective and therapeutic vaccines.
Keywords: SARS DNA vaccine, SCID-PBL/hu, Human neutralizing antibody against M
1. Introduction
The causative agent of severe acute respiratory syndrome (SARS) has been identified as a new type of corona virus, SARS corona virus (SARS CoV) [1], [2], [3]. SARS has infected more than 8400 patients in about 7 months in over 30 countries and caused more than 800 deaths. However, no SARS vaccine is currently available for clinical use. Therefore, we have developed novel vaccine candidates against SARS CoV using cDNA constructs encoding the structural antigens; S, M, E, or N protein. In immunized mice, neutralizing antibodies against the virus and T cell immunity against virus-infected-cells were studied, since these immunities play important roles in protection against SARS CoV and many virus infections. In particular, CD8+ CTL plays an important role in T cell immunity dependent protection against virus infections and the eradication of murine and human cancers [4], [5]. In the present study, the SCID-PBL/hu model, which is capable of analyzing in vivo human immune response, was used because it is a more relevant translational model for human cases [4]. These vaccines induce neutralizing antibody and CTL. This is the first report inducing antibody against SARS M. Theses vaccines should provide useful tool for development of protective vaccines in human.
2. Materials and methods
Three kinds of SARS CoV strains: HKU39849 [1], TW-1 and FFM-1 [2] and their cDNAs were used. S, M, N or E cDNA was transferred into pcDNA 3.1(+) vector [4]. PBL from healthy human volunteers were administered i.p. into IL-2 receptor γ-chain disrupted NOD SCID mice [IL-2R(−/−) NOD-SCID], and SCID-PBL/hu mice were constructed [4]. pcDNA 3.1(+) vector, 50 μg each, containing SARS S, M, N, or E DNA was injected i.m. into SCID-PBL/hu three times, at an interval of 7 days. Neutralizing antibodies against SARS CoV in the serum from the mice were assayed by use of Vero-E6 cell. CTL activity against SARS CoV was studied using human cells, expressing SARS antigens. CTL activity of human CD8-positive lymphocytes in the spleen from SCID-PBL/hu was assessed using 51Cr-release assay [5], [6]. Human monoclonal antibodies were produced from B cell hybridoma using P3U1 myeloma cell and spleen cells from human immunoglobulin transchromosomic mice (KM mice) [7].
3. Results
3.1. Induction of human immune responses against SARS CoV using SCID-PBL/hu
The production of neutralizing antibodies against SARS CoV was observed in the serum from mice immunized with S DNA vaccine SARS (M) DNA vaccine and N DNA vaccine induced murine T cell responses against SARS [4]. To analyze the human immune responses, SCID-PBL/hu were constructed and human CD3-positive T lymphocytes and human B cells and macrophages were replaced in the spleen cells and PEC from these SCID-PBL/hu mice, as shown in Fig. 1 .
Fig. 1.
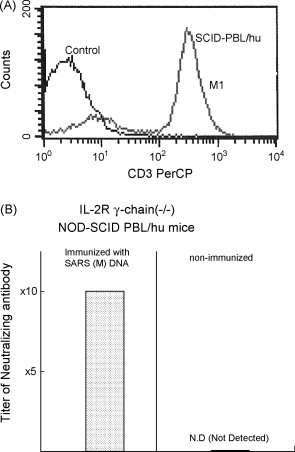
(A) Human CD3-positive T cells in the spleen from SCID-PBL/hu mice. 1 × 107 PBL from healthy human volunteers were administered i.p. into IL-2R(−/−) NOD-SCID. The number of human CD3-positive T cells were assessed by using anti-human CD3 antibody and FACS. (B) Induction of human neutralizing antibody against SARS coronavirus M protein in SCID PBL/hu mice by SARS (M) DNA vaccination. Titration of neutralizing antibody against SARS CoV in the serum from these mice was assessed by Vero-E6 cells.
3.2. SARS M DNA vaccine induced the production of human neutralizing antibodies against SARS CoV in SCID-PBL/hu model
Human neutralizing antibodies were induced from SCID-PBL/hu mice vaccines with SARSS [6] and M DNA vaccines (Fig. 2 ). Titer of neutralizing antibody in the serum from SCID-PBL/hu mice immunized with SARS (M) DNA vaccine was 1:10. In contrast, inhibition of cytopathic effect was observed even in 100-fold dilution of the same serum. Furthermore, B cell hybridoma producing human monoclonal antibodies were established by the fusion of P3U1 cells and spleen cells from human immunoglobulin transchromosomic mice immunized with SARS antigens (Table 1 ). Specificity is now being studied.
Fig. 2.
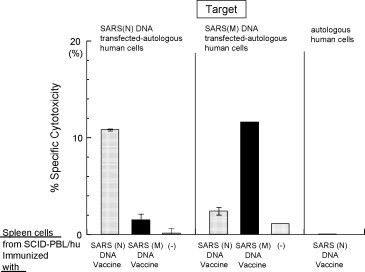
SARS (N) DNA vaccine and SARS (M) DNA vaccine induces in vivo human CTL against SARS CoV in the SCID-PBL/hu human immune systems. Autologous B blastoid cells transfected with SARS (N) DNA or SARS (M) DNA were used as target cells using 51Cr release assay. E/T ration was 10:1.
Table 1.
Method for establishment of hybridoma producing human monoclonal antibody against SARS CoV
| Humanized monoclonal antibody against SARS-S protein |
|---|
| SARS TW1 strain |
| S protein (S431-447-KLH) |
| ↓ |
| Human immunoglobulin gene transchromosomic mice (KM mouse) |
| ↓ |
| Spleen + P3U1 |
| ↓ |
| Hybridoma (Screening) |
| Humanized monoclonal antibody against SARS S (431–447) peptide: clones (21 clones) |
SARS S peptide (S431-447 KLH) was immunized to KM mouse. Hybrid clones producing human monoclonal antibody against SARS S peptide were selected.
3.3. SARS M DNA and N DNA vaccines induced human T cell immune responses (CTL and proliferation) in SCID-PBL/hu model
The M DNA vaccine enhanced the CTL activity against antologous B blast cells transfected with SARS M DNA but not with SARS N DNA in SCID-PBL/hu mice (Fig. 2). On the other hand, SARS N DNA vaccine augmented the CTL activity specific for autologous B blast cells transfected with SARS N DNA.
4. Discussion
We have demonstrated that SARS (M) DNA and (N) DNA vaccines induce virus-specific immune responses (CTL and T cell proliferation) in the mouse systems using type II lung alveolar T cell lines in clone target models [4]. Gao et al. developed adenovirus based a SARS DNA vaccine encoding S1 polypeptide was capable of inducing neutralizing antibody, while another SARS DNA vaccine encoding N protein generated IFN-γ producing T cells in rhesus monkeys [8]. SARS S DNA vaccine which elicits effective neutralizing antibody responses that generate protective immunity in a mouse model [9]. However its immunogenicity in humans has yet to be established. Therefore, it is very important to evaluate the efficacy of SARS DNA vaccine in a SCID-PBL/hu mice, which is a highly relevant translational model for demonstrating human immune responsiveness.
In the present study, SARS M DNA vaccine and N DNA vaccine induced human CTL specific for SARS M antigen and SARS N antigen, respectively. Furthermore, SARS M DNA as well as SARSS DNA vaccine induce human neutralizing antibodies against SARS CoV by the SCID-PBL/hu model. Antibody against SARS M antigen exerted high inhibitory activity against cytopathic effect by SARS CoV. It was reported that monoclonal antibodies against external domain of M neutralize murine hepatitis corona virus, but only in the presence of complement, and both E and M proteins are required for budding of virions [10]. Neutralizing antibody activity against M protein was not at all eliminated by the inactivation of complement. Antibody against M protein might inhibit the growth of SARS CoV in the cells. Therefore, the effect of combination immunization with such SARS vaccines (M vaccine and S vaccine) and the specificity of human monoclonal neutralizing antibodies are now being studied. These vaccines are expected to provide useful tool for development of therapeutic vaccines.
Acknowledgements
This study was supported by Grant-in-Aid for the science and technology and Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education Culture Sports, Science and Technology, Japan. This study also supported by a Heath and Labour Science Research Grant from the Ministry of Health, Labour, and Welfare, Japan.
References
- 1.Peiris J.S. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 4.Okada M., Takemoto Y., Okuno Y., Hashimoto S., Yoshida S., Fukunaga Y. The development of vaccines against SARS corona virus in mice and SCID-PBL-hu mice. Vaccine. 2005;23:2269–2272. doi: 10.1016/j.vaccine.2005.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka F., Abe M., Akiyoshi T., Nomura T., Sugimachi K., Kishimoto T. The anti-human tumor effect and generation of human cytotoxic T cells in SCID mice given human peripheral blood lymphocytes by the in vivo transfer of the Interleukin-6 gene using adenovirus vector. Cancer Res. 1997;57(7):1335–1343. [PubMed] [Google Scholar]
- 6.Okada M., Yoshimura N., Kaieda T., Yamamura Y., Kishimoto T. Establishment and characterization of human T hybrid cells secreting immunoregulatory molecules. Proc Natl Acad Sci USA. 1981;78(12):7717–7721. doi: 10.1073/pnas.78.12.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida I., Tomizuka K., Yoshida H., Tahara T., Takahashi N., Ohguma A. Production of human monoclonal and polyclonal antibodies in TransChromo (TC) animals. Clon Stem Cells. 2002;4:91–102. doi: 10.1089/153623002753632084. [DOI] [PubMed] [Google Scholar]
- 8.Gao W., Tamin A., Soloff A., D’Aiuto L., Kwanegbo E., Robbins P.D. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362(9399):1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai M.M.C., Holmes K.V. Coronaviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. 4th ed. vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1163–1203. (Fields Virology). [Google Scholar]


