Highlights
-
•
Vaccination in the first week after hatching generates poorly protective immune responses to IBV.
-
•
A delay in humoral immune response to IBV is observed when vaccinated in the first two weeks of life.
-
•
Vaccinating 1-day-old birds generates lower avidity IgG antibodies than in 4 week old birds to IBV.
-
•
Our data strongly argues to change the practice of vaccinating for IBV immediately after hatching.
Keywords: Age-dependent immunity, Infectious bronchitis virus, Antibody kinetics, Mucosal immunity, Avidity index, Avian coronavirus
Abbreviations: Ark DPI, Arkansas Delmarva Poultry Industry; CALT, conjunctiva-associated lymphoid tissue; ELISPOT, enzyme-linked immunospot; HG, Harderian gland; HALT, head-associated lymphoid tissues; HRP, horseradish peroxidase; IBV, infectious bronchitis virus; EID50, median embryo infectious dose; TLR, Toll-like receptor
Abstract
Infectious bronchitis virus (IBV) is an endemic disease of chickens and a major contributor to economic losses for the poultry industry despite vaccination. Recent observations indicated that chicks may have an immature immune system immediately after hatching when vaccinated for IBV. Therefore we hypothesized that early IBV vaccination will generate an immature, poorly protective IBV-specific immune response contributing to immune escape and persistence of IBV. To test this hypothesis the IBV-specific immune response and immune protection were measured in chicks vaccinated at different ages. This demonstrated a delayed production of IgG and IgA plasma antibodies in the 1, 7 and 14-day-old vaccination groups and also lower IgA antibody levels were observed in plasma of the 1-day-old group. Similar observations were made for antibodies in tears. In addition, IgG antibodies from the 1-day-old group had lower avidity indices than day 28 vaccinated birds. The delayed and/or lower antibody response combined with lower IgG avidity indices coincided with increased tracheal inflammation and depletion of tracheal epithelia cells and goblet cells upon IBV field strain challenge. The lack of vaccine-mediated protection was most pronounced in the 1-day-old vaccination group and to a lesser extent the 7-day-old group, while the 14-day-old and older chickens were protected. These data strongly support IBV vaccination after day 7 post hatch.
1. Introduction
IBV is endemic and currently one of the most important causes of economic losses for the poultry industry and represents a continuous threat for this industry. In the past, it was estimated that with the best possible management of flocks IBV infection will reduce income by approximately 3% when compared to an IBV-free flock [1]. There is approximately a 50% vaccine failure for Arkansas (Ark) serotype of IBV [2], the most prevalent vaccine serotype used in the USA. Symptoms of IBV infection include, but are not limited to, wet eyes, swollen face, tracheal and kidney lesions, respiratory disease, reduced weight gain in broilers, decreasing and poor egg quality in layers [3], [4]. The existence of various IBV serotypes as well as antigenic variants [4] complicates vaccination programs. Since immunity induced by vaccination against a single serotype generally provides insufficient protection against other serotypes [5], [6].
Mucosal immunity plays a role in the control of IBV in chickens as was demonstrated using IBV-resistant and IBV-susceptible inbred chicken lines [7]. This combined with the finding of Gelb et al. [8], in which ocular immunization with the Massachusetts Connaught strain of IBV only on day 1 or on day1 plus day 14 followed by challenge with Massachusetts 41 provided protection of 8% and 50% of the chickens, respectively, while the same SPF White leghorns only ocularly immunized on day 14 were 100% protected [8]. BSA immunization of 1, 7 and 12 day old broiler chickens obtained very similar results [9]. This raises questions pertaining the maturity of the immune system and in particular the mucosal immune system, and the ability of chicks to generate a protective immune response when vaccinated at a very young age.
Conjunctiva-associated lymphoid tissue (CALT) and Harderian glands do not fully mature as a lymphoid organ until weeks after hatching [5], [6], [10], [11], [12]. This combined with the practice of immunizing and boosting for IBV early after hatching may set up the immune response for failure to protect. The second IBV immunization on day 14 of age, which by itself is fully protective, does not completely compensate for the premature priming on day 1 [8]. Field studies by de Wit et al. [3] demonstrated a lack of protective immunity when birds were boosted between day 8 through day 13 of age. The percentage of birds in a commercial flock positive for IBV-specific IgM antibodies was correlated with vaccine protection and increased with the age of boosting. This data supports the notion that early vaccination and boosting of the IBV immune response may limit induction of protective immune responses to IBV. Unlike the study by Gelb et al. [8], the de Wit et al. [3] study can also be interpreted that maternal antibodies interfere with vaccine delivery during the first 2 weeks of life [13].
Further evidence that the immune response may be limited during the first weeks of life comes from the observation that IgA levels are undetectable in plasma the first week of life and IgM levels are low [14]. This indicates that immunoglobulin class switching and production of antibodies is very limited during the first week post hatch and therefore chicks are highly dependent on maternal IgY antibodies for protection against IBV, which drops ∼50% during the first week of life [14].
Besides diminished B cell response after vaccination, splenic T cells from one week old chickens are also less responsive to polyclonal activation than that of older chickens. The splenic T cells from 1 day old chicks even produce inhibitory factors for proliferation of mature T cells in vitro [15]. Furthermore, splenic lymphocytes of 40 day old chicken displayed better antigen specific proliferation after oral Salmonella exposure than 10 day old chicken [16]. When measuring gene expression in lung and trachea in 1 and 4 week old birds after avian influenza exposure a reduced expression of immune-related genes was shown and included innate immune response genes in the younger birds [17]. Additional evidence that innate immune mechanisms are diminished in young chickens was demonstrated by a lower Salmonella phagocytic index of heterophils during the first few days of life [18]. Thus, early exposure to pathogens or vaccines may induce suboptimal innate and adaptive immune responses.
Based on these observations we hypothesized that early IBV vaccination, i.e., within the first week after hatching, will generate an immature, poorly protective IBV-specific immune response contributing to IBV immune escape and persistence. Therefore, the ability of SPF chickens of different age to induce an IBV-specific antibody response and protect against challenge with an IBV field strain was measured. Our data indicate that early vaccination is suboptimal for induction of IBV-specific immune responses and immune protection.
2. Materials and methods
Chickens: Specific-pathogen-free (SPF) white leghorn eggs were obtained from Sunrise Farms, Inc., Catskill, NY, hatched and used in all experiments. All hatched chickens were used for the below outlined experiments regardless of sex. Chickens were housed in cages in BSL 2 facilities for the duration of the experiment. Food and water were provided ad libitum. All experimental procedures and animal care were performed in compliance with all applicable federal and institutional animal use guidelines. Auburn University College of Veterinary Medicine is an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited institution.
IBV-vaccination and challenge: SPF chickens were ocularly vaccinated with 3 × 105 50% embryo infectious doses (EID50) of a live attenuated ArkDPI IBV vaccine strain (Zoetis, New York, NY) in 50 μl PBS, which was expanded in our laboratory. Chickens were vaccinated 1 day of age and 1, 2, 3 or 4 weeks of age. All groups were challenged ocularly with 7.3 × 105 EID50 of the AL/4614/98 IBV field strain 21 days after vaccination
Sample collection: Tears were collected as previously described [19]. Blood samples were obtained by puncturing the brachial vein with a sterile 20G needle into Kendall monoject, EDTA containing, blood collection tubes (Tyco Healthcare Group LP, Mansfield, MA) and incubated on ice. Blood samples were centrifuged at 500 × g for 30 min. Plasma was collected and stored at −80 °C until tested.
IBV propagation and purification for ELISA: IBV was propagated in SPF White Leghorn embryonated chicken eggs (Sunrise Farms, Inc., Catskills, NY) by inoculation on day 10 of embryonation as previously reported [20]. Supernatants were titrated for the IBV virus using the Reed and Muench method [21]. IBV was treated with 0.1% β-propriolactone for 30 min at 37 °C [22]. Inactivation of the virus was confirmed by injection into embryonated eggs. The inactivated IBV was purified based on a previously published protocol [23]. The virus was then stored at −80 °C until used.
2.1. IBV-specific ELISA
In order to measure IgG (IgY), IgA and IgM antibody levels in plasma and tears of chicken, an IBV-specific enzyme-linked immunosorbent assay (ELISA) was developed as previously described [20]. In Brief, ELISA plates were coated with β-propriolactone killed, purified IBV at 2 μg/ml in carbonate buffer. The plates were blocked with PBS-BSA (1%) after which the samples were loaded at two-fold dilutions. Binding of chicken antibodies was detected using biotinylated anti-chicken-IgG, -IgA and -IgM monoclonal antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL) followed by streptavidin-horseradish peroxidase. The plates were developed using TMB (3,3′,5,5-Tetramethylbensidine; Invitrogen corp., Frederick, MD) substrate. The highest sample dilution with at least an optical density of 0.100 above background level at 450 nm was defined as the endpoint-titer. Ten to thirteen chickens were analyzed per group. The control group consisted out 3 chicken from each age group for IgA and IgG, which were pooled in one group of 15 since no differences were observed between the controls. For IgM levels in plasma the controls (each group containing 5 chickens except day 28 which had 3) were displayed separate for each age group. This was done because significant differences were observed between control IgM levels to IBV in different age groups.
2.2. Avidity index
The avidity index was determined as previously described [24], [25] using the above described IBV-specific ELISA. Plasma and tears were diluted 1:64 in ELISA buffer and were loaded on β-propiolactone killed IBV coated ELISA plates (2 μg/ml) [20]. After overnight incubation of these samples at 4 °C, 100 μl of increasing concentrations of potassium thiocyanate (0.00, 0.094, 0.187, 0.375, 0.75, 1.50, 3.0 M KSCN) were loaded into the wells and incubated for 30 min at room temperature. After washing the plates, the detection of IBV-specific antibodies was accomplished as previously reported [20]. The data were normalized to percent inhibition relative to samples not exposed to KSCN. The concentration of KSCN to inhibit 50% of the reactivity of the ELISA was defined as the avidity index [24], [25]. The OD450 read-out for the KSCN inhibition data was curve-fitted using 3-order polynomial regression analyses in Microsoft office Excell program. The Excell provided formula for the inhibition curve was used to determine the x values of y = 0.5, which are the concentrations of KSCN inhibiting 50% of the ELISA reactivity, representing the avidity indices of those samples. All samples were analyzed in triplicates and 4–5 samples were analyzed per group.
2.3. Histomorphometrics and histopathology
The cranial 1/3 of tracheae was collected 4 days after IBV challenge with 7.3 × 105 EID50 of AL/4614/98 IBV field strain. The tracheae were formalin-fixed and embedded in paraffin. Longitudinal 5 μm sections were made and were hematoxylin and eosin (H&E) stained and analyzed for mucosal thickness using Aperio Scan Scope and the Image J morphometry program (rsb.info.nih.gov/ij/download.html). To measure the mucosal thickness 5 measurements were made at regular intervals on one tracheal ring (see Supplemental Fig. 1).
As stated above, histopathology was analyzed in H&E stained tracheal slides 4 days after IBV challenge. Besides mucosal thickness (see supplemental Fig. 1), deciliation (see supplemental Fig. 2), goblet cells (see supplemental Fig. 3) and lymphocytes scores (see supplemental Fig. 1) of the tracheal mucosa were evaluated blindly and scored 1 through 5 based on severity (i.e., normal, mild, moderate, marked, severe). Five chickens were used as positive and negative controls, i.e., one of each age group, and 10–13 chickens were analyzed for each age group. A visual depiction of the scoring of these parameters is provided in the supplemental data (supplemental Figs. 1–3).
Statistical analysis: Data were analyzed using a one-way ANOVA test with Newman–Keuls multi-comparison test or the t-test using GraphPad Prism5 software. Groups were considered significantly different when P < 0.05.
3. Results
To determine whether age of IBV vaccination affected the humoral immune response, plasma samples were collected 14 and 21 days and tears 14 days after vaccination with 3 × 105 EID50 of a live-attenuated ArkDPI IBV vaccine strain on 1, 7 14, 21 or 28 days of age. The IBV-specific IgG endpoint titers in plasma 14 days after vaccination are significantly lower for day 1 vaccinated birds with a mean of 7.8 ± 1.0 when compared to day 14, 21 and 28 vaccinated birds, which means vary between 10.2–11.4. The 7-day-old group does not differ significantly from day 1 or later vaccinated bird (Fig. 1A). The IgG IBV-specific plasma levels 21 days after vaccination demonstrate, that the day 21 and 28 old vaccination groups stayed the same, while IgG antibody titers in groups vaccinated on day 1, 7 and 14 still increased (Fig. 1C). This shows that early vaccination causes a delay in the IBV-specific IgG antibody kinetics.
Fig. 1.
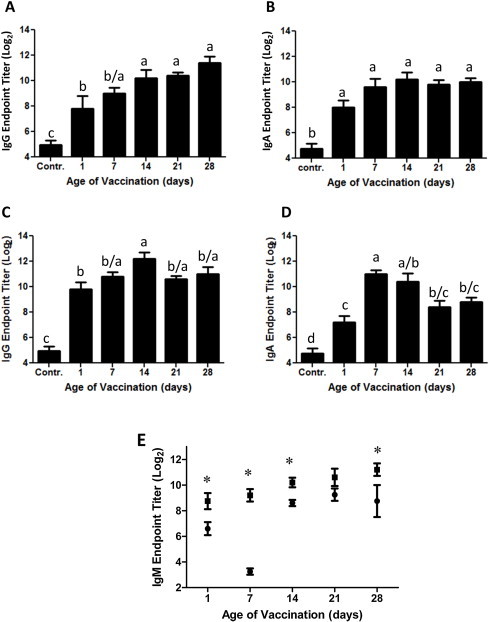
The IBV-specific IgG, IgA and IgM response in plasma. Endpoint titers of IBV-specific IgG on day 14 (A) and 21 (C) as well as IgA on day 14 (B) and 21 (D) and IgM on day 7 post IBV vaccination (squares) and in controls (circles) (E) were measured by ELISA. Chickens were vaccinated at day 1, 7, 14, 21 or 28 of age. Unvaccinated chickens of the different age groups served as negative control. The data was analyzed by one way ANOVA with the Newman–Keuls post-test. The control group contains 3 data points for each age group, which were pooled in one group (n = 15). All vaccinated age groups contained between 10 and 13 chickens for IgG and IgA. A significant difference is observed at P < 0.05 and is indicated by different letters. IgM levels in IBV vaccinated birds are depicted by squares (n = 5) and the controls by circles (n = 5, d28 group n = 3). For the IgM controls the different age groups are shown separately since significant differences were observed between them. A significant increase (P < 0.05) of IgM levels in IBV vaccinated birds over their control group is indicated by a (*).
The IBV-specific IgA plasma antibody titers are not significantly different between groups, although the day 1 vaccinated group mean antibody titer is the lowest of all groups (Fig. 1B) and at least 3-fold lower than the next lowest group. Unlike the IgG antibody titers only the day 7 vaccination group increases in mean IgA plasma titer on day 21 post vaccination, while day 14 group stays the same and the day 21, 28 and 1 groups decline in mean IgA titer. Thus, only the day 7 group increases antibody titers on day 21 when compared to older birds. Thus, the day 7 group displays a delayed response in antibody production compared to older birds. The day 7 and day 14 groups have plasma IgA titers to IBV that are comparable to, or higher than, the day 21 and 28 groups on day 21 of the response. Unlike the day 7 and day14 vaccination groups the day 21 and 28 groups are declining on day 21 of the response compared to the immune response on day 14. This indicates that they are past their peak response on day 21 and possibly even on day 14 based on previous observations [20]. These data are consistent with a delay in the IgA plasma response to IBV in birds vaccinated at a younger age and a non-significant decline in mean IgA titers in the 1-day-old group.
IBV-specific IgM antibody titers were measured in plasma. The plasma samples analyzed were collected on day 7 post vaccination. This time point was selected based on the literature in which the peak IgM response was observed between 5–9 days after virus challenge or live virus vaccination [26], [27], [28]. Due to the variability of IBV-reactive IgM in the controls between different age groups, independent controls were included for each age group. The IgM levels in the controls decreased considerably by ∼2 week of age after (7 days after the day 7 old chick vaccination) which increased one week later and stabilized in older age control groups (Fig. 1E). The day 14 through day 28 control IgM levels were significantly higher than in the day 1 and day 7 age group controls. And the day 7 control was significantly lower than the day 1 control IgM levels. All IgM titers from the IBV vaccinated age groups were significantly higher when compared to their controls (P < 0.05) with exception of the day 21 age groups (P = 0.08). This was due to higher control values, which were ∼1.6 fold higher than in the age 14 or 28 days old groups. This may reflect an initial peak of natural antibodies induced to IBV before stabilizing. The IBV vaccinated age groups did not differ significantly in IgM antibody levels to IBV with exception of the day 1 IBV vaccinated group, which mean ± SE was 8.7 ± 0.6 had significantly lower IBV-specific IgM levels in plasma than the day 14 (10.2 ± 0.4) day 21 (10.6 ± 0.7) and day 28 (11.2 ± 0.5) vaccinated birds but did not differ significantly from the day 7 (9.2 ± 0.5) vaccinated birds (Fig. 1E). The day 7 IgM antibody titers were also significantly lower than those in the day 28 vaccination group but not compared to the other age groups.
In tears the IBV-specific IgG response is significantly higher in the day 21 and 28 vaccinated groups than in the day 1 and 7 vaccinated chickens for day 14 of the immune response (Fig. 2A). The IgG endpoint titer in the day 1 immunized group is even significantly lower than the day 7 immunized group, while the day 14 immunized group is intermediate between the day 7 group and chickens vaccinated at an older age. Thus, a correlation between age of vaccination and the magnitude of the IBV-specific IgG response in tears is observed on day 14 of the IBV-specific immune response.
Fig. 2.
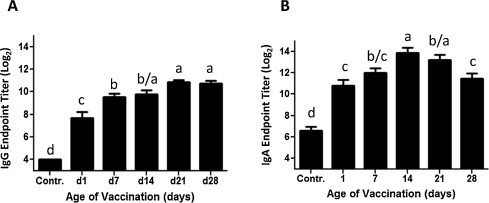
The IBV-specific IgG and IgA response in tears. Endpoint titers of IBV-specific IgG (A) and IgA (B) on day 14 of the immune response were measured by ELISA. Chickens were vaccinated on day 1, 7, 14, 21 or 28 of age. Unvaccinated chickens of the different age groups served as negative control. Depicted are the means and standard error of each age group and the control group, and different age groups are as described in Fig. 1 (n = 10–14 per group). The data was analyzed by one way ANOVA with the Newman–Keuls post-test. Differences were considered significant at P < 0.05 and are indicated by different letters.
The day 14 and 21 groups have significantly higher IgA anti-IBV responses in tears on day 14 after vaccination compared to the day 1 group. The day 1 group is not significantly different from the day 7 and 28 vaccination groups. The day 28 group is also significantly lower than the day 14 and 21 groups (Fig. 2B). This is likely due to the day 28 group displaying faster kinetics for IgA antibody levels in tears after vaccination, rather than a lower response [20]. The day 14 group IgA response is significantly higher than the day 1 and day 7 groups consistent with a delay or deficiency in the mucosal antibody response when vaccinated at an earlier age.
Our data indicate there is a delay in antibody production when vaccinated at a younger age. The day 1 vaccination group not only displays a delay but also lower levels of antibody production. To determine whether there are not only quantitative differences between antibodies produced when vaccinated on day 1 but also qualitative differences we compared avidity indices for IgG and IgA antibodies from plasma and tears generated in 1 day old versus fully matured 28 day old birds 14 days after IBV vaccination. As is illustrated in Fig. 3A,B a significantly higher avidity index is observed for IgG plasma antibodies for the day 28 vaccination group when compared to the day 1 vaccinated birds, while no significant difference is observed for IgA plasma antibodies. The same observations are also made for tear IgG and IgA antibodies (Fig. 3C,D).
Fig. 3.

Avidity indices of IBV-specific IgG and IgA antibodies in plasma and tears from chickens vaccinated at 1 and 28 days of age. Avidity indices were determined using KSCN inhibition of the IBV ELISA. Depicted are the means and standard error of plasma IgG (A) and IgA (B) and tear IgG (C) and IgA (D) of n = 4–5 observations per group. The data were analyzed using the Student t-test. Differences were considered significant at P < 0.05.
Ciliated cells and goblet cells are the primary target of IBV in the respiratory tract [29]. Fig. 4 displays the deciliation (Fig. 4A) and goblet cell (Fig. 4B) scores 4 days after IBV challenge. IBV challenge decreased ciliated epithelial cells and goblet cell score. Ciliated cells were fully protected when vaccinated on day 7 of age or later but not when vaccinated on day 1 of age (Fig. 4A). The protection of goblet cells increases with the age of vaccination and were fully protected when vaccinated on day 21 of age or older (Fig. 4B).
Fig. 4.
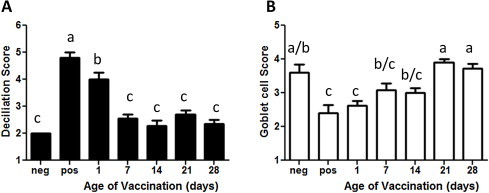
Tracheal deciliation and goblet cell depletion after IBV challenge. To measure the degree of protection against IBV challenge after vaccination tracheal deciliation and goblet cell depletion were measured. Chickens were vaccinated at day 1, 7, 14, 21 or 28 of age and challenged 21 days later. Unvaccinated/unchallenged chickens of all age groups served as negative control and unvaccinated/IBV challenge chickens as positive control. Trachea were collected 4 days post challenge. Depicted are the mean and one standard error. For the negative and positive controls n = 5 for the different age groups n = 10–13. The data were analyzed by one way ANOVA with the Newman–Keuls post-test. Significant difference was observed at P < 0.05 and are indicated by different letters.
The lymphocytes score and mucosal thickness were also measured in tracheal samples on day 4 post AL/4614/98 IBV field strain challenge as indicators of inflammation. As is illustrated in Fig. 5A, a significant decrease in lymphocyte score was observed in the day 7 and 14 vaccination groups when compared to the day 1 group. The day 1 vaccination group did not significantly differ from the day 21 and 28 vaccination groups due to a small increase in lymphocyte score in the latter two groups (Fig. 5A). The day 1 group displayed the highest lymphocytes score from all vaccination groups, which is consistent with the highest inflammatory response to IBV challenge. This was also supported by a significant increase in mucosal thickness in the day 1 vaccinated group when compared with the day 14, 21 and 28 groups but not day 7 group, which was intermediate between the day 1 and groups vaccinated at an older age (Fig. 5B).
Fig. 5.
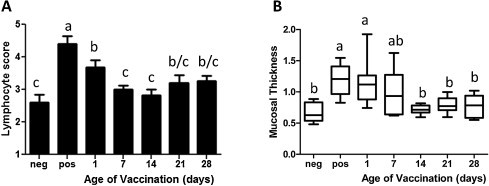
Tracheal lymphocyte infiltration and mucosal thickness after IBV challenge. To measure the degree of inflammation induced by IBV challenge tracheal lymphocyte infiltration and mucosal thickness were measured. Chickens were vaccinated at day 1, 7, 14, 21 or 28 of age and challenged 21 days later. Unvaccinated/unchallenged chickens of all age groups served as negative control and unvaccinated/IBV challenged chickens as positive control. Trachea were collected 4 days post challenge. Depicted are the mean and one standard error in panel A and the mean, 95–5% interval (boxes) and the maximum and minimum (whiskers) in panel B. The data were analyzed by one way ANOVA with the Newman–Keuls post-test. Significant difference was observed at P < 0.05 and are indicated by different letters. For the negative and positive controls n = 5 for the different age groups n = 10–13.
4. Discussion
Based on our data, the hypothesis that early IBV vaccination will generate an immature, poorly protective IBV-specific immune response, is confirmed. IBV vaccination on day 1 of age, which is routinely performed in the poultry industry, will not be fully protective and as a consequence the chicks remain vulnerable to IBV exposure. Thus, early vaccination perpetuates the IBV problems and is a factor in the estimated $48 million or more annual loss to the poultry industry due to IBV infection [30]. Our measurements of mucosal and systemic antibody levels demonstrates a delayed production of IgG and IgA plasma antibodies in the day 1, day 7 and 14 of age vaccination groups. IgA antibody levels in the day 1 group, unlike IgG antibodies, do not recover later in the response. Besides delayed IgG kinetics, the day 1 group displays also a lower avidity index than the day 28 vaccinated group. Lower avidity index is not observed in IgA antibodies. The delayed and/or lower antibody response and lower IgG avidity index translated in increased tracheal inflammation and depletion of tracheal epithelia cells and goblet cells upon IBV challenge when compared to chicks vaccinated later in life. A lack of vaccine-mediated protection is most noticeable in the 1 day of age vaccination group and to a lesser extend the day 7 vaccination group, while the day 14 and older vaccinated chickens are protected.
The IgM antibodies specific for IBV were significantly elevated above controls on days 7 of the IBV immune response in all age groups except the day 21 group. The day 21 group was not quite significant (P = 0.08) because of higher IgM levels in the control group. This could be due to an initial surge of natural IgM antibodies to IBV in this age group. A significant decline in the 7 day old group is observed when comparing the IgM antibody levels to IBV in the control day 1 group. This would be consistent with a drop of presumably natural maternal IBV-specific IgM antibodies in these SPF chickens in the day 7 control age group. These IgM antibodies rapidly increases in the day 14 group after which they stabilize in the older age groups. This indicates that a considerable portion of IgM antibodies in plasma from the older vaccination groups reacts with IBV without seeing the virus, indicating these are natural antibodies to IBV, which only increase after day 14 of age. Bacterial colonization of the intestinal tract of chickens is established during the first two weeks post-hatch [31]. This, combined with the observation that probiotics enhance natural antibodies in chicken [32], indicates that IBV-specific natural IgM antibodies to IBV are possibly generated following intestinal colonization presumably by stimulating B1 cells, which are the main producers of natural IgM antibodies in sera of mammals [33]. In the IBV vaccinated groups we see a steadily incline of IgM IBV-specific antibodies in plasma with age as has been reported by De Wit et al. [3]. Only the day 1 age group displays significantly lower IgM antibody levels when compared with the day 14–28 age groups but not with the day 7 age group. This is consistent with an early in life deficiency or delay in the IgM response.
The lower avidity index for the IgG antibodies in 1-day-old chicks is an important factor contributing to decreased protection to IBV challenge. Increased antibody affinity maturation to virus vaccines strongly correlated with better protection [34], [35]. A lack of antibody affinity maturation observed following vaccination against respiratory syncytial virus was due to a lack of TLR stimulation [35]. This indicates that 1-day-old birds may be deficient in TLR expression. Evidence that this is the case comes from a recent publication [36] demonstrating, that significantly lower levels of TLR7 expression in spleen and small intestines were observed when comparing 1-day-old chicks with 4- to 5-week-old chickens. Inclusion of TLR activating adjuvants could alleviate the problems of early vaccination by boosting antibody production and affinity maturation in 1-day-old chicks. Neither monomeric plasma nor dimeric tears-derived IgA [37] displays this drop in avidity index for the day 1 vaccinated group (Fig. 3B,D). Although there is a lower level of IgA antibodies produced by the day 1 vaccinated birds compared to the older groups, which is consistent with a delay in class-switching in the day 1 old group, it is not clear why the lack of a mature mucosal immune system in the 1-day-old group [5], [6], [11], [12], [13] did not result in lower affinity maturation of mucosal IgA antibodies compared to the 4-week-old group.
Another factor influencing early vaccination is the level of maternal antibodies, an issue not addressed in this study. There exists a linear relationship with the hens’ plasma antibody levels and transfer of IgY to the chicks’ circulation [14]. Chicks were over 95% protected against IBV challenge on day 1 if they had high levels of maternal antibodies but less than 30% protected when challenged on day 7. This protection correlated with local respiratory antibodies and not serum antibodies [38]. IBV-specific maternal antibodies decreased the induction of neutralizing antibodies following boosting [38]. Despite this inhibition by maternal antibodies of the memory response, low or erratic maternal antibody titers to IBV in broiler flocks are associated with IBV-induced economic losses [39]. This further supports that protection by maternal antibodies, which are predominantly of the IgG isotype, is important to prevent activation of an immature immune system that is not capable generating a fully protective immune response early in life.
In several studies IBV vaccination was effective against IBV challenge in both SPF chickens and commercial broilers when the initial vaccination was performed on day 1 [40], [41], [42]. However, in these studies day 1 vaccination was followed with a second vaccination two weeks later for optimal protection against challenge, which would have masked the relative poor IBV-specific immune responses after the day 1 immunization. Extensive immunization on the day of hatch containing three different live attenuated viruses, which caused severe vaccine symptoms, provided similar protection as two single live attenuated IBV virus vaccines on day of hatch and day 14 of age when challenged with a heterologous virus [42]. This seems to indicate that induction of cross-protective immunity may be less impaired when vaccinated early in life. However, no direct comparison of the same vaccination protocol was analyzed between these two challenge groups, which makes this data harder to interpret in the context of age-dependent immune responses.
A decreased humoral immune response to vaccines early in life as seen in chickens is also observed in humans. Neonates are highly dependent upon passively acquired maternal antibodies, since their humoral immune system remains underdeveloped [43]. These passively obtained antibodies in infants can alter humoral and antibody-dependent immune responses to vaccines [43], [44]. In broiler chicks maternal antibodies to pathogens persisted only for ∼10 days [45]. In our study interference of maternal antibodies was excluded. The observation that 1-day-old and 7-day-old birds are not fully protected when vaccinated are confirming the importance of pathogen-specific maternal antibodies during this period.
In summary we can say that IBV vaccination of chickens on day 1 of age contribute to the IBV problem in the poultry industry by inducing lower levels and/or slower kinetics of antibody production as well as lower avidity IgG antibodies. This results in a poorly protective immune response as is demonstrated by subsequent IBV field strain challenge. Therefore, it is advisable for the poultry industry based on our data to change their practice from vaccinating chicks on day 1 of age to vaccinating after day 7 of age.
Acknowledgements
We thank the Alabama Agricultural Experiment Station for funding this research ALA052-2-14016.
Footnotes
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.04.026.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.McMartin D.A. Infectious bronchitis. In: McFerran J.B., McNulty M.S., editors. Virus infections of birds. Elsevier Science Publishers; Amsterdam: 1993. pp. 249–274. [Google Scholar]
- 2.Jackwood M.W., Hilt D.A., McCall A.W., Polizzi C.N., McKinley E.T., Williams S.M. Infectious bronchitis virus field vaccination coverage and persistence of Arkansas-type viruses in commercial broilers. Avian Dis. 2009;53:175–183. doi: 10.1637/8465-090308-Reg.1. [DOI] [PubMed] [Google Scholar]
- 3.De Wit J.J., Swart W.A.J.M., Fabri T.H.F. Efficacy of infectious bronchitis virus vaccinations in the field: association between the alpha-IBV IgM response, protection and vaccine application parameters. Avian Pathol. 2010;39:123–131. doi: 10.1080/03079451003604639. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh D., Davis P.J. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J Gen Virol. 1986;67:1443–1448. doi: 10.1099/0022-1317-67-7-1443. [DOI] [PubMed] [Google Scholar]
- 5.Albini B., Wick G. Delineation of B and T lymphoid cells in the chicken. J Immunol. 1974;112:444–450. [PubMed] [Google Scholar]
- 6.Wight P.A., Burns R.B., Rothwell B., Mackenzie G.M. The Harderian gland of the domestic fowl. I. Histology, with reference to the genesis of plasma cells and Russell bodies. J Anat. 1971;110:307–315. [PMC free article] [PubMed] [Google Scholar]
- 7.Cook K.A., Otsuki K., Martins N.R., Ellis M.M., Huggins M.B. The secretory antibody response of inbred lines of chicken to avian infectious bronchitis virus infection. Avian Pathol. 1992;21:681–692. doi: 10.1080/03079459208418890. [DOI] [PubMed] [Google Scholar]
- 8.Gelb J., Jr., Nix W.A., Gellman S.D. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis. 1998;42:364–374. [PubMed] [Google Scholar]
- 9.Mast J., Goddeeris B.M. Development of immunocompetence of broiler chickens. Vet Immunol Immunopath. 1999;70:245–256. doi: 10.1016/s0165-2427(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 10.Maslak D.M., Reynolds D.L. B cells and T-lymphocyte subsets of the head-associated lymphoid tissues of the chicken. Avian Dis. 1995;39:736–742. [PubMed] [Google Scholar]
- 11.Fix A.S., Arp L.H. Quantification of particle uptake by conjunctiva-associated lymphoid tissue (CALT) in chickens. Avian Dis. 1991;35:174–179. [PubMed] [Google Scholar]
- 12.Albini B., Wick G., Rose E., Orlans E. Immunoglobulin production in chicken Harderian glands. Int Arch Allergy Appl Immunol. 1974;47:23–34. doi: 10.1159/000231198. [DOI] [PubMed] [Google Scholar]
- 13.Tizard I. The avian antibody response. Semin Avian Exot Pet Med. 2002;1:2–14. [Google Scholar]
- 14.Hamal K.R., Burgess S.C., Pevzner I.Y., Erf G.F. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult Sci. 2006;85:1364–1372. doi: 10.1093/ps/85.8.1364. [DOI] [PubMed] [Google Scholar]
- 15.Lowenthal J.W., Connick T., McWaters P.G., York J.J. Development of T cell immune responsiveness in the chicken. Immunol Cell Biol. 1994;72:115–122. doi: 10.1038/icb.1994.18. [DOI] [PubMed] [Google Scholar]
- 16.Beal R.K., Powers C., Wigley P., Barrow P.A., Kaiser P., Smith A.L. A strong antigen-specific T cell response is associated with age and genetically dependent resistance to avian enteric salmonellosis. Infect Immun. 2005;73:7509–7516. doi: 10.1128/IAI.73.11.7509-7516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reemers S.S., van Leenen D., Groot Koerkamp M.J., van Haarlem D., van de Haar P., van Eden W. Early host responses to avian influenza A virus are prolonged and enhanced at transcriptional level depending on maturation of the immune system. Mol Immunol. 2010;47:1675–1685. doi: 10.1016/j.molimm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Wells L.L., Lowry V.K., DeLaoch J.R., Kogut M.H. Age-dependent phagocytosis and bactericidal activities of the chicken heterophil. Dev Comp Immunol. 1998;22:103–109. doi: 10.1016/s0145-305x(97)00024-4. [DOI] [PubMed] [Google Scholar]
- 19.Toro H., Lavaud P., Vallejos P., Ferreira A. Transfer of IgG from serum to lachrymal fluid in chickens. Avian Dis. 1993;37:60–66. [PubMed] [Google Scholar]
- 20.Orr-Burks N., Gulley S.L., Gallardo R.A., Toro H., van Ginkel F.W. IgA as an early humoral responder after mucosal avian coronavirus vaccination. Avian Dis. 2014;58:279–286. doi: 10.1637/10740-120313-Reg.1. [DOI] [PubMed] [Google Scholar]
- 21.Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 22.King D.J. Evaluation of different methods of inactivation of Newcastle disease virus and avian influenza virus in egg fluids and serum. Avian Dis. 1991;35:505–514. [PubMed] [Google Scholar]
- 23.Sylvester S.A., Kataria J.M., Dhama K., Rahul S., Bhardwaj N., Tomar S. Purification of infectious bronchitis virus propagated in embryonated chicken eggs and its confirmation by RT-PCR. Ind J Comp Mircobiol Immunol Infect Dis. 2003;24:143–147. [Google Scholar]
- 24.Jones C.L., MacDonald R.A., Hosking C.S., Roberton D.M. Estimating the relative avidity of mucosal IgA for antigen. J Immunol Methods. 1987;105:111–117. doi: 10.1016/0022-1759(87)90420-0. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald R.A., Hosking C.S., Jones C.L. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods. 1988;8:356–361. doi: 10.1016/0022-1759(88)90196-2. [DOI] [PubMed] [Google Scholar]
- 26.Cook J.A.K., Da Silva Martins N.R., Mockett A.P.A., Barrett A.D.T. Local and systemic antibody class responses to an infectious laryngotracheitic virus vaccine strain. Avian Pathol. 1992;21:97–106. doi: 10.1080/03079459208418822. [DOI] [PubMed] [Google Scholar]
- 27.Da Silva Martins N.R., Mockett A.P.A., Cook J.K.A. The immunoglobulin M response in chicken serum to infectious bursal disease virus. Avian Pathol. 1992;21:517–521. doi: 10.1080/03079459208418871. [DOI] [PubMed] [Google Scholar]
- 28.Rathnaprada S., Dhinakar Raj G., Thiagarajan V. Development of monoclonal antibodies against chicken IgM and its application in immunity studies. Indian J Biotechnol. 2007;6:187–193. [Google Scholar]
- 29.Abd El Rahman S., El-Kenawy A.A., Neumann U., Herrler G., Winter C. Comparative analysis of the sialic acid binding activity and the tropism for the respiratory epithelium of four different strains of avian infectious bronchitis virus. Avian Pathol. 2009;38:41–45. doi: 10.1080/03079450802632049. [DOI] [PubMed] [Google Scholar]
- 30.Webb D. DTZ institute for animal health report. DTZ Institute; 2010. The economic and social impact of the institute for animal health's work on avian diseases; pp. 1–14. [Google Scholar]
- 31.Amit-Romach E., Sklan D., Uni Z. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult Sci. 2004;83:1093–1098. doi: 10.1093/ps/83.7.1093. [DOI] [PubMed] [Google Scholar]
- 32.Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Zhou H., Sanei B. Probiotics stimulate production of natural antibodies in chickens. Clin Vaccine Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berland R., Wortis H.H. Origins and functions of B1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 34.Khuranaa S., Coyle E.M., Verma S., King L.R., Manischewitz J., Crevar C.J. H5 N-terminal sheet promotes oligomerization of H7-HA1 that induces better antibody affinity maturation and enhanced protection against H7N7 and H7N9 viruses compared to inactivated influenza vaccine. Vaccine. 2014;32:6421–6432. doi: 10.1016/j.vaccine.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 35.Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Zea Hernandez J., Batalle J.P. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brownlie R., Zhu J., Allan B., Mutwiri G.K., Babiuk L.A., Potter A. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligonucleotides. Mol Immunol. 2009;46:3163–3170. doi: 10.1016/j.molimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 37.van Ginkel F.W., Tang D.C., Gulley S.L., Toro H. Induction of mucosal immunity in the avian Harderian gland with a replication deficient Ad-5 vector expressing avian influenza H5 hemagglutinin. Dev Comp Immunol. 2009;33:28–34. doi: 10.1016/j.dci.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mondal S.P., Naqi S.A. Maternal antibody to infectious bronchitis virus: its role in protection against infection and development of active immunity to vaccine. Vet Immunol Immunopathol. 2001;79:31–40. doi: 10.1016/S0165-2427(01)00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Herdt P., Ducatelle A.R., Uyttebroek A.E., Sneep A., Torbeyns R. Infectious bronchitis serology in broilers and broiler breeders: correlations between antibody titers and performance in vaccinated flocks. Avian Dis. 2001;45:612–619. [PubMed] [Google Scholar]
- 40.Cook J.K.A., Orbell S.J., Woods M.A., Huggins M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999;28:477–485. doi: 10.1080/03079459994506. [DOI] [PubMed] [Google Scholar]
- 41.Terregino C., Toffan A., Beato M.S., De Nardi R., Vascellari M., Meini A. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37:487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- 42.De Wit J.J., Nieuwenhuisen-van Wilgen J., Hoogkamer A., van de Sande H., Zuidam G.J., Fabri T.H. Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathol. 2011;40:463–471. doi: 10.1080/03079457.2011.599060. [DOI] [PubMed] [Google Scholar]
- 43.Jaspan H.B., Lawn S.D., Safrit J.T., Bekker L-G. The maturing immune system: implications for development and testing HIV-1 vaccines for children and adolescents. AIDS. 2006;20:483–494. doi: 10.1097/01.aids.0000210602.40267.60. [DOI] [PubMed] [Google Scholar]
- 44.Siegrist C.A., Cordova M., Brandt C., Barrios C., Berney M., Tougne C. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine. 1998;16:1409–1414. doi: 10.1016/s0264-410x(98)00100-5. [DOI] [PubMed] [Google Scholar]
- 45.Gharaibeh S., Mahmoud K. Decay of maternal antibodies in broiler chickens. Poultry Sci. 2013;92:2333–2336. doi: 10.3382/ps.2013-03249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


