Highlights
-
•
Ov-ASP-1, an onchocerca volvulus protein, has good adjuvanticity for protein antigens.
-
•
A truncated Ov-ASP-1 (ASPPR) maintains adjuvanticity as the full-length of Ov-ASP-1.
-
•
ASPPR augments humoral and cellular immune responses elicited by protein antigens.
-
•
ASPPR has potential to be further developed as a novel adjuvant for human use.
Abbreviations: Ov-ASP-1, Onchocerca volvulus activation-associated secreted protein-1; ASPPR, PR-1 domain of Ov-ASP-1; CAP, CRISPS, Ag 5 and PR-1; CRISPS, cysteine-rich secretory protein; Ag 5, antigen 5; PR-1, pathogenesis-related 1; OVA, ovalbumin; HBsAg, Hepatitis B virus surface antigen; FBS, fetal bovine serum
Keywords: Adjuvant, Ov-ASP-1, Pathogenesis-related-1 family, Th1/Th2 immune responses
Abstract
The Onchocerca volvulus activation-associated secreted protein-1 (Ov-ASP-1) has good adjuvanticity for a variety of antigens and vaccines, probably due to its ability activate antigen-processing cells (APCs). However, the functional domain of Ov-ASP-1 as an adjuvant is not clearly defined. Based on the structural prediction of this protein family, we constructed a 16-kDa recombinant protein of Ov-ASP-1 that contains only the core pathogenesis-related-1 (PR-1) domain (residues 10–153), designated ASPPR. We found that ASPPR exhibits adjuvanticity similar to that of the full-length Ov-ASP-1 (residues 10–220) for various antigens, including ovalbumin (OVA), HBsAg protein antigen, and the HIV peptide 5 (Pep5) antigen, but it is more suitable for vaccine design in ASPPR-antigen fusion proteins, and more stable in PBS than Ov-ASP-1 stored at −70 °C. These results suggest that ASPPR might be the functional region of Ov-ASP-1 as an adjuvant, and therefore could be developed as an adjuvant for human use.
1. Introduction
Vaccination has been the most effective and economical strategy to prevent or treat a variety of infections [1]. In recent years, the development of protein- or peptide-based subunit vaccines has become of great interest due to their improved safety; however, they typically elicit a weak or modest antibody response with little, or no, T cell response on their own [2], [3], [4]. Thus, adjuvants have to be added to their formulations to improve the efficacy of these new generation subunit vaccines [1].
Adjuvants currently licensed for human vaccines in the U.S. and/or Europe include aluminum (alum) salts, oil-in-water emulsion (MF59, AS03 and AF03), virosomes and AS04 (monophosphoryl lipid A (MPL) with alum salt) [5]. As a widely used adjuvant, alum can induce good Th2-associated antibody response, but it has little capacity to stimulate strong cellular immune responses, and it is not effective for peptide antigens [5], [6]. Oil-in-water emulsion adjuvants, such as MF59, AS03 and AF03 as well as AS04 are used in vaccine formulations against influenza virus or HPV and HBV, respectively. They are able to permit dose reduction and induce longer duration of antibody responses, and could not enhance the immunogenicity of peptide antigens [7], [8], [9]. The AS03 adjuvant has recently undergone an increased scrutiny because of the adverse reactions, including increased risk of narcolepsy, induced in children, adolescents and young people [10]. Accordingly, new generations of adjuvants, which are capable of safely inducing both humoral and cellular immune response with proper magnitude, breadth and potency are needed for the vaccine development of future subunit vaccines [1].
Immunostimulatory adjuvants are direct activators of innate immune receptors such as toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs) [1], and the cytosolic DNA sensor of the endoplasmic reticulum (ER)-resident transmembrane protein stimulator of IFN genes (STING) [11]. The innate adjuvants include the TLR4 ligand MPL [12], the TLR3 ligand poly (I:C) [13], the TLR5 ligand Flagellin [14], [15], the TLR7/8 ligand Imiquimod and R848, and the TLR9 ligand CpG ODN [16]. Apart from these immunostimulatory molecules, several helminth-proteins were reported to have adjuvant effects when used with different vaccine models; however, most of them induced Th2-type immune responses [17], [18], [19]. Our previous studies have shown that a 22-kDa protein named activation-associated secreted protein-1 (Ov-ASP-1) from Onchocerca volvulus is a potent adjuvant for various vaccine antigens, including synthetic peptide-based antigens [20], [21], [22], [23]. Importantly, Ov-ASP-1 can be formulated with a vaccine antigen either in aqueous admixtures or as a fusion protein. The attributes of Ov-ASP-1 adjuvant include: (i) easy formulation; (ii) enables reduction in dosage of the vaccine antigen; (iii) has the capacity to accelerate and enhance antibody responses against the target vaccine antigens; (iv) able to elicit mixed Th1/Th2 with Th1-biased antibody responses and cellular immune responses against some antigens; (v) elicits a balanced immune responses against various vaccines; and (vi) augments recall responses to the vaccine antigens [22], [23], [24], [25], [26], [27]. Ov-ASP-1 was shown to bind specifically to human antigen presenting cells (APCs) and to trigger Th1-biased proinflammatory cytokine production in naïve PBMCs, most likely through its ability to activate monocyte-derived dendritic cells, TLR2 and TLR4 [28].
However, the functional region of Ov-ASP-1 as an adjuvant was not yet defined. In this study, we found that a truncated fragment of Ov-ASP-1 (residues 10–153, 16 kDa), which contains the core pathogenesis-related-1 (PR-1) domain of Ov-ASP-1, designated ASPPR, exhibited adjuvanticity similar to that of the full-length Ov-ASP-1 (residues 10–220).
2. Material and methods
2.1. Mice
Female 6- to 8-week-old BALB/c mice (Beijing Experimental Animal Center, Beijing, China) were used for all mouse experiments. The experimental protocols were approved by The Laboratory Animal Center, State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology IACUCs (Permit number, BIME 2012–11).
2.2. Design, expression and identification of ASPPR
By analyzing the structure of the pathogenesis-related 1 (PR-1) family of proteins, in particular the crystal structure of Na-ASP-2, a homologues protein derived from the parasitic nematode Necator americanus [29] using the SWISS-MODEL Workspace program [30], the predicted PR-1 domain of Ov-ASP-1 was identified. Accordingly, the truncated fragment, ASPPR, containing residues 10–153 of Ov-ASP-1 was designed; its E. coli codon-optimized cDNA was synthesized and inserted into the pQE30 expression vector. The pQE-ASPPR recombinant construct was expressed in E. coli as previously described [24]. ASPPR expression was analyzed by SDS-PAGE, and confirmed by Western blot, using an Ov-ASP-1-specific monoclonal antibody (mAb) prepared in our laboratory.
ASPPR was purified by Ni-NTA chromatography as previously described [24] with few modifications. The major difference is the conditions of its purification: the recombinant protein was dialyzed at the end with PBS, whereas, previously the protein was stopped in the Laemmli buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8·3). Briefly, inclusion bodies containing the target recombinant protein were dissolved in 8 M urea overnight at 4 °C; the soluble denatured protein was then purified by Ni-EF (GenScript) using denatured condition and subsequently refolded by gradient dialysis in 8, 6, and 4 M urea in Tris–glycine buffer followed by Laemmli buffer and PBS, for 12 h at each step. The purified protein was passed through the lipopolysaccharide (LPS)-removing gel (Detoxigel™, Pierce Biotechnology, Rockford, IL) to remove any contaminating LPS and analyzed by SDS-PAGE. Full-length Ov-ASP-1 was prepared by using the same protocol. The final recombinant ASPPR and Ov-ASP-1 proteins used in the following experiments were negative for bacterial endotoxin, as determined by the Limulus amebocyte lysate (LAL) assay [28].
2.3. Stability analysis of ASPPR protein
The purified ASPPR and Ov-ASP-1 control in endotoxin-free PBS were aliquoted in sterile tubes, and stored at −70 °C in freezer. The protein concentration in the samples taken out at 0, 1, and 3 months, respectively, after frozen were measured by Bradford assay, and calculated according to the standard curve.
2.4. Flow cytometry-based binding assay
Splenocytes from 6- to 8-week-old female BALB/c mice were isolated and counted. 1 × 106 cells were incubated with purified ASPPR or Ov-ASP-1 (final concentration 10 μg/ml) at room temperature for 30 min, followed by incubation with antibody cocktails of APC-anti-CD3, PE-Cy5-anti-CD45R/B220, PE-anti-CD14 (BD Bioscience) and FITC-anti-His (1:200 diluted, Miltenyi Biotec, Inc.) antibody for another 30 min. The stained cells were collected by flow cytometry (BD FACSCalibur™, BD Biosciences), and analyzed using FlowJo 7.6.
2.5. Mouse immunization and sample collection
Adjuvanticity of ASPPR was evaluated in BALB/c mice using two proteins (OVA and HBsAg) and one peptide (HIV-1 Pep5, an identified Th-epitope rich region which is located on HIV-1 gp120, gp120206–234 PKVSFEPIPIHYCAPAGFAILKCNNKTFN,) as the model vaccine antigens. Six female BALB/c mice per group were intramuscularly administrated with OVA (1 μg), HBsAg (1 μg) or HIV-1 Pep5 (20 μg) in endotoxin-free PBS (GIBCO) and formulated with ASPPR or Ov-ASP-1 (25 μg), or with Alum (InvivoGen; ratio of Alum to antigen is 1:9) and boosted once with the same antigens and adjuvants 3-week later. Either antigen alone or endotoxin-free PBS (GIBCO) was used as the control. Mouse sera were collected before immunization and 3 weeks after final immunization to detect specific antibodies. Splenocytes were isolated at the third week after boost to evaluate cellular immune responses.
2.6. Measurement of serum antibody by ELISA
ELISA was used to measure serum IgG, IgG1 and IgG2a antibodies against each antigen in the mice vaccinated with three tested antigens; ovalbumin (OVA), HBsAg, or HIV-1 Pep5, plus respective adjuvants, as described above, using a protocol similar to that described previously [31]. OVA, HBsAg, HIV Pep5, ASPPR or Ov-ASP-1 at a final concentration of 1 μg/ml was used as the capture antigen for ELISA.
2.7. IFN-γ and IL-4 ELISPOT
Specific IFN-γ- and IL-4-producing splenocytes from the vaccinated mice were measured by mouse IFN-γ or IL-4 ELISPOT according to the manufacturer's recommendations (BD Biosciences). Briefly, splenocytes (5 × 105 cells/well) were incubated for 48 h with or without peptide antigens HBsAg (S28–39, IPQSLDSWWTSL [32]) or HIV-1 (PKVSFEPIPIHYCAPAGF) at a final concentration of 10 μg/ml. The spots were counted by ELISPOT Reader (CTL) and expressed as the number of spot-forming cells (SFC)/106 cells.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (AVOVA) was used for comparison of mean or geometric mean from multiple groups. A P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. ASPPR was efficiently expressed in E. coli
ASPPR, a 144-aa fragment (residues 10–153) containing the core PR-1 domain of Ov-ASP-1, was expressed in E. coli. Similar to Ov-ASP-1, ASPPR was expressed mainly in the form of inclusion bodies. Recombinant ASPPR in the native form was obtained after purification and refolding by gradient dialysis to PBS (Fig. 1A). It was recognized by anti-Ov-ASP-1 mAb, as detected by Western blot (Fig. 1B), indicating that ASPPR was successfully expressed in E. coli. We noticed that ASPPR in PBS stored at −70 °C for 3 months is more stable than Ov-ASP-1 that was kept under the same condition (Fig. S1).
Fig. 1.
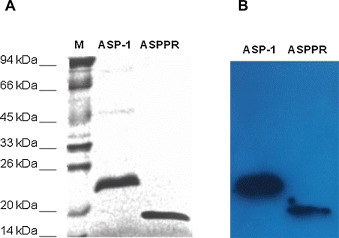
Expression of ASPPR and Ov-ASP-1. (A) SDS-PAGE analysis of the purified Ov-ASP-1 protein and ASPPR truncated fragment after renaturing. (B) Western blot analysis of ASPPR using anti-Ov-ASP-1 mAb.
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.02.053.
3.2. ASPPR has bioactivity similar to that of Ov-ASP-1
To determine whether the truncated ASPPR fragment would function in a manner similar to Ov-ASP-1, we first compared the binding profile of ASPPR and Ov-ASP-1 to splenocytes of healthy BALB/c mice using FITC-labeled anti-His antibody, which recognized the His-tag at the N-terminus of ASPPR and Ov-ASP-1. While Ov-ASP-1 and ASPPR shared similar binding profiles, ASPPR demonstrated a higher binding ability to T, B, and NK cells, as well as the macrophages within the splenocytes (Fig. 2 ), suggesting that ASPPR might be the functional core of Ov-ASP-1.
Fig. 2.
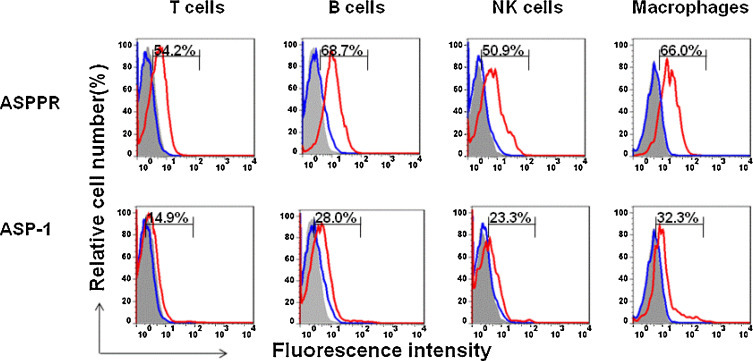
Bioactivity of the truncated fragment ASPPR in vitro. Splenocytes from healthy BALB/c mice were isolated to analyze the bioactivity of ASSPR. Purified ASPPR or Ov-ASP-1 (final concentration 10 μg/ml) was added to 1 × 106 of naive mouse splenocytes in 5 ml round bottom tubes and incubated at room temperature for 30 min, followed by incubation with antibody cocktails of APC-anti-CD3, PE-Cy5-anti-CD45R/B220, PE-anti-CD14 and FITC-anti-His antibody (1/200 dilution) for another 30 min. The stained cells were analyzed by flow cytometry, and the overlay analysis was done by FlowJo 7.6. Gray shade stands for blank control, blue line is His-FITC control, and red line is ASPPR (or Ov-ASP-1) + His-FITC. The presented data were representative results from three mice (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.3. ASPPR retains the adjuvanticity of Ov-ASP-1 for the model antigen OVA
We first compared the adjuvanticity of ASPPR with Ov-ASP-1 and alum using OVA, a model antigen frequently used to evaluate adjuvanticity. Similar to Ov-ASP-1 and alum, our results showed that ASPPR significantly augmented IgG antibody responses to OVA vs. OVA alone (P < 0.001) (Fig. 3A). Further comparison of IgG subtypes revealed that the IgG1 antibody was similarly augmented in the presence of ASPPR, Ov-ASP-1 and alum (Fig. 3B), but that the IgG2a antibody response was elicited only in the ASPPR and Ov-ASP-1 vaccination groups (P < 0.05) (Fig. 3 C). These results indicate that both ASPPR and Ov-ASP-1 are able to elicit a mixed Th1 and Th2 responses, which is different from the skewed Th2 response induced by alum, suggesting that ASPPR has the ability to maintain the balanced adjuvanticity elicited by Ov-ASP-1.
Fig. 3.
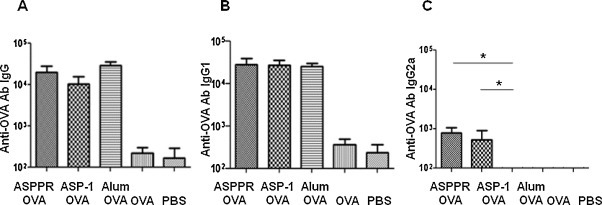
Anti-OVA antibody in BALB/c mice immunized with ASPPR and OVA. Six-week-old female BALB/c mice were immunized intramuscularly with 10 μg of endotoxin-free OVA and 25 μg of recombinant ASPPR and boosted once 3 week later; mice were immunized with OVA with Ov-ASP-1, OVA with Alum, OVA alone and PBS as control. Serum was collected on the third week after the final immunization, and anti-OVA- specific antibodies, including IgG (A), IgG1 (B) and IgG2a (C), were measured by ELISA. The data are presented as geometric mean ± SD.
3.4. ASPPR augments specific anti-HBsAg humoral and cellular immune responses
To observe the adjuvant effect of ASPPR with a recombinant vaccine protein, we used the recombinant protein HBsAg (the protective antigen of hepatitis B virus), in the presence of ASPPR to immunize mice. ASPPR not only augmented the IgG antibody responses (Fig. 4A), but also increased both Th2-associated IgG1 (Fig. 4B) and Th1-associated IgG2a (Fig. 4 C) antibody responses, which reflects a bioactivity profile much different from that of alum. Of note, ASPPR induced significantly higher antibody IgG2a responses than Ov-ASP-1 (P < 0.05) with similar levels of antibody IgG and IgG1. ELISPOT assay revealed that ASPPR as well as Ov-ASP-1 elicited higher production of HBsAg peptide-specific IFN-γ (Fig. 4D), but ASPPR also induced higher IL-4 (Fig. 4E) responses; whereas, alum stimulated only significantly higher IL-4. Taken together, these results indicated that ASPPR not only augmented protein antigen-induced antibody response, but also enhanced specific cellular immune responses, suggesting that ASPPR retains the adjuvanticity of Ov-ASP-1 for recombinant protein antigen such as HBsAg.
Fig. 4.
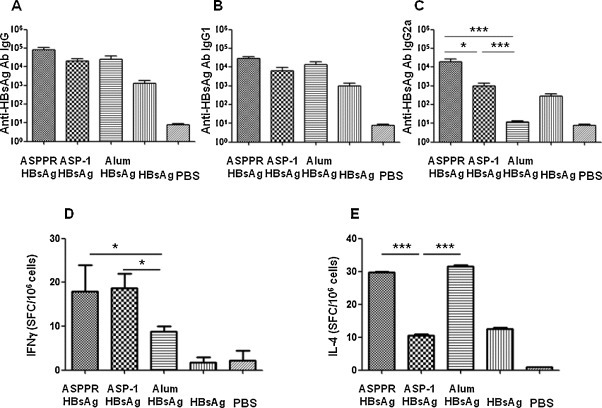
Anti-HBsAg antibody and cellular immune responses in BALB/c mice immunized with ASPPR and HBsAg. BALB/c mice were intramuscularly immunized with 1 μg of recombinant HBsAg and 25 μg of recombinant ASPPR and boosted once 3 weeks later. Mice were immunized with HBsAg plus Ov-ASP-1, HBsAg plus Alum, HBsAg alone, or PBS alone were used as the control. Upper: antibody levels in mouse sera. Sera were collected on the third week after the final immunization, and anti-HBsAg-specific antibody IgG (Fig. 4A), IgG1 (Fig. 4B), and IgG2a (Fig. 4C) were measured by ELISA. Antibody titers are expressed as geometric mean ± SD. Lower: ELISPOT Assay. Splenocytes from the immunized mice were isolated and stimulated with 10 μg/ml of the peptide specific to HBsAg, S28–39IPQSLDSWWTSL, to observe specific cellular immune response by counting spots on the plate of IFNγ (Fig. 4D) and IL-4 (Fig. 4E). The representative data of one of six mice is presented.
3.5. ASPPR assists HIV-1 peptide to elicit both humoral and cellular immune response
We then tested whether ASPPR would act as an effective adjuvant for a synthetic peptide using a 29-aa HIV-1 gp160 peptide, Pep5, as the model vaccine peptide. ELISA results revealed that ASPPR augmented humoral and cellular immune responses similar to those of Ov-ASP-1, and both stimulated significantly higher IgG (Fig. 5A), IgG1 (Fig. 5B) and IgG2a (Fig. 5 C) antibody responses than alum did. ELISPOT results showed that both ASPPR and Ov-ASP-1 augmented IFN-γ production (Fig. 5D), but only alum induced significant IL-4 (Fig. 5E) response in the immunized mice. These findings demonstrated that ASPPR, similar to Ov-ASP-1, is an effective adjuvant also for the selected peptide antigen, peptide 5.
Fig. 5.
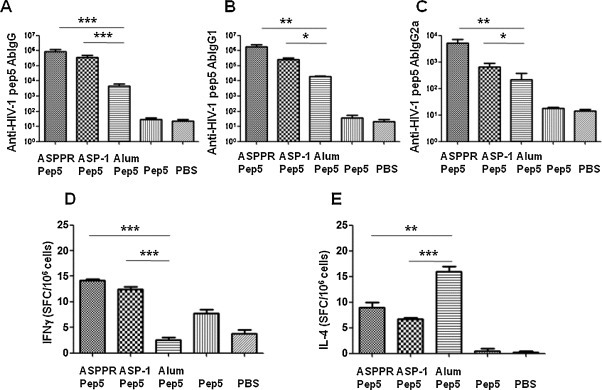
Anti-HIV Pep5 antibody and cellular immune responses in BALB/c mice immunized with ASPPR and HIV Pep5. BALB/c mice were intramuscularly immunized with 20 μg of the synthesized HIV-1 Pep5 (PKVSFEPIPIHYCAPAGFAILKCNNKTFN) and 25 μg of recombinant ASPPR and boosted once 3 weeks later. Mice were immunized with HIV-1 Pep5 with Ov-ASP-1, Pep5 with Alum, Pep5 alone and PBS as control. Upper panel: antibody IgG and isotype in mouse sera. Sera were collected on the third week after the final immunization, and anti-HIV-1 Pep5-specific antibody IgG (Fig. 5A), IgG1 (Fig. 5B), and IgG2a (Fig. 5C) were measured by ELISA and expressed as geometric mean ± SD. Lower panel: ELISPOT assay. Splenocytes from the immunized mice were isolated and stimulated with 10 μg/ml of the specific peptide to observe the specific cellular immune response by counting the spots on the plate of IFNγ (Fig. 5D) and IL-4 (Fig. 5E).
4. Discussion
Adjuvants have been proven to be very important components in vaccines, making it essential to explore and identify novel adjuvants that are suitable for various types of vaccine antigens [1], [33]. In particular, safe and nontoxic adjuvants able to stimulate both humoral and cellular immune responses for different types of antigens are sought. Our previous studies have shown that Ov-ASP-1 in aqueous formulation with bystander antigens is an efficient adjuvant for several vaccine antigens, including recombinant proteins, synthetic peptides and commercially inactivated vaccines [24]. Moreover, Ov-ASP-1 induced a mixed Th1/Th2 response with a Th1-biased antibody profile against many antigens. However, its functional region is still unknown. In this study, we show the ASPPR might be the functional fragment of Ov-ASP-1, as it retains the potent adjuvanticity demonstrated for the full length Ov-ASP-1, but ASPPR is more stable than Ov-ASP-1 in PBS.
The genealogical tree analysis of OV-ASP-1 sequence revealed that OV-ASP-1 belongs to the CAP superfamily [34], which also includes Na-ASP-2. Alignment of Ov-ASP-1 and Na-ASP-2 amino acid sequences showed that Ov-ASP-1 has a major conserved domain that forms a similar tertiary structure as that published for Na-ASP-2 [29]. Using the crystal structure of Na-ASP-2 as a model, we designed a potential functional fragment, ASPPR, that corresponds to the PR-1 structurally conserved domain and which was successfully expressed in an E. coli system. Further investigation revealed that ASPPR retained the cell binding profile of Ov-ASP-1, but has a higher binding capacity, as demonstrated by its ability to bind to mouse T, B, and NK cells, as well as macrophages. Previously it was shown that Ov-ASP-1 binds mostly to human B cells and monocytes [28].
We have previously shown that full-length Ov-ASP-1 can enhance both humoral and cellular immune responses against protein antigens [22], [24]. Our results proved that ASPPR retains the adjuvanticity of Ov-ASP-1 for the common used model antigen OVA. Then, we tested the adjuvanticity of ASPPR using HBsAg, an important protective antigen of HBV and the key component of the HBV vaccine. Immunization with HBsAg using alum induced only Th2-biased antibody response [35], which is not beneficial for HBV clearance. As expected, ASPPR effectively augmented both balanced Th1/Th2-associated humoral and cellular immune responses against HBV, indicating that ASPPR retains the adjuvanticity of Ov-ASP-1 as an adjuvant for recombinant proteins.
Peptide-based subunit vaccines have the advantage of ease of design, modification and production. However, because of their relatively low immunogenicity, they have to be formulated with adjuvants to enhance their immunogenicity. Thus far, licensed adjuvants for human use, such as alum, do not effectively enhance peptide-induced immune responses. Some new adjuvants, such as poly (I:C), an agonist of TLR3, have been proven to be effective for peptide antigens, but induced adverse effects, which may limit their application [13]. In our previous studies, we showed that Ov-ASP-1 is an efficient adjuvant also for SARS and HIV peptide antigens in aqueous [24], or for influenza H5N1 peptide in a recombinant fusion protein form by linking influenza M2e to Ov-ASP-1 [23]. In the present study, we have demonstrated that the truncated ASPPR protein maintains the adjuvanticity of the full-length Ov-ASP-1 also when used with the HIV-1 peptide Pep5.
Since both ASPPR and Ov-ASP-1 are proteins, they can induce anti-self antibodies in the vaccinated mice, thus raising the question of whether preexisting antibodies against these protein adjuvants may suppress their adjuvanticity when they are used in subsequent vaccine formulations. Our previous study have shown that the antibodies induced against Ov-ASP-1 (1:256,000–1:512,000) had no impact on its ability to induce immune responses against bystander antigens (e.g., the RBD of SARS-CoV spike protein and a commercial influenza virus HA vaccine) when used as an adjuvant in a sequential vaccine. Moreover, we have shown that the anti-Ov-ASP-1 anti-self-antibodies elicited during three immunization of non-human primate are much reduced, thus pointing to the possibility that the level of anti-self-antibodies could be species dependent and presently less of an issue with moving forward to the development of Ov-ASP-1 as an adjuvant [27]. Notably, another protein adjuvant, flagellin, has been used in clinics or clinical trials [36], [37], [38]. To the best of our knowledge, there has been no report to show that the anti-flagellin antibodies have significant impact on its adjuvanticity. As a protein-based adjuvant, Ov-ASP-1 can be fused with a protein antigen as a subunit vaccine consisting of both immunogen and adjuvant. Since ASPPR has much shorter sequence than Ov-ASP-1, we believe that ASPPR is more suitable than the full-length of Ov-ASP-1 for construction and expression of the fusion protein-based subunit vaccine.
In summary, we demonstrated that the truncated fragment of Ov-ASP-1, ASPPR containing only the PR-1 domain, maintains the same adjuvanticity as the full-length Ov-ASP-1, suggesting that ASPPR can be developed as an adjuvant for human use.
Acknowledgement
This work was supported by a grant from NSFC #31000412.
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
Zhihua Kou, Email: zh_69kou@163.com.
Yusen Zhou, Email: yszhou@bmi.ac.cn.
References
- 1.Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 2.Sharma C., Khan M.A., Mohan T., Shrinet J., Latha N., Singh N. A synthetic chimeric peptide harboring human papillomavirus 16 cytotoxic T lymphocyte epitopes shows therapeutic potential in a murine model of cervical cancer. Immunol Res. 2014;58(1):132–138. doi: 10.1007/s12026-013-8447-2. [DOI] [PubMed] [Google Scholar]
- 3.Torresi J., Fischer A., Grollo L., Zeng W., Drummer H., Jackson D.C. Induction of neutralizing antibody responses to hepatitis C virus with synthetic peptide constructs incorporating both antibody and T-helper epitopes. Immunol Cell Biol. 2007;85(2):169–173. doi: 10.1038/sj.icb.7100021. [DOI] [PubMed] [Google Scholar]
- 4.Pollard R.B., Rockstroh J.K., Pantaleo G., Asmuth D.M., Peters B., Lazzarin A. Safety and efficacy of the peptide-based therapeutic vaccine for HIV-1, vacc-4x: a phase 2 randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2014;14(4):291–300. doi: 10.1016/S1473-3099(13)70343-8. [DOI] [PubMed] [Google Scholar]
- 5.Alving C.R., Peachman K.K., Rao M., Reed S.G. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24:310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marichal T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 7.Vono M., Taccone M., Caccin P., Gallotta M., Donvito G., Falzoni S. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci U S A. 2013;110:21095–21100. doi: 10.1073/pnas.1319784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox C.B., Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccin. 2013;12:747–758. doi: 10.1586/14760584.2013.811188. [DOI] [PubMed] [Google Scholar]
- 9.Sow P.S., Watson-Jones D., Kiviat N., Changalucha J., Mbaye K.D., Brown J. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10-25-year-old HIV-Seronegative African girls and young women. J Infect Dis. 2013;207:1753–1763. doi: 10.1093/infdis/jis619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nohynek H., Jokinen J., Partinen M., Vaarala O., Kirjavainen T., Sundman J. AS03 adjuvanted H1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X.D., Wu J., Gao D., Wang H., Sun L., Chen Z.J. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireton G.C., Reed S.G. Adjuvants containing natural and synthetic toll-like receptor 4 ligands. Expert Rev Vaccin. 2013;12(7):793–807. doi: 10.1586/14760584.2013.811204. [DOI] [PubMed] [Google Scholar]
- 13.Cui Z., Qiu F. Synthetic double-stranded RNA poly(I:C) as a potent peptide vaccine adjuvant: therapeutic activity against human cervical cancer in a rodent model. Cancer Immunol Immunother. 2006;55:1267–1279. doi: 10.1007/s00262-005-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Yglesias A.H., Zhao X., Quarles E.K., Lai M.A., Vandenbos T., Strong R.K. Flagellin induces antibody responses through a TLR5- and inflammasome-independent pathway. J Immunol. 2014;192:1587–1596. doi: 10.4049/jimmunol.1301893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizel S.B., Bates J.T. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody M.A., Santra S., Vandergrift N.A., Sutherland L.L., Gurley T.C., Drinker M.S. TLR-7/8 and 9 agonists cooperate to enhance HIV-1 envelope antibody responses in Rhesus Macaques. J Virol. 2014;88:3329–3339. doi: 10.1128/JVI.03309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland M.J., Harcus Y.M., Riches P.L., Maizels R.M. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–1987. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Segura-Velazquez R.A., Perez-Torres A., Rosas G., Toledo A., Restelli M., Acosta E. A novel synthetic adjuvant effectively enhances the immunogenicity of the influenza vaccine. Vaccine. 2006;24:1073–1080. doi: 10.1016/j.vaccine.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Okano M., Satoskar R., Nishizaki K., Harn D.A., Jr. Lacto-N-fucopentaose III found on Schistonsoma mansoni egg antigens function as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167:442–450. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald A.J., Turaga P.S., Harmon-Brown C., Tierney T.J., Bennett K.E., McCarthy M.C. Differential cytokine and antibody responses to adult and larval stages of Onchocerca volvulus consistent with the development of concomitant immunity. Infect Immun. 2002;70:2796–2804. doi: 10.1128/IAI.70.6.2796-2804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustigman S., MacDonald A.J., Abraham D. CD4-dependent immunity to Onchocerca volvulus third-stage larvae in humans and the mouse vaccination model: common ground and distinctions. Int J Parasitol. 2003;33:1161–1171. doi: 10.1016/s0020-7519(03)00170-x. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald A.J., Cao L., He Y., Zhao Q., Jiang S., Lustigman S. rOv-ASP-1, a recombinant secreted protein of the helminth Onchocerca volvulus, is a potent adjuvant for inducing antibodies to ovalbumin, HIV-1 polypeptide and SARS-CoV peptide antigens. Vaccine. 2005;23:3446–3452. doi: 10.1016/j.vaccine.2005.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao G., Du L., Xiao W., Sun S., Lin Y., Chen M. Induction of protection against divergent H5N1 influenza viruses using a recombinant fusion protein linking influenza M2e to Onchocerca volvulus activation associated protein-1 (ASP-1) adjuvant. Vaccine. 2010;28(44):7233–7240. doi: 10.1016/j.vaccine.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 24.Xiao W., Du L., Liang C., Guan J., Jiang S., Lustigman S. Evaluation of recombinant Onchocerca volvulus activation associated protein-1 (ASP-1) as a potent Th1-biased adjuvant with a panel of protein or peptide based antigens and commercial inactivated vaccines. Vaccine. 2008;26:5022–5029. doi: 10.1016/j.vaccine.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDonald A.J., Libri N.A., Lustigman S., Barker S.J., Whelan M.A., Semper A.E. A novel, helminth-derived immunostimulant enhances human recall responses to hepatitis C virus and tetanus toxoid and is dependent on CD56+ cells for its action. Clin Exp Immunol. 2008;152:265–273. doi: 10.1111/j.1365-2249.2008.03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J., Fisher E.M., Hensley S.E., Lustigman S., Dm M., Shen H. Antigen sparing and enhanced protection using a novel r-Ov-ASP-1 adjuvant in aqueous formulation with influenza vaccines. Vaccine. 2014;32:2696–2702. doi: 10.1016/j.vaccine.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Tricoche N., Du L., Hunter M., Zhan B., Goud G. The adjuvanticity of an O. volvulus-derived rOv-ASP-1 protein in mice using sequential vaccinations and in non-human primates. PLoS ONE. 2012;7(5):e37019. doi: 10.1371/journal.pone.0037019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Y., Barker S.J., MacDonald A.J., Yu Y., Cao L., Li J.J. Recombinant Ov-ASP-1, A Th1-biased protein adjuvant derived from helminth Onchocerca volvulus, can directly bind and activate antigen-presenting cells. J Immunol. 2009;182:4005–4016. doi: 10.4049/jimmunol.0800531. [DOI] [PubMed] [Google Scholar]
- 29.Asojo O.A., Goud G., Dhar K., Loukas A., Zhan B., Deumic V. X-ray structure of Na-ASP-2, a pathogenesis-related -1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. J Mol Biol. 2005;346:801–814. doi: 10.1016/j.jmb.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Arnold K., Bordoli L., Kopp J., Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 31.Wen J., Yang Y., Zhao G., Tong S., Yu H., Jin X. Salmonella typhi Ty21a bacterial ghost vector augments HIV-1 gp140 DNA vaccine-induced peripheral and mucosal antibody responses via TLR4 pathway. Vaccine. 2012;30:5733–5739. doi: 10.1016/j.vaccine.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Song E.S., Park S.A., Kim S.H., Cho Y.J., Ahn B.Y., Ahn B.C. Adjuvant effect of CIA07, a combination of Escherichia coli DNA fragments and modified lipopolysaccharides, on the immune response to hepatitis B virus surface antigen. FEMS Immunol Med Microbiol. 2007;51:496–504. doi: 10.1111/j.1574-695X.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 33.Levitz S.M., Golenbock D.T. Beyond empiricism: informing vaccine development through innate immunity research. Cell. 2012;148:1284–1292. doi: 10.1016/j.cell.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibbs G.M., Roelants K., O’Bryan K. The CAP superfamily: cysteine-rich secretary proteins, antigen 5, and pathogenesis-related 1 protein-roles in reproduction, cancer, and immune defense. Endocr Rev. 2008;29:865–897. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- 35.Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treanor J.J., Taylor D.N., Tussey L., Hay C., Nolan C., Fitzgerald T. Safety and immunognicity of a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine. 2010;28:8268–8274. doi: 10.1016/j.vaccine.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Taylor D.N., Treanor J.J., Strout C., Johnson C., Fitzgerald T., Kavita U. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29:4897–4902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Taylor D.N., Treanor J.J., Sheldon E.A., Johnson C., Umlauf S., Song L. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine. 2012;30:5761–5769. doi: 10.1016/j.vaccine.2012.06.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


