Abstract
Severe acute respiratory syndrome (SARS) caused by a newly identified coronavirus (SARS-CoV) remains a threat to cause epidemics as evidenced by recent sporadic cases in China. In this communication, we evaluated the efficacy and safety of two SARS vaccine candidates based on the recombinant modified vaccinia Ankara (MVA) expressing SARS-CoV spike or nucleocapsid proteins in ferrets. No clinical signs were observed in all the ferrets challenged with SARS-CoV. On the other hand, vaccination did not prevent SARS-CoV infection in ferrets. In contrast, immunized ferrets (particularly those immunized with rMVA-spike) exhibited significantly stronger inflammatory responses and focal necrosis in liver tissue after SARS-CoV challenge than control animals. Thus, our data suggest that enhanced hepatitis is linked to vaccination with rMVA expressing SARS-CoV antigens.
Keywords: Vaccinia virus, SARS, Ferrets, Vaccine, Hepatitis
1. Introduction
The first worldwide severe acute respiratory syndrome (SARS) epidemic between late 2002 and the first half of 2003 caused severe stress in every aspect of our society, particularly in those epidemic areas. The causative agent was quickly identified and characterized as a new member of the family Coronaviridae, the SARS-associated coronavirus (SARS-CoV) [1], [2]. As evidenced by sporadic cases reported in late 2003 (http://www.wpro.who.int/sars/docs/pressreleases/pr_27122003.asp and http://www.who.int/csr/don/2004_04-23/en/), SARS-CoV remains a constant threat to cause another epidemic. Therefore, it is urgently needed to develop an effective vaccine to contain future SARS outbreak.
In the present study, we used the highly attenuated vaccinia virus, modified vaccinia Ankara (MVA), as a vector to construct recombinant MVA expressing SARS-CoV spike (S) and nucleocapsid (N) proteins, analogues of which are the two major antigenic proteins responsible for inducing protective immune responses against coronaviruses [3], [4]. Since it has been reported that ferrets were susceptible to SARS-CoV infection [5], we used ferrets as an animal model to evaluate the efficacy and safety of rMVA based SARS vaccines.
2. Materials and methods
2.1. Cells, viruses
BHK21 and Vero E6 cells were used to grow MVA (kindly provided by Dr. Bernard Moss at NIH) and Tor2 isolate of SARS-CoV (isolated at the NML), respectively.
2.2. Animals
Six to 10 weeks old (350–500 g) male (castrated) ferrets (Mustela putorius furo) were purchased from Marshall Farm Pet Supplies (Wolcott, New York). Animal housing and all the animal manipulations were approved by the Animal Care Committee of the Canadian Science Centre for Human and Animal Health and met the Canadian Council on Animal Care guidelines.
2.3. Construction of recombinant MVA expressing SARS-CoV S and (N) proteins
SARS-CoV (Tor2 isolate) S and N genes were synthesized using standard RT-PCR protocol and the sequence was confirmed by comparison to the Genbank sequence (accession number NC_004718).
SARS-CoV S and N genes were further cloned into vaccinia recombinant and expression vector pJS5 provided by Dr. Bernard Moss of NIH [6]. The recombinant MVA expressing the S (rMVA-S) and N (rMVA-N) proteins were selected with the mycophenolic acid selection medium using the standard protocol for the construction of recombinant poxviruses [7].
2.4. Manipulation of ferrets
All animal work was performed in biosafety level 3 (BSL3, immunization stage) and BSL4 (challenge stage) containment laboratory of the Canadian Food Inspection Agency at the Canadian Science Centre for Human and Animal Health. Ferrets were immunized with 108 plaque-forming-units (pfu) of rMVA-S, rMVA-N, the parental MVA (control) or PBS by intraperitoneal and subcutaneous routes. Two weeks after the prime immunization, ferrets received a booster immunization of 5 × 107 pfu of the corresponding viruses or PBS by the same inoculation routes. Animals were monitored daily and the blood samples were collected on days 0, 7, 14, 21 and 28 days post vaccination for analyzing antibody responses by enzyme-linked immunosorbent assay (ELISA) and micro-neutralization assays.
Four weeks after the prime immunization, ferrets were challenged with 106 pfu of the Tor2 isolate of SARS-CoV by intranasal route. Animals were monitored and the temperature was taken daily. Feces and oral/throat swabs specimens were collected daily. The blood samples were taken between days 3 and 5, 7 and 9, 12 and 14, 19 and 21, 26 and 28 post SARS-CoV challenge. All the animals were euthanised 28 or 29 days after challenge with SARS-CoV and necropsies were performed.
2.5. ELISA and micro plaque reduction neutralization test (mPRNT)
For ELISA assay, SARS-CoV infected or mock-infected Vero-E6 cell lysate were used to coat 96-well ELISA plates (Falcon 353911). Ferret sera were heat-inactivated (56 °C for 30 min) and diluted in PBS containing 0.5% Tween 20 and 5% skimmed milk (1/100, in triplicates). The HRP conjugated secondary antibody goat anti-ferret (IgG) was purchased from the Immunology Consultant Laboratory (Newberg, OR, USA). The ABTS/H2O2 system from KPL was used as a substrate. The OD readings from the reaction between sera and the SARS-CoV infected Vero-E6 cell lysate (positive control) and the mock-infected lysate (negative control) were compared and the positive/negative (P/N) ratio was calculated to determine the ELISA results. Mean negative P/N ratio was established using a total of 60 sera provided by Marshall Farms and was determined as 1.43 with a standard deviation of 0.36. Thus, in our ELISA data, P/N ratio less than 1.79 was interpreted as negative while the ratio greater than (or equal to) 1.79 was interpreted as positive. The mPRNA was performed essentially as previously reported [8].
2.6. Detection of viral RNA by RT-PCR
Viral RNA was extracted from blood, pharyngeal swab and feces using the TriPure Isolation reagents (Roche). Two sets of primers were used in a one-step RT-PCR test [9]: nucleocapsid primers—forward primer 5′-ATAATACTGCGTCGTCTTGGTTC-3′ and reverse primer 5′-TGGCAATGTTGTTCCTTGAG-3′; BNI polymerase primers (developed at the Bernhard-Nocht Institute for Tropical Medicine, Hamburg, Germany, http://www.bni-hamburg.de/)—BNI OUT S25′-ATGAATTACCAAGTCAATGGTTAC-3′ (forward) and BNI OUTAS 5′-CATAACCAGTCGGTACAGCTAC-3′ (reverse). Samples from ferrets which had not received SARS-CoV were used as negative control. Virus collected from infected cell supernatant was used as positive control.
2.7. Blood chemistry
Ferret blood samples were analyzed for the levels of alkaline phosphatase, alanine aminotransferase, albumin, creatinine, total bilirubin, total protein and urea using VET/TEST® blood chemistry analyzer (IDEXX Laboratories Inc., USA) with the reagents supplied by the manufacturer.
2.8. Histopathology
Standard histopathological procedures were used to prepare the formalin fixed tissues for histopathological examination. Tissues were stained with hematoxylin and eosin.
3. Results and discussion
3.1. Expression of SARS-CoV S and N proteins
The expression of SARS-CoV S and N proteins was confirmed by Western blot with S specific monoclonal antibody or SARS patient serum (Fig. 1 ).
Fig. 1.

Expression of SARS-CoV N and S proteins by rMVA-N and S recombinant viruses determined by immunoblot. Panel A: detection of SARS-CoV nucleocapsid protein (with a human patient serum); panel B: detection of SARS-CoV spike protein (with a SARS-CoV spike specific mouse monoclonal antibody); lane 1: BHK21 cell control, lane 2: rMVA-N infected BHK21 cell lysate, lane 3: rMVA-S infected BHK21 cell lysate.
3.2. Immunization of ferrets with rMVA-S and rMVA-N
All ferrets were SARS-CoV negative based on the serological and RT-PCR tests performed before the experiment. Twelve ferrets were divided into four groups of three animals, immunized with PBS, parental MVA, rMVA-S or rMVA-N, respectively. As shown in Table 1A, antibody was detected in the three ferrets (#7, 8 and 9) vaccinated with the rMVA-S after the booster immunization by ELISA. Neutralizing activity was also detected in the sera collected 7 days after booster immunization with the rMVA-S virus while the titre declined to the undetectable level 14 days after the booster in the micro-neutralization assay (Table 1B). In comparison, no detectable antibody response was observed in ferrets that received the rMVA-N even following the booster immunization.
Table 1.
Antibody response and virus detection following rMVA immunization and SARS-CoV challenge
| Ferret numbers | Immunogens | Days post vaccination |
Days post challenge |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14a | 21 | 28 | 3–5 | 7–9 | 12–14 | 19–21 | 27–29 | ||
| Part A | |||||||||||
| 1 | MVA | NA | − | − | − | + | ++ | ++ | NA | +++ | ++++ |
| 2 | MVA | − | + | − | − | − | + | ++ | ++ | ++++ | ++ |
| 3 | MVA | − | − | − | − | + | − | + | +++ | ++++ | ++++ |
| 4 | rMVA-N | − | − | − | − | − | − | + | ++ | ++++ | ++ |
| 5 | rMVA-N | − | − | − | − | − | − | ++ | ++++ | +++ | ++ |
| 6 | rMVA-N | − | − | − | − | − | − | − | ++++ | ++++ | ++++ |
| 7 | rMVA-S | − | − | − | − | + | ++ | ++++ | ++++ | +++ | +++ |
| 8 | rMVA-S | − | + | + | + | − | − | +++ | +++ | ++++ | +++ |
| 9 | rMVA-S | − | + | + | ++ | ++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 10 | PBS | − | − | − | − | − | NA | + | ++ | ++++ | ++ |
| 11 | PBS | − | − | − | − | − | − | ++ | +++ | ++++ | +++ |
| 12 | PBS | − | − | − | − | − | + | + | +++ | +++ | ++++ |
| Part B | |||||||||||
| 1 | MVA | − | − | − | − | − | − | 320 | 320 | 160 | 320 |
| 2 | MVA | − | − | − | 20 | − | − | 160 | 160 | 640 | 640 |
| 3 | MVA | − | − | − | − | − | 40 | 160 | 320 | 640 | 640 |
| 4 | rMVA-N | − | − | − | − | − | − | 320 | 320 | 160 | 1280 |
| 5 | rMVA-N | − | − | − | − | − | − | 320 | 160 | 640 | 640 |
| 6 | rMVA-N | − | − | − | − | − | 40 | 80 | 1280 | 1280 | 1280 |
| 7 | rMVA-S | − | − | − | 40 | − | 20 | 1280 | 1280 | 640 | 640 |
| 8 | rMVA-S | − | 20 | − | 40 | − | 80 | 1280 | 640 | 640 | 1280 |
| 9 | rMVA-S | − | − | − | 20 | − | 640 | 2560 | 320 | 1280 | 1280 |
| 10 | PBS | - | − | − | − | − | − | 320 | 320 | 1280 | 1280 |
| 11 | PBS | − | − | − | − | − | − | 320 | 320 | 640 | 1280 |
| 12 | PBS | − | − | − | − | − | 20 | 80 | 320 | 1280 | 1280 |
| Ferret numbers |
Immunogen |
Blood* |
Pharyngeal* |
Feces* |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–3 | 4–6 | 8–10 | 13–15 | 20–22 | 27–29 | 1–3 | 4–6 | 8–10 | 13–15 | 20–22 | 27–29 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Part C | ||||||||||||||||||||
| 1 | MVA | − | − | + | − | + | − | + | − | + | − | − | − | − | − | − | + | + | + | − |
| 2 | MVA | − | − | − | − | + | − | + | + | + | − | − | − | + | + | + | + | + | + | − |
| 3 | MVA | − | − | − | + | − | − | + | + | − | − | − | − | − | − | − | + | − | − | − |
| 4 | rMVA-N | − | − | + | + | − | − | − | + | + | − | − | − | − | − | − | + | + | − | − |
| 5 | rMVA-N | − | − | + | + | + | − | + | + | − | + | + | − | − | − | − | + | + | − | − |
| 6 | rMVA-N | − | − | − | − | − | − | + | − | + | − | − | − | + | + | − | + | + | − | − |
| 7 | rMVA-S | − | − | + | + | − | − | + | + | + | − | − | − | + | − | − | + | + | − | − |
| 8 | rMVA-S | − | − | + | + | + | − | + | + | + | + | + | − | + | − | + | − | + | + | − |
| 9 | rMVA-S | − | − | − | + | − | − | + | + | + | − | − | − | − | + | − | + | + | − | − |
| 10 | PBS | − | − | + | + | − | − | + | + | − | − | − | − | − | − | − | + | + | − | − |
| 11 | PBS | − | − | − | + | + | − | + | + | + | + | − | − | + | + | + | + | + | − | − |
| 12 | PBS | − | − | − | − | − | − | + | + | + | − | − | − | + | − | + | + | − | − | − |
Part A: antibody response determined by ELISA; a, the day booster immunization given; the ELISA results were determined as P/N ratios (positive/negative ratios): ‘–‘=P/N < 1.79, ‘+’=P/N between 1.79 and 4.00, ‘++’=P/N between 4.00 and 8.00, ‘+++’=P/N between 8.00 and 12.00, ‘++++’= P/N > 12.00. Part B: neutralizing antibody responses determined by mPRNT; the lowest dilution used was 1/20; ‘–‘=negative. Part C: detection of viral RNA by RT-PCR from blood, pharyngeal swabs and feces; ‘–’=negative with both primer pairs (as described in Section 2); ‘+’=positive with one or both of the primer pairs; ‘*’, specimens collected days post challenge.
3.3. SARS-CoV challenge of immunized ferrets
Since it has been reported that ferrets were susceptible to SARS-CoV infection [5], we challenged the vaccinated and control animals with 106 pfu of the SARS-CoV Tor2 isolate by the intranasal route 2 weeks after the booster immunization. No clinical signs (elevated temperature, altered behaviour including feeding) were observed up to 29 days post challenge in any of the animals (data not shown). However, the viral RNA was detected in feces, pharyngeal swabs and blood samples by RT-PCR (Table 1C). The viral RNA could be detected in pharyngeal swabs and feces from all ferrets within the first 7 days of the challenge. Moreover, infectious virus could be isolated from selected pharyngeal swabs early in the infection (up to 5 days post infection, data not shown). The viral RNA declined in feces from all ferrets to undetectable level after 6 days post challenge. In contrast, most of the ferrets (10 out of 12) continued to shed virus in their pharyngeal secretion 10 days post challenge. In fact, two ferrets (#5 vaccinated with the rMVA-N and #8 vaccinated with rMVA-S) still shed virus in the pharyngeal excretion up to 22 days post challenge. Interestingly, no viral RNA was found in blood specimens until 8 days post challenge (except ferret #10, a PBS control for vaccination). However, on average the viral RNA persisted in blood longer than in pharyngeal excretion and feces (except ferret #12 in which no viral RNA was detected in blood through the whole course of the study). Our data indicate that SARS-CoV replicates in ferrets and that replication can last for up to 3 weeks. However, further studies are required to elucidate the kinetics of the virus replication in ferrets.
The antibody response after challenge with SARS-CoV was examined with ELISA and mPRNT tests. Most notably, a neutralizing antibody response was observed in the sera from ferrets vaccinated with the rMVA-S as early as 3 days after the SARS-CoV challenge while neutralizing antibody was only detected in other ferrets 7 days after SARS-CoV inoculation. This shows that rapid memory immune response occurred in the ferrets immunized with rMVA-S following SARS-CoV challenge. Furthermore, ferrets immunized with the rMVA-S developed peak neutralizing antibody titre between days 7 and 9 post challenge with SARS-CoV (Table 1C). In contrast, other challenged ferrets developed comparable levels of neutralizing antibodies between 19 and 21 days after the SARS-CoV challenge (Table 1B). The neutralizing antibody response corresponds with the serum IgG titre determined by ELISA (Table 1A). The rapid and vigorous neutralizing antibody response induced by immunization with the rMVA-S did not lead to the prevention of SARS-CoV dissemination as evidenced by the presence of virus in all the clinical specimens (Table 1C).
3.4. Blood chemistry and histopathology
Although no obvious clinical signs were observed following the challenge with SARS-CoV, further biochemical tests of blood samples and histological examination of various tissue sections were performed to investigate any pathological effects as consequences of rMVA vaccination and SARS-CoV challenge. Using the VetTest® dry chemistry analyzer (IDEXX Laboratories Inc., USA), blood samples taken at various time points were examined for the levels of alkaline phosphatase (an indicator of hepatic disease involving the biliary system), alanine aminotransferase (ALT, an indicator of hepatic parenchymal lesions), albumin (an indicator of abnormality of hepatic and renal function), creatinine (an indicator of renal disease), total bilirubin (an indicator of obstructive liver disease), total protein (indicator of abnormality of hepatic and renal function) and urea (an indicator of renal disease). Surprisingly, ferrets vaccinated with rMVA-N or rMVA-S demonstrated a significantly higher level of ALT after challenge with SARS-CoV than the control ferrets (Fig. 2 , panels A–E). The elevated level of ALT was evidenced by day 5 post SARS-CoV challenge and lasted until day 21. All the other parameters tested fell into the normal or slightly higher (alkaline phophatase) physiological range compared to the reference value (IDEXX Laboratories Inc., USA, data not shown).
Fig. 2.
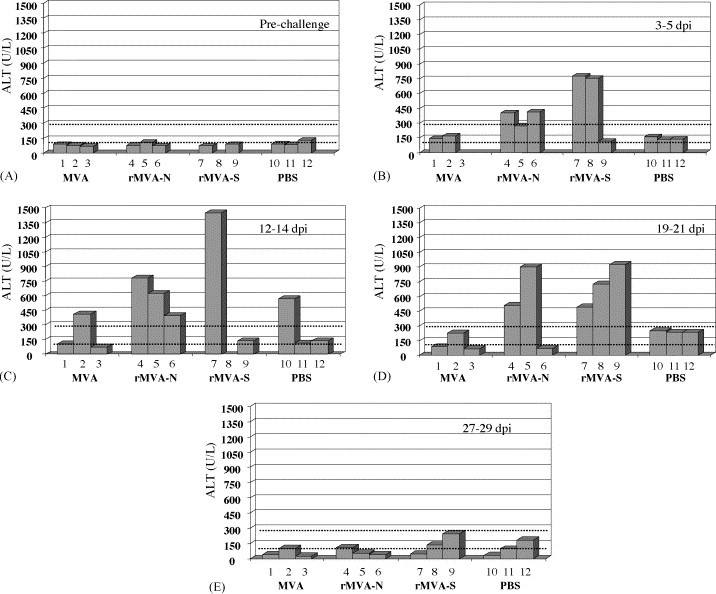
ALT level following rMVA immunization and SARS-CoV challenge. Panel A: pre-challenge (ferret #8 sample not available); panel B: 3–5 days post infection (dpi) with SARS-CoV (ferret #3 sample not available); panel C: 12–14 dpi (ferret #8 sample not available); panel D: 19–21 dpi; panel E: 27–29 dpi; the ALT value between the dotted scale line is considered as normal reference value.
Histopathological evaluation of liver tissues revealed that all the animals infected with SARS-CoV developed periportal and pan-lobular hepatitis. In correlation with the elevated ALT level, ferrets immunized with rMVA-S developed significantly more severe lesions including focal liver cell necrosis than all the other infected animals (Fig. 3 ). In particular, ferret #9, which developed the most rapid and vigorous antibody response, had the most severe hepatitis. In contrast, only mild hepatitis was observed in control animals receiving parental MVA or PBS. Although ferrets immunized with rMVA-N also demonstrated elevated level of ALT, only one ferret (#4) developed more severe hepatitis than control animals (severity between the rMVA-S immunized ferrets and the controls as observed post mortem). It should be mentioned that the tissue specimen for the pathological sectioning was collected post mortem (27–29 days after the challenge); by then the ALT level had already declined to (or slightly below) the normal range (Fig. 2, panel E). Therefore, it is likely that the liver inflammation shown in Fig. 3 may not truly reflect the severity of the hepatitis associated with rMVA-S or rMVA-N vaccination and SARS-CoV challenge. Detailed pathological examination at the time when the ALT level is at the highest should be performed in the future studies. In correlation with the data shown in Table 1C, no viral RNA was found in any tissue collected from the post mortem examination (28 and 29 days after the challenge). Other organs were only mildly affected by SARS-CoV infection (data not shown).
Fig. 3.
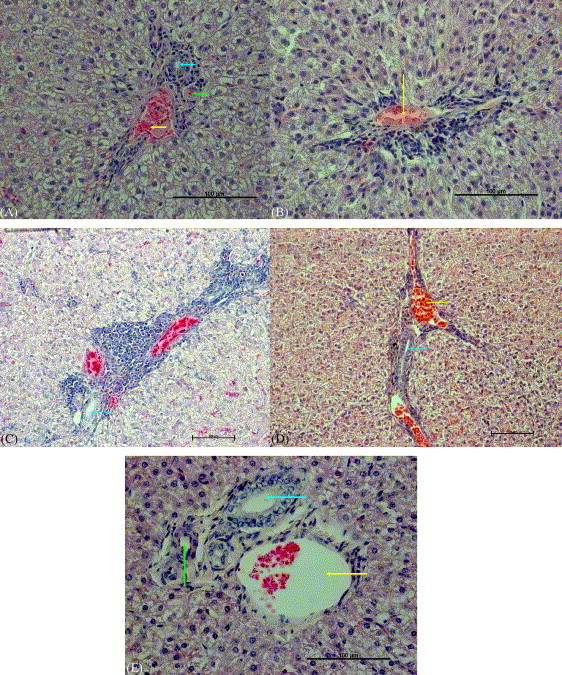
Representative pictures of livers from ferrets. Perivascular mononuclear infiltrates were present in all livers from ferrets exposed to SARS-CoV: (A) MVA, mild hepatitis; (B) rMVA-SARS-N; (C) rMVA-SARS-S, severe hepatitis with focal necrosis; (D) PBS control, mild hepatitis; (E) non-infected, no significant lesions. Arrows: vein (green); artery (yellow); and bile duct (green).
Antibody-dependent enhancement (ADE) of viral infectivity has been described for several viruses [10], [11]. It was well documented that neutralizing antibody induced by the spike protein of feline infectious peritonitis virus (FIPV, also a coronavirus) failed to protect cats from the virus challenge [12]. On the contrary, antibodies acquired either through a passive transfer of immune serum against the spike protein of FIPV [13] or by immunization with a recombinant vaccinia virus expressing the spike protein [14] often lead to accelerated infection by the mechanism of ADE of the virus infectivity. More recently, the enhanced susceptibility to FIPV has also been linked to the immune responses induced by the virus membrane and nucleocapsid protein co-delivered with interleukin-12 [15]. SARS-CoV has been shown to infect hepatocytes and cause hepatitis in humans [16]. Therefore, our observation that immunization with rMVA-S induced enhanced hepatitis in ferrets after SARS-CoV challenge is in line with the previous reports on ADE of FIPV infection.
A failure to observe detectable immune response induced by rMVA-N and an inconsistent link between the rMVA-N vaccination and enhanced liver inflammation may be due to the lack of optimization of the immunization regimen in this experiment. Further investigation to improve the immune responses by the use of different immunization regimen, more ferrets (which would allow a post mortem examination at various time points after vaccination and challenge), more detailed analysis of the immune responses and immunohistological studies should aid in understanding the link between the immune responses induced by SARS-CoV antigens and the enhanced liver inflammation. Moreover, to further confirm the observation of vaccination enhanced hepatitis in ferrets after challenge with SARS-CoV, other vaccination strategies, e.g. inactivated vaccine, recombinant adenovirus based vaccine, subunit vaccine, should be examined in the similar fashion as reported in this communication.
In conclusion, we would like to summarize our initial evaluation of rMVA based SARS vaccine as follows. First, rMVA-S can induce rapid and vigorous neutralizing antibody response in ferrets challenged with SARS-CoV; however, such neutralizing antibody did not prevent virus infection and spreading. Second, vaccination with SARS-CoV S and/or N protein may lead to enhanced pathology during SARS-CoV infection of liver and may cause damage of the liver. Third, although SARS-CoV does not cause clinical disease in ferrets, our results suggest that ferret may be a useful model for evaluating the safety of the vaccination strategy. Finally, we would like to suggest that extra caution must be taken in future human trials of SARS vaccines due to the potential organ damage resulting from immunizations.
Acknowledgements
This work was supported by Health Canada and Canadian Food Inspection Agency. We would like to thank Dr. Tim Booth, Dr. Michael Carpenter and Dr. Steven Jones for their critical review of the manuscripts. We thank the continuous support from Dr. Frank Plummer, Dr. Paul Kitching and Dr. Tim Booth. We also thank James Neufeld, Peter Marszal, Jason Gren, Greg Smith, Shane Jones, Roxanne Proulx, Yvonne Deschambault, Elsie Grudeski, Shelley Ganske, Lisa Manning, Anton Andonov, Runtao He, Yan Li, John Copps, Allen Grolla, Daryl Dick, Jody Berry, Dr. Peter Wright and Ms. Nicole Beausoleil for their help.
References
- 1.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S. The genome sequence of the SARS-associated coronavirus. Science. 2003;300(5624):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 2.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 3.Collins A.R., Knobler R.L., Powell H., Buchmeier M.J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming J.O., Stohlman S.A., Harmon R.C., Lai M.M., Frelinger J.A., Weiner L.P. Antigenic relationships of murine coronaviruses: analysis using monoclonal antibodies to JHM (MHV-4) virus. Virology. 1983;131(2):296–307. doi: 10.1016/0042-6822(83)90498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martina B.E., Haagmans B.L., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425(6961):915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti S., Sisler J.R., Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23(6):1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 7.Falkner F.G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weingartl H.M, Drebot M.A., Hubalek Z., Halouzka J., Andonova M., Dibernardo A. Comparison of assays for the detection of West Nile virus antibodies in chicken serum. Can J Vet Res. 2003;67(2):128–132. [PMC free article] [PubMed] [Google Scholar]
- 9.Weingartl H.M., Copps J., Drebot M.A., Marszal P., Smith G., Gren J. Susceptibility of pigs and chickens to SARS coronavirus. Emerg Infect Dis. 2004;10(2):179–184. doi: 10.3201/eid1002.030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tirado S.M., Yoon K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 11.Takada A., Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13(6):387–398. doi: 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- 12.Olsen C.W. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet Microbiol. 1993;36(1–2):1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss R.C., Scott F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis. 1981;4(2):175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vennema H., de Groot R.J., Harbour D.A., Dalderup M., Gruffydd-Jones T., Horzinek M.C. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J Virol. 1990;64(3):1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glansbeek H.L., Haagmans B.L., te Lintelo E.G., Egberink H.F., Duquesne V., Aubert A. Adverse effects of feline IL-12 during DNA vaccination against feline infectious peritonitis virus. J Gen Virol. 2002;83(Pt 1):1–10. doi: 10.1099/0022-1317-83-1-1. [DOI] [PubMed] [Google Scholar]
- 16.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]


