Abstract
The novel H7N9 avian influenza A virus has caused human infections in China since 2013; some isolates from the fifth wave of infections have emerged as highly pathogenic avian influenza viruses. Recombinant hemagglutinin proteins of H7N9 viruses can be rapidly and efficiently produced with low-level biocontainment facilities. In this study, recombinant H7 antigen was obtained from engineered stable clones of Chinese Hamster Ovary (CHO) cells for subsequent large-scale production. The stable CHO cell clones were also adapted to grow in serum-free suspension cultures. To improve the immunogenicity of the recombinant H7 antigens, we evaluated the use of a novel combination adjuvant of PELC and CpG (PELC/CpG) to augment the anti-H7N9 immune responses in mice. We compared the effects with other adjuvants such as alum, AddaVax (MF59-like), and several Toll-like receptor ligands such as R848, CpG, and poly (I:C). With the PELC/CpG combination adjuvant, CHO cell-expressed rH7 antigens containing terminally sialylated complex type N-glycans were able to induce high titers of neutralizing antibodies in sera and conferred protection following live virus challenges. These data indicate that the CHO cell-expressed recombinant H7 antigens and a PELC/CpG combination adjuvant can be used for H7N9 subunit vaccine development.
Keywords: CHO cells, Hemagglutinin, H7N9 vaccine, PELC/CpG adjuvant
1. Introduction
Influenza A viruses are negative-sense RNA viruses that belong to the Orthomyxoviridae family [1]. Hemagglutinin (HA) and neuraminidase (NA) are the two major viral envelope proteins that are generally used to classify different influenza A serotypes. There are 18 (H1–H18) and 11 (N1–N11) different types of HA and NA, respectively [2], [3]. The novel H7N9 avian influenza A virus isolated in 2013 China outbreaks [4], [5] has already caused five waves of human infections [6]. The fifth epidemic wave in 2016–2017 was the most severe with 759 confirmed human cases, probably due to the geographical spread of the virus from eastern China throughout the whole country [7]. More importantly, the H7N9 viruses isolated during the fifth epidemic wave have evolved into two distinct lineages, namely, the Yangtze River Delta and Pearl River Delta lineages [8], [9], [10]. Some of these fifth-epidemic-wave H7N9 isolates of Yangtze River Delta lineage carried polybasic amino acids at the HA1/HA2 cleavage site, a characteristic of highly pathogenic avian influenza viruses [11]. Recent evidence indicating that the A/Shanghai/2/2013 vaccine strain may not elicit the cross-reactivity of the immune response towards recent H7N9 isolates, the World Health Organization (WHO) has suggested two additional candidate vaccine strains: A/Guangdong/17SF003/2016 and A/Hong Kong/125/2017, besides for two previous vaccine strains: A/Shanghai/2/2013 and A/Anhui/1/2013 (https://www.who.int/influenza/vaccines/virus/candidates_reagents/summary_a_h7n9_cvv_20181108.pdf?ua = 1). The development of effective H7N9 vaccines is the best way to prevent further spread of the virus and curtail potential pandemic.
The inactivated or split H7N9 vaccines require various adjuvant systems, including alum [12], [13], [14], MF-59 or MF59-like adjuvant [13], [15], [16], [17], [18], [19], and AS03 adjuvant [17], [18], [20], to boost immunogenicity. Preparation of inactivated or split H7N9 vaccines is essentially carried out under biosafety level 2+ or 3 facilities for egg-based or cell culture-based virus propagation, inactivation, and purification processes. Recombinant H7N9 subunit vaccines can be rapidly and efficiently produced and require low-level biocontainment for vaccine manufacturing. Recombinant H7 (rH7) antigens have been successfully expressed in E. coli, insect cells and mammalian cells; however, these antigens need adjuvant formulations to boost anti-H7N9 immunity as reported for RIG-1 ligand [21], flagellin [22], polyethylenimine [22], poly (I:C) [23], alum [24], and squalene-based oil-in-water emulsion systems [25], [26]. As the antigenic structures of influenza HA proteins are complex and require extensive N-linked glycosylation to maintain their immunogenicity, one approach is to obtain engineered stable clones of Chinese Hamster Ovary (CHO) cells for the long-term expression of recombinant HA antigens in culture supernatants. Such a production method may allow semi-continuous or continuous harvesting of recombinant glycoproteins within an extended period time, thereby providing the advantage of the development of more cost-effective industrial processes [27], [28]. Using CHO cell technology, we have recently obtained the high-yield producer stable cell clones for production of glycan-masking Pan-H5 vaccines [29]. Similar strategies have been also reported for production of a homogeneous HIV-1 envelope SOSIP trimer vaccine [30] and a MERS-coronavirus vaccine antigen [31].
In this study, we expressed rH7 antigens from engineered stable clones of CHO cells through single cell cloning and standard dhfr-methotrexate (MTX) gene amplification and selection procedures, and screened and evaluated the immunogenicity of their formulations with different adjuvant systems in mice. The anti-H7N9 antibody responses were analyzed by determining the titers of IgG and IgG subtypes, hemagglutination inhibition (HI), and microneutralization (MN) in sera; protective immunity was also assessed following live virus challenges. Our findings indicate that CHO-rH7 formulated with PELC/CpG combination adjuvant can be used for H7N9 subunit vaccine development.
2. Materials and methods
2.1. Expression and purification of rH7 from stable clones of CHO cells
The coding sequence of rH7 protein carried the ectodomain of HA sequence (A/Shanghai/2/2013 (H7N9), a GCN4pII trimerization motif, and a 6x histidine tag. The gene was cloned into a pIS2ID expression vector (containing the pCMV promoter, IVS, internal ribosome entry site (IRES)-driven dihydrofolate reductase (DHFR), and pSV40-driven zeocin resistance gene) to obtain a high-yield stable clone of CHO cells, as previously described [32], [33], [34]. CHO/dhFr- (dhfr deficient) cells (ATCC CRL-9096, Bioresource Collection and Research Center in Taiwan) were maintained in Minimum Essential Medium Alpha medium (MEMα) with ribonucleosides and deoxyribonucleosides (Invitrogen), supplemented with 10% fetal bovine serum. To amplify dhfr/H7-expressing CHO cells, selection was performed in MEMα, supplemented with 10% dialyzed fetal bovine serum (Invitrogen) without RNS or dRNS. The pIS2ID expression vector was transfected to CHO/dhFr- cells using lipofectamine 2000 (Invitrogen) to establish permanent clones. At 24 h post-transfection, cells from 24-well plates were subcultured in 3 duplicate wells in 6-well plates. Medium in each well was replaced with MEMα without RNS and dRNS supplemented with 10% dialyzed fetal bovine serum (Invitrogen) and 200 μg/ml Zeocin (Invitrogen). Following 2 weeks of selection, remaining cells were diluted and inoculated into 96-well plates for single clone selection. After 2 weeks of incubation at 37 °C, single cell clones were selected by visual inspection under microscopy. Additional cell clone selection was performed by gradually increasing the concentration of MTX (Sigma), which was an inhibitor of DHFR protein, from 0.02 to 0.08 to 0.32 to 1.0 μM. Selection at each concentration occurred over a 2–3 week period. Cell clones that survived following treatment with 1 µM MTX were collected and analyzed by Western blotting using anti-rH7 antibodies (GeneTex Inc.) to confirm CHO-rH7 expression. The final selected rH7-expressing clones were cultured, and the culture supernatants were harvested for rH7 purification using nickel-chelated resin affinity chromatography (Tosoh), as previously described [29], [30], [31]. The purified rH7 proteins were treated with endoglycosidase H (Endo H) (New England BioLabs) or N-glycosidase F (PNGase F) (New England BioLabs) for Western blotting characterization. To obtain CHO-rH7 with high-mannose (mannose-terminated) type N-glycans, we also grew the same stable CHO cell clones in medium containing 100 μM kifunensine (KIF), a mannosidase inhibitor capable of blocking the high-mannose trimming process during complex type N-glycan biosynthesis, as the procedures we previously reported [34]. To obtain CHO-rH7 with single-N-acetylglucosamine (GlcNAc)-type N-glycans, the purified rH7 proteins obtained form KIF-treated CHO cell cultures were reacted with Endo H (New England Biolab) for 3 h at 37 °C, where Endo H was used to cleave high mannose and some hybrid oligosaccharides from N-linked glycoproteins within the chitobiose core [34].
2.2. Mouse immunizations
Female BALB/c mice (6–8 weeks old, 5 mice per group) were intramuscularly immunized twice over a 3-week interval. Groups of mice were immunized with phosphate-buffered saline (PBS); 0.2, or 2 μg of rH7 proteins without or with 300 μg of alum adjuvant (Adju-Phos, Brenntag); 10 μg of R848 (InvivoGen); 10 μg of CpG K3 oligodeoxynucleotides, 50% of AddaVax (InvivoGen), 10 μg of poly (I:C) (InvivoGen), or PELC/CpG (10% PELC with 10 μg CpG). All procedures involving animals were performed in accordance with the guidelines established by the Laboratory Animal Center of National Tsing Hua University (NTHU). Animal protocols were reviewed and approved by the NTHU Institutional Animal Care and Use Committee.
2.3. Determination of serum IgG and IgG subtype, HI, and MN titers
Plates were coated with 0.2 µg rH7 proteins overnight and blocked with PBS buffer containing 1% BSA. Serum samples were two-folds serially diluted and incubated for 1 h at room temperature. Plates were subsequently incubated with 100 µL of a HRP-conjugated anti-mouse IgG and IgG subtype antibody, followed by treatment with 100 µL of TMB substrate (BioLegend). The reaction was stopped with the addition of 2 N H2SO4. Optical density (450 nm) was measured with a TECAN spectrophotometer. For HI assay, serum samples were overnight treated with a receptor-destroying enzyme (Denka Seiken), two-fold,serially diluted, and incubated with four HA units of H7N9 virus (A/Taiwan/01/2013). Turkey RBCs (0.5%) were added to the treated serum samples, and HI titers were determined as the reciprocal of the highest dilution that could completely inhibit hemagglutination. For MN assay, serum samples were serially diluted two-fold, co-incubated with equal volumes of the H7N9 virus diluent (A/Taiwan/01/2013; 100 TCID50/well) for 1 h at 37 °C, and incubated with the prepared MDCK cells. Infectivity was determined as the detection of cytopathic effect on day 4. Neutralizing titers were defined as the reciprocals of the highest serum dilutions that could neutralizing 50% of H7N9 virus infectivity in the infected cells compared with that in the uninfected cells.
2.4. Virus challenges
Three weeks after final immunization, mice were anesthetized and intranasally challenged with 10 LD50 of the H7N9 virus (A/Taiwan/01/2013) in a volume of 50 μL. Mouse survival and weight loss were monitored daily over 14 days. As per Institutional Animal Care and Use Committee (IACUC) guidelines, body weight loss >25% served as the end-point. Mouse challenge experiments were evaluated and approved by the IACUC of Academia Sinica. Surviving mice from the immunization experiments were sacrificed using carbon dioxide, according to the ISCIII IACUC guidelines.
2.5. Adaptation of CHO cell clones to serum-free medium
The CHO cell clones were adapted from MEM-α medium (Gibco) supplemented with 10% fetal bovine serum (Biological Industries) to serum-free BalanCD CHO growth A medium (IrvineScientific) containing 2x GlutaMAX (Gibco) in T-75 flasks. CHO cell clones were adapted to serum-free medium by gradually decreasing the volume of serum-containing medium (100%, 50%, 20%, and 0%) and increasing the volume of serum-free medium (0%, 50%, 80%, and 100%). The cells were detached from T-75 flasks and suspended in serum-free medium. These cells were transferred to a 125 mL spinner flask and cultured at 60 rpm (working volume was 50 mL). The medium was replaced with fresh medium after every 3 days. The suspended cells were counted using a hemocytometer. The cell counts from days 1 to 9 of culture were determined for plotting the cell growth curve.
2.6. Determination of rH7 concentration in cell culture supernatant
The concentration of rH7 in serum-free cell culture supernatant was measured with an ELISA using an influenza A H7N9(A/Anhui/1/2013) hemagglutinin/HA ELISA pair set kit (Sino Biological) as described in the manufacturer’s protocol. In brief, the 96-well plates were coated with 100 µL of capture antibody (0.5 µg/mL in PBS) at 4 °C overnight. After three times washes with TBST (20 mM Tris, 150 mM NaCl, and 0.05% Tween20), the wells were blocked for 1 h with TBST containing 2% BSA. A total of 100 µL of culture supernatant samples collected on days 3 to 9 were added to individual wells, and incubated for 1 h. After three washes, 100 µL of an HRP-conjugated detection antibody (0.5 µg/ml in TBST with 0.5% BSA) was added, and the plates were incubated for 1 h. After three washes, TMB substrate was added to each well to develop the reaction, and 2 N H2SO4 was used to eventually stop the reaction. The absorbance at 450 nm wavelength was recorded and fitted to the standard curve of H7 proteins to measure the protein concentration.
2.7. Statistical analyses
All results were analyzed with one-way ANOVA and Tukey’s test (GraphPad Prism v5.03). A value of p < 0.05 indicated statistical significance. All experiments were performed at least twice each.
3. Results
3.1. Expression, purification, and characterization of rH7 protein in stable CHO cell clones
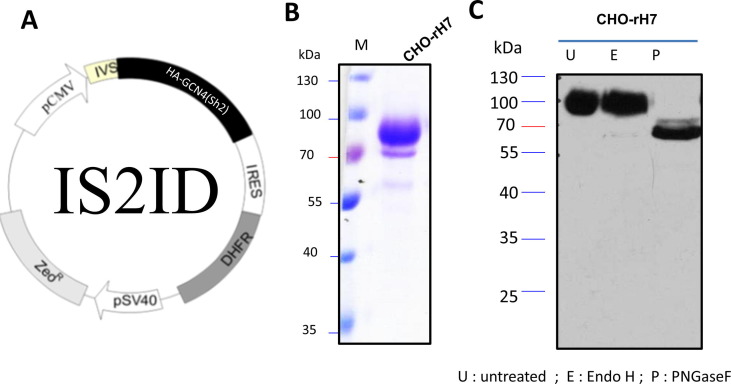
The gene encoding HA of A/Shanghai/2/2013 (H7N9) virus strain, with an additional GCN4-pII sequence and a 6 × histidine tag, was cloned into a pIS2ID expression vector containing a pCMV promoter, IVS, IRES-driven DHFR, and pSV40-controlled zeocin-resistant gene (Fig. 1 A). DHFR-deficient CHO cells (CHO/dhFr-) were transfected with pIS2ID plasmids and the positive transformants were selected with zeocin. Single cell cloning for DHFR/MTX-stepwise selection was performed to obtain CHO-rH7 stable clones. After the stepwise increase of MTX selection procedures, the survived clones at 1 µM MTX were maintained at least more than five passages to ensure their stabilities for rH7 expression. The rH7 proteins were purified from cell culture supernatants with nickel affinity chromatography. The results of SDS-PAGE and Coomassie Brilliant Blue staining showed that the molecular mass of CHO-rH7 was ∼70 kDa (Fig. 1B). The N-glycan pattern of rH7 protein was predominantly complex type, owing to its resistance to Endo H, but not PNGase, as evidence from Western blotting results (Fig. 1C). Additionally, we have already reported CHO-rH7 N-glycan profiling using hydrophilic interaction liquid chromatography- high-performance liquid chromatography [34]. The major glycoforms of CHO-rH7 contained tri- and tetra-antennary structures with one or more terminal sialic acids for 36.6% Sia1-3Gal3Man3GlcNAc5Fuc and 27.0% Sia1-4Gal4Man3GlcNAc6Fuc [34].
Fig. 1.
Expression and characterization of rH7 in stable clones of CHO cells. (A) CHO cell vector for rH7 expression; (B) purified rH7 protein analysis on SDS-PAGE gels with Coomassie blue staining; (C) Western blot analysis of purified rH7 protein after Endo H and PNGase F treatments.
3.2. Serum titers of total IgG and IgG subtype and induction of HI and MN antibodies upon immunizations with rH7 formulated with various types of adjuvants
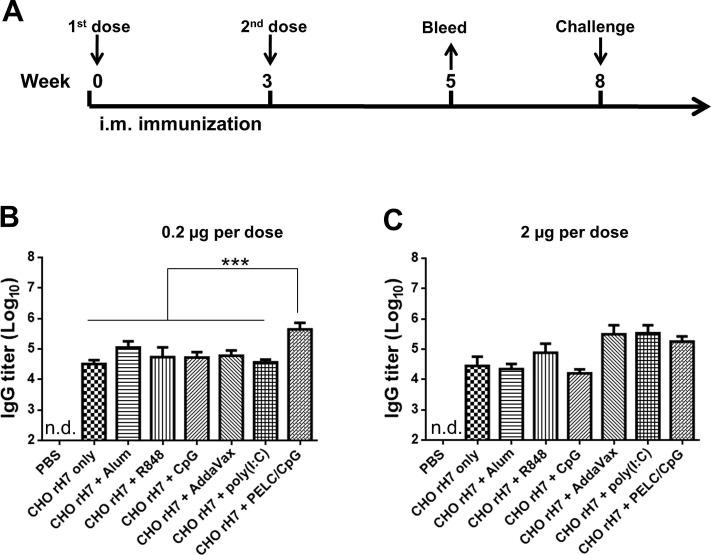
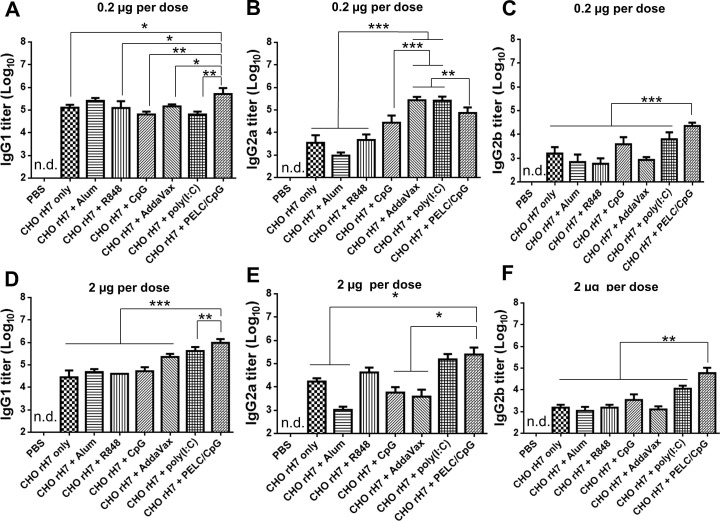
Groups of BALB/c mice were intramuscularly immunized with 0.2 µg or 2 µg of CHO-rH7 formulated with alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvants within a 3-week period (Fig. 2 A). Antisera were collected 2 weeks after the second dose of immunization. At 0.2 µg dose of CHO-rH7, the total IgG antibody titer was significantly higher with PELC/CpG adjuvant than with other adjuvants (Fig. 2B). Furthermore, at 2 µg dose of CHO-rH7, AddaVax, poly (I:C), and PELC/CpG adjuvants showed slightly higher IgG titers than the titer observed with R848, but the difference was not statistically significant (Fig. 2C). Therefore, the formulation containing CHO-rH7 in combination with PELC/CpG triggered the strongest IgG response. The titer of IgG1 subtype induced by PELC/CpG adjuvant was significantly higher than that induced by alum, R848, CpG, AddaVax, and poly (I:C) adjuvants at 0.2 and 2 µg doses of CHO-rH7 (Fig. 3 A, D). Furthermore. PELC/CpG and poly (I:C) adjuvants yielded higher titers of IgG2a than alum, R848, and CpG adjuvants (Fig. 3B, E). Our results also showed that 0.2 and 2 µg doses of CHO-rH7 formulated with PELC/CpG adjuvant had significantly higher IgG2b titers than those induced by alum, R848, CpG, AddaVax, and poly (I:C) adjuvants (Fig. 3C, F). Since, Th1 and Th2 are known to drive the induction of IgG1 and IgG2a secretion, adjuvanting with PELC/CpG appears to enhance both Th1 and Th2 cellular responses. Use of PELC/CpG adjuvant also resulted in higher IgG2a and IgG2b titers that are more capable of activating Fc effector functions such as antibody-dependent cellular cytotoxicity.
Fig. 2.
Total IgG titers elicited by intramuscular immunization with rH7 formulated with PELC/CpG combination adjuvant. (A) Groups of BALB/c mice were intramuscularly immunized with CHO-rH7 formulated with alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvant for two doses within a 3-week interval. Total IgG titers in sera were determined by ELISA for (B) 0.2 µg and (C) 2 µg CHO-rH7 antigen without or with alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvant. * indicates P < 0.05; ** indicates P < 0.01, ***, indicates P < 0.001.
Fig. 3.
IgG1, IgG2a, and IgG2b subtypes in sera. IgG1 titers in antisera from mice immunized with the formulation of alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvant with (A) 0.2 µg and (D) 2 µg dose of CHO-rH7 antigen. The IgG2a titers in antisera from mice immunized with the formulation of alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvant with (B) 0.2 µg and (E) 2 µg dose of CHO-rH7 antigen. The IgG2b titers in antisera from mice immunized with the formulation of alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvant with (C) 0.2 µg and (F) 2 µg dose of CHO-rH7 antigen. * indicates P < 0.05; ** indicates P < 0.01, ***, indicates P < 0.001.
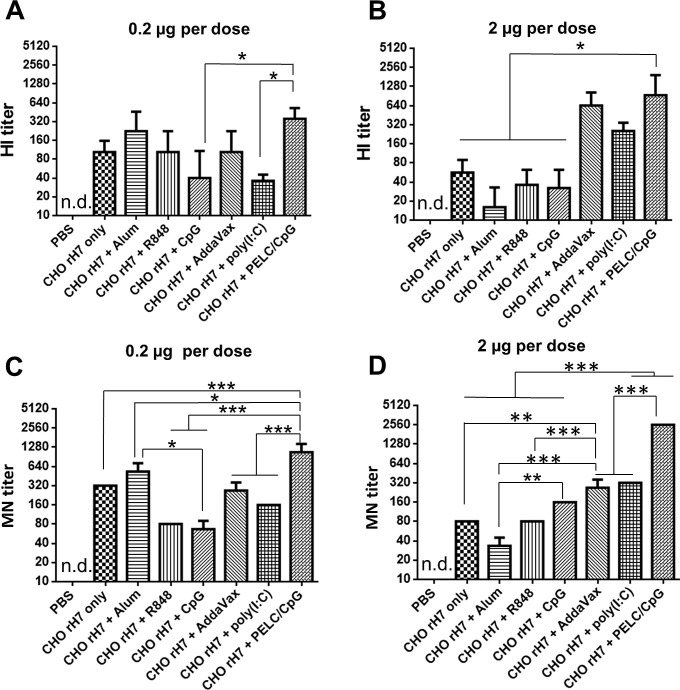
Antisera were also analyzed for the titers of anti-H7N9 HI and MN antibodies. At 0.2 µg CHO-rH7, the use of alum and PELC/CpG adjuvants had higher HI titers compared to CHO-rH7 without adjuvant use or with the use of R848, CpG, AddaVax, and poly (I:C) adjuvants (Fig. 4 A). At 2 µg CHO-rH7, the HI titer was significantly higher with PELC/CpG than with alum, R848, and CpG adjuvants (Fig. 4B). We further evaluated the titer of MN in the antisera and found that the highest titer was reported in the presence of PELC/CpG adjuvant than with other adjuvants at CHO-rH7 doses of 0.2 and 2 µg (Fig. 4C, D). Therefore, the use of PELC/CpG adjuvant clearly improved the titers of HI and MN antibodies than the alum adjuvant for CHO-rH7 immunizations in mice.
Fig. 4.
HI and MN antibody titers in sera. HI titers in antisera from mice immunized with the formulation of alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvant with (A) 0.2 µg and (B) 2 µg dose of CHO-rH7 antigen. The MN titers in antisera from mice immunized with the formulation of alum, R848, CpG, AddaVax, poly (I:C), or PELC/CpG adjuvant with (C) 0.2 µg and (D) 2 µg dose of CHO-rH7 antigen. * indicates P < 0.05; ** indicates P < 0.01, ***, indicates P < 0.001.
3.3. Protection after live virus challenge
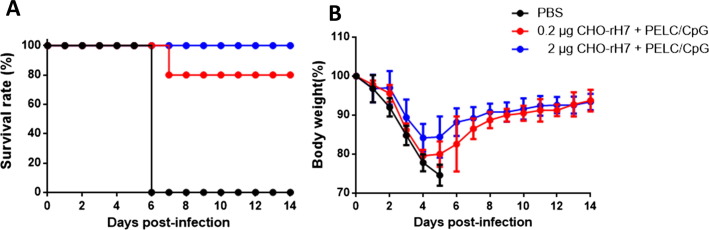
To analyze the protective immunity, the groups of mice immunized with 0.2 µg and 2 µg of CHO-rH7 in combination with PELC/CpG and those treated with PBS-control group were challenged with 10 LD50 of H7N9 virus (A/Taiwan/01/2013). The survival rates slightly decreased from 100% to 80% as the dose of CHO-rH7 was lowered from 2 to 0.2 µg with PELC/CpG as the adjuvant (Fig. 5 A). The body weight loss also increased as the CHO-rH7 dose was reduced from 2 to 0.2 µg with PELC/CpG adjuvant; however, all formulations used mediated complete recovery (Fig. 5B). This observation indicates that immunization with 2 µg and 0.2 µg of CHO-rH7 in combination with PELC/CpG adjuvant provided protection in 100% and 80% survival of mice against live virus challenge, respectively.
Fig. 5.
Protective immunity against the H7N9 virus. Three weeks after second dose immunization, mice were anesthetized and subjected to intranasal challenge with 10 LD50 of H7N9 virus (A/Taiwan/01/2013) in a volume of 50 μL. (A) Survival rate and (B) body weight loss were monitored for 14 days. Mice whose body weights fell below 75% of their initial weights were sacrificed.
3.4. Immunogenicity of CHO-rH7 with different N-glycan patterns
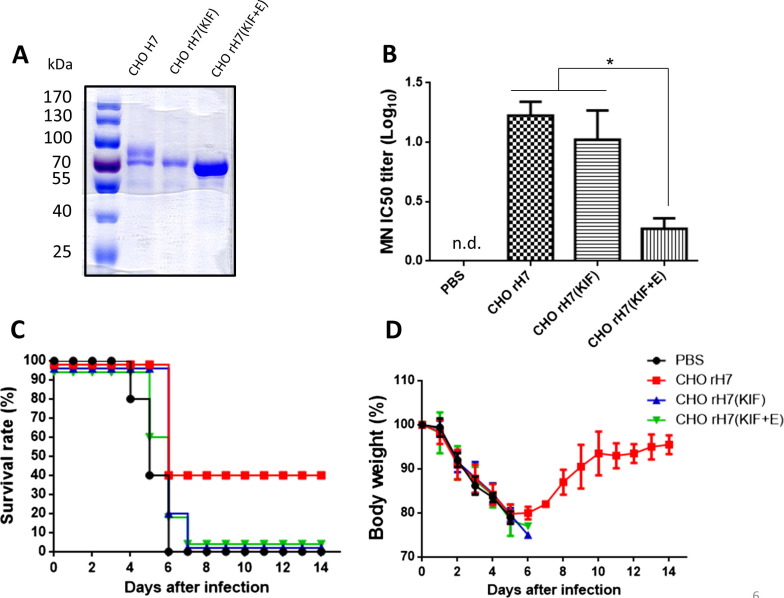
Since the CHO cell-expressed recombinant H7 antigens contained most terminally sialylated complex type N-glycans, we can also obtain other two types of rH7 with different N-glycan patterns: (i) CHO-rH7 (KIF) for rH7 with high mannose-type (mannose-terminated) N-glycans using KIF treatments, and (ii) CHO-rH7 (KIF + E) for rH7 with single GlcNAc-type N-glycans using KIF treatment and Endo H digestion as we previously reported [34]. We observed the gradually reduced molecular weights from CHO-rH7 to CHO-rH7 (KIF) and CHO-rH7 (KIF + E) in SDS-PAGE gels staining with Commassie blue (Fig. 6 A). Groups of BALB/c mice were immunized with 0.2 µg of CHO-rH7, CHO-rH7 (KIF), and CHO-rH7 (KIF + E) with PELC/CpG adjuvant following the same immunization and virus challenge regimens. Results indicated that reduced MN titers (ID50 dose) by CHO-rH7 (KIF), and to a larger degree by CHO-rH7 (KIF + E), as compared by CHO-rH7 immunization (Fig. 6B). Following live virus challenges, only the immunization with CHO-rH7 plus PELC/CpG,resulted in protection with a 40% survival rate and the full body weight recovery (Fig. 6C and D). No protection was observed for the immunization with either 0.2 µg CHO-rH7 (KIF) or 0.2 µg CHO-rH7 (KIF + E) formulated in PELC/CpG adjuvants (Fig. 6C and D). Therefore, only rH7 with terminally sialylated complex type N-glycans, can provide protection against live virus challenge.
Fig. 6.
Immunogenicity of CHO-rH7 with different N-glycan patterns. (A) purified CHO-rH7, CHO-rH7 (KIF), and CHO-rH7 (KIF + E) protein analysis on SDS-PAGE gels with Coomassie blue staining; (B) MN titers (ID-50) in antisera from mice immunized with 0.2 µg CHO-rH7, CHO-rH7 (KIF), or CHO-rH7 (KIF + E) antigen plus PELC/CpG adjuvant; (C) Survival rate and (D) body weight loss from immunized mice following live virus challenge were monitored for 14 days. Mice whose body weights fell below 75% of their initial weights were sacrificed.
3.5. Adaptation of stable CHO cell clone in serum-free suspension cultures
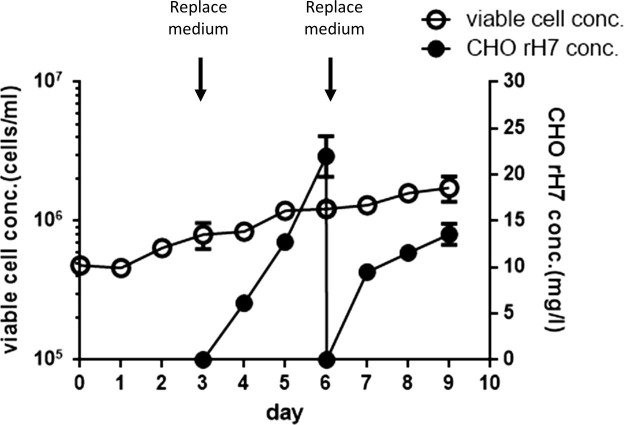
To meet the requirement of industrial application, the rH7-expressing stable CHO cell clones were adapted to serum-free conditions by gradually decreasing the FBS content of the medium from 10% to 5% and eventually to 2% and 0%. The adapted CHO cell clones were cultured as a single cell suspension in a serum-free medium in spinner flasks and the cell growth and rH7 production were monitored for 9 days. The culture medium was completely replaced with fresh medium at day 3 and 6 to maintain the cell viability around 90% (Fig. 7 ). As a result, the cell density increased from 4.8 × 105 cells/mL at day 1 to 1.7 × 106 cells/mL at day 9 (Fig. 7). The cell viability for the adapted cell clones was all above 90% during the 9-day serum-free suspension cultures; however, the cell growth was slow with a doubling time around 3 days. The titers of rH7 production in culture supernatants increased to 20 mg/L from day 3 to 6, and to 15 mg/L from day 6 to day 9 (Fig. 7). The final yield of rH7 production was ∼8.2 mg/L, approximately double the amount for adherent cells from serum-containing cultures. These results indicated that the rH7-producing CHO cell clone was adapted to grow in serum-free suspension cell cultures.
Fig. 7.
Stable CHO cell clone in serum-free suspension cultures. Cell density and rH7 production titer of the adapted stable CHO cell clones cultured in serum-free suspension cultures. The culture medium was completely replaced with fresh medium at day 3 and 6 to maintain the cell viability around 90%.
4. Discussion
Here, we reported the success to obtain engineered stable clones of CHO cells for the production of rH7 antigens, which may be used for the development of H7N9 recombinant HA vaccines. The stable clones have been successfully adapted to grow well in serum-free suspension cultures; this property may allow large-scale production of these clones. The yield of rH7 produced from the engineered stable cell clones was approximately twice the amount obtained for serum-containing adherent cultures. Further improvements in the CHO cell culture processes via medium optimizations and fed-batch cultivation with extended culture period may increase the yield of antigen production from milligram to gram per liter as reported for HIV-1 envelope antigen production from stable CHO cells [35].
We evaluated the ability of the PELC/CpG combination adjuvant to augment rH7 immunogenicity in mice. The non-pathogen-associated molecular pattern (non-PAMP) adjuvants such as alum and AddaVax (MF59-like), or the PAMP adjuvants like Toll-like receptor (TLRs) ligands for R848, CpG, and poly (I:C) were used as adjuvants for parallel comparison. PELC and MF59-like AddaVax adjuvants are formulated using squalene-based oil-in-water emulsions that are similar in composition to MF59 adjuvant (4.3% squalene, 0.5% Span85, and 0.5% Tween 80 in citrate buffer) [36]. PELC formulation had only one different composition, obtained by replacing Tween 80 with a biodegradable poly(ethylene glycol)-block-poly(lactide-co-ε-caprolactone) (PEG-b-PLACL) polymer, as compared with MF59-like AddaVax adjuvant [37]. The effects of these adjuvants on the augmentation of rH7 immunogenicity were compared with alum, an adjuvant that is known to cause cell death and release numerous molecules that act as damage-associated molecular patterns (DAMPs) to alert the innate immune systems [38]. Alum can also activate NLRP3 inflammasomes for the induction of effective B-cell responses and Th2 biased cellular immune response [39]. MF59 or AddaVax (MF-59 like) is a more potent adjuvant that elicits a broader immune responses than alum adjuvant [40]. Our studies show that the combination PELC/CpG adjuvant elicited significantly higher titers of anti-H7 IgG, IgG1, gG2a antibodies, and HI- and MN-neutralizing antibodies in mice than alum, AddaVax (MF59-like), and several TLR ligands investigated in this study. These results are in concordance with those reported in studies for an inactivated H5N1 vaccine with the PELC/CpG combination adjuvant [37], or a trivalent influenza vaccine with the combination of alum, calcium phosphate, MF59, or poly-(lactide co-glycolide) with CpG adjuvant [41], to act synergistically for the elicitation of anti-influenza immunity.
The antigenic properties of influenza HA include binding to RBCs for agglutination as well as to the sialic acids present in bovine fetuin glycoproteins. We have previously reported that CHO-rH7 antigens lost the ability to agglutinate turkey RBCs as well as to bind to sialic acids-containing bovine fetuin glycproteins, whereas Sf9 insect cell-derived rH7 (Sf9-rH7) antigens retained both these properties [26]. Although CHO-rH7 antigens were unable to agglutinate RBCs or bind sialic acids-containing fetuin, the data established in the present study clearly demonstrated that CHO-rH7 antigens were immunogenic for inducing anti-H7N9 immune responses in mice. We also obtained two other N-glycan types of CHO-rH7 antigen: (i) CHO-rH7 (KIF) with high mannose-type N-glycans and (ii) CHO-rH7 (KIF + E) with single GlcNAc-type N-glycans. Only the CHO-rH7 (KIF + E) antigen, but not the CHO-rH7 (KIF) antigen, was able to regain a weak property for RBC agglutination (data not shown). With PELC/CpG combination adjuvant, a 0.2 µg dose of CHO-rH7 but not CHO-rH7 (KIF) and CHO-rH7 (KIF + E) elicited a protective response and conferred protection in survival and body weight recovery of the immunized mice following live virus challenges. The results are in contradictory to HIV-1 gp120 antigen produced in CHO cells where limiting N-linked glycosylation to early oligomannose glycans can enhance the HIV-1 subunit vaccine efficacy [42]. Our present results demonstrated that CHO cell-expressed rH7 antigens containing terminally sialylated complex type N-glycans, but not high mannose-type or single GlcNAc-type N-glycans, can provide protection against H7N9 infections. Furthermore, we have previously reported that CHO-rH5 antigens for the removal of terminal sialic acid moieties can improve the immunity, when compared with the N-linked complex type CHO-rH5 antigens or the paucimannose-type Sf9/Mimic cell-derived rH5 antigens [33]. Ongoing studies investigating CHO-rH7 antigens after the removal of sialic acid moieties may further improve their anti-H7N9 immunity.
In conclusion, the present study demonstrated a new method for the production of CHO-rH7 vaccines using engineered stable clones of CHO cells. These clones expressed antigens and were adapted to grow in serum-free suspension cultures, which may be useful for the large-scale industrial production of H7N9 subunit vaccines. With the PELC/CpG combination adjuvant, CHO cell-expressed rH7 antigens containing terminally sialylated complex type N-glycans were able to induce high titers of neutralizing antibodies in sera and conferred protection following live virus challenges. These data indicate that CHO-rH7 antigens coupled with PELC/CpG combination adjuvant formulation may be useful for the development of an effective H7N9 subunit vaccine
Conflict of interest
The authors have declared that no competing interests exist.
Acknowledgements
The authors wish to thank Ken J. Ishii of the National Institute of Biomedical Innovation and Osaka University, Japan for providing us with the CpG K3 ODN used in this research. This work was supported by the Ministry of Science and Technology, Taiwan(MOST107-2321-B-007-003, MOST107-2321-B-002-011).
REFERENCES
- 1.Horimoto T., Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 2.Tong S., Li Y., Rivailler P., Conrardy C., Castillo D.A., Chen L.M. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci USA. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu X., Yu W., McBride R., Li Y., Chen L.M., Donis R.O. Hemagglutinin homologue from H17N10 bat influenza virus exhibits divergent receptor-binding and pH-dependent fusion activities. Proc Natl Acad Sci USA. 2013;110:1458–1463. doi: 10.1073/pnas.1218509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 5.Huo X., Chen L., Qi X., Huang H., Dai Q., Yu H. Significantly elevated number of human infections with H7N9 virus in Jiangsu in eastern China, October 2016 to January 2017. Euro Surveill. 2017;22(13) doi: 10.2807/1560-7917.ES.2017.22.13.30496. pii: 30496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Yang L., Zhu W., Zhang Y., Zou S., Bo H. Two outbreak sources of influenza A (H7N9) viruses have been established in China. J Virol. 2016;90:5561–5573. doi: 10.1128/JVI.03173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kile J.C., Ren R., Liu L., Greene C.M., Roguski K., Iuliano A.D. Update: Increase in Human Infections with Novel Asian Lineage Avian Influenza A(H7N9) Viruses During the Fifth Epidemic—China, October 1, 2016-August 7, 2017. Morb Mortal Wkly Rep. 2017;66:928–932. doi: 10.15585/mmwr.mm6635a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L., Ren R., Yang L., Bao C., Wu J., Wang D. Sudden increase in human infection with avian influenza A(H7N9) virus in China, September-December 2016. Western Pac Surveill Response J. 2017;8:6–14. doi: 10.5365/WPSAR.2017.8.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su S., Gu M., Liu D., Cui J., Gao G.F., Zhou J. Epidemiology, evolution, and pathogenesis of H7N9 influenza viruses in five epidemic waves since 2013 in China. Trends Microbiol. 2017;5:713–728. doi: 10.1016/j.tim.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Quan C., Shi W., Yang Y., Yang Y., Liu X., Xu W. New threats from H7N9 influenza virus: spread and evolution of high- and low-pathogenicity variants with high genomic diversity in wave five. J. Virol. 2018;92:e00301–e318. doi: 10.1128/JVI.00301-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iuliano A.D., Jang Y., Jones J., Davis C.T., Wentworth D.E., Uyeki T.M. Increase in human infections with avian influenza A(H7N9) virus during the fifth epidemic - China, October 2016-February 2017. MMWR Morb Mortal Wkly Rep. 2017;66:254–255. doi: 10.15585/mmwr.mm6609e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan W., Han L., Dong Z., Niu X., Li Z., Bao L. Induction of neutralizing antibodies to influenza A virus H7N9 by inactivated whole virus in mice and nonhuman primates. Antiviral Res. 2014;107:1–5. doi: 10.1016/j.antiviral.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Wu C.Y., Chang C.Y., Ma H.H., Wang C.W., Chen Y.T., Hsiao P.W. Squalene-adjuvanted H7N9 virus vaccine induces robust humoral immune response against H7N9and H7N7 viruses. Vaccine. 2014;32(35):4485–4494. doi: 10.1016/j.vaccine.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Wu U.I., Hsieh S.M., Lee W.S., Wang N.C., Kung H.C., Ou T.Y. Safety and immunogenicity of an inactivated cell culture-derived H7N9 influenza vaccine in healthy adults: A phase I/II, prospective, randomized, open-label trial. Vaccine. 2017;35(33):4099–4104. doi: 10.1016/j.vaccine.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Bart S.A., Hohenboken M., Della Cioppa G., Narasimhan V., Dormitzer P.R. Kanesa-Thasan N. A cell culture-derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Sci Transl Med. 2014;6(234):234ra55. doi: 10.1126/scitranslmed.3008761. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan M.J., Bernstein D.I., Winokur P., Rupp R., Anderson E., Rouphael N., DMID 13–0032 H7N9 Vaccine Study Group Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA. 2014;312(14):1409–1419. doi: 10.1001/jama.2014.12854. [DOI] [PubMed] [Google Scholar]
- 17.Wong S.S., Kaplan B., Zanin M., Debeauchamp J., Kercher L., Crumpton J.C. Impact of adjuvants on the immunogenicity and efficacy of split-virion H7N9 vaccine in ferrets. J Infect Dis. 2015;212(4):542–551. doi: 10.1093/infdis/jiv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson L.A., Campbell J.D., Frey S.E., Edwards K.M., Keitel W.A., Kotloff K.L. Effect of varying doses of a monovalent H7N9 influenza vaccine with and without AS03 and MF59 adjuvants on immune response: a randomized clinical trial. JAMA. 2015;314(3):237–246. doi: 10.1001/jama.2015.7916. [DOI] [PubMed] [Google Scholar]
- 19.Ou H., Yao H., Yao W., Wu N., Wu X., Han C. Analysis of the immunogenicity and bioactivities of a split influenza A/H7N9 vaccine mixed with MF59 adjuvant in BALB/c mice. Vaccine. 2016;34(20):2362–2370. doi: 10.1016/j.vaccine.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Madan A., Segall N., Ferguson M., Frenette L., Kroll R., Friel D. Immunogenicity and safety of an AS03-adjuvanted H7N9 pandemic influenza vaccine in a randomized trial in healthy adults. J Infect Dis. 2016;214(11):1717–1727. doi: 10.1093/infdis/jiw414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao W., Liepkalns J.S., Kamal R.P., Reber A.J., Kim J.H., Hofstetter A.R. RIG-I ligand enhances the immunogenicity of recombinant H7HA protein. Cell Immunol. 2016;304–305:55–58. doi: 10.1016/j.cellimm.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L., Xiong D., Hu M., Kang X., Pan Z., Jiao X. Immunopotentiation of different adjuvants on humoral and cellular immune responses induced by HA1-2 subunit vaccines of H7N9 influenza in mice. PLoS ONE. 2016;11:e0150678. doi: 10.1371/journal.pone.0150678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krammer F., Albrecht R.A., Tan G.S., Margine I., Hai R., Schmolke M. Divergent H7 immunogens offer protection from H7N9 virus challenge. J Virol. 2014;88(8):3976–3985. doi: 10.1128/JVI.03095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L., Chang T.Z., He Y., Kim J.R., Wang S., Mohan T. Coated protein nanoclusters from influenza H7N9 HA are highly immunogenic and induce robust protective immunity. Nanomedicine. 2017;13(1):253–262. doi: 10.1016/j.nano.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khurana S., Coyle E.M., Verma S., King L.R., Manischewitz J., Crevar C.J. H5 N-terminal beta sheet promotes oligomerization of H7-HA1 that induces better antibody affinity maturation and enhanced protection against H7N7 and H7N9 viruses compared to inactivated influenza vaccine. Vaccine. 2014;32:6421–6432. doi: 10.1016/j.vaccine.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 26.Chen T.H., Liu Y.Y., Jan J.T., Huang M.H., Spearman M., Butler M. Recombinant hemagglutinin proteins formulated in a novel PELC/CpG adjuvant for H7N9 subunit vaccine development. Antiviral Res. 2017;146:213–220. doi: 10.1016/j.antiviral.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Matthews C.B., Wright C., Kuo A., Colant N., Westoby M., Love J.C. Reexamining opportunities for therapeutic protein production in eukaryotic microorganisms. Biotechnol Bioeng. 2017;114(11):2432–2444. doi: 10.1002/bit.26378. [DOI] [PubMed] [Google Scholar]
- 28.Romanova N., Noll T. Engineered and natural promoters and chromatin-modifying elements for recombinant protein expression in CHO cells. Biotechnol J. 2018;13(3):e1700232. doi: 10.1002/biot.201700232. [DOI] [PubMed] [Google Scholar]
- 29.Chen T.H., Liu W.C., Lin C.Y., Liu C.C., Jan J.T., Spearman M. Glycan-masking hemagglutinin antigens from stable CHO cell clones for H5N1 avian influenza vaccine development. Biotechnol Bioeng. 2018 doi: 10.1002/bit.26810. [DOI] [PubMed] [Google Scholar]
- 30.Bale S., Martiné A., Wilson R., Behrens A.J., Le Fourn V., de Val N. Cleavage-independent HIV-1 trimers from CHO cell lines elicit robust autologous tier 2 neutralizing antibodies. Front Immunol. 2018;9:1116. doi: 10.3389/fimmu.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyon M.P., Du L., Tseng C.K., Seid C.A., Pollet J., Naceanceno K.S. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine. 2018;36(14):1853–1862. doi: 10.1016/j.vaccine.2018.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin S.C., Leng C.H., Wu S.C. Generating stable Chinese hamster ovary cell clones to produce a truncated SARS-CoV spike protein for vaccine development. Biotechnol Prog. 2010;26(6):1733–1740. doi: 10.1002/btpr.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S.C., Jan J.T., Dionne B., Butler M., Huang M.H., Wu C.Y. Different immunity elicited by recombinant H5N1 hemagglutinin proteins containing pauci-mannose, high-mannose, or complex type N-glycans. PLoS ONE. 2013;8(6):e66719. doi: 10.1371/journal.pone.0066719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W.C., Lin Y.L., Spearman M., Cheng P.Y., Butler M., Wu S.C. Influenza virus hemagglutinin glycoproteins with different N-glycan patterns activate dendritic cells in vitro. J Virol. 2016;90(13):6085–6096. doi: 10.1128/JVI.00452-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Rourke S.M., Byrne G., Tatsuno G., Wright M., Yu B., Mesa K.A. Robotic selection for the rapid development of stable CHO cell lines for HIV vaccine production. PLoS ONE. 2018;13(8):e0197656. doi: 10.1371/journal.pone.0197656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calabro S., Tritto E., Pezzotti A., Taccone M., Muzzi A., Bertholet S. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine. 2013;31:3363–3369. doi: 10.1016/j.vaccine.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Huang M.H., Lin S.C., Hsiao C.H., Chao H.J., Yang H.R., Liao C.C. Emulsified nanoparticles containing inactivated influenza virus and CpG oligodeoxynucleotides critically influences the host immune responses in mice. PLoS ONE. 2010;5:e12279. doi: 10.1371/journal.pone.0012279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maricha I.T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K., Lekeux P., Coban C., Akira S., Ishii K.J., Bureau F., Desmet C.J. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 39.Ruwona T.B., Xu H., Li X., Taylor A.N., Shi Y.C., Cui Z. Toward understanding the mechanism underlying the strong adjuvant activity of aluminum salt nanoparticles. Vaccine. 2016;34:3059–3067. doi: 10.1016/j.vaccine.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Hagan D.T., Ott G.S., De Gregorio E., Seubert T. The mechanism of action of MF59 – an innately attractive adjuvant formulation. Vaccine. 2012;30(29):4341–4348. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 41.Wack A., Baudner B.C., Hilbert A.K., Manini I., Nuti S., Tavarini S. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26(4):552–561. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 42.Byrne G., O'Rourke S.M., Alexander D.L., Yu B., Doran R.C., Wright M. CRISPR/Cas9 gene editing for the creation of an MGAT1-deficient CHO cell line to control HIV-1 vaccine glycosylation. PLoS Biol. 2018;16(8):e2005817. doi: 10.1371/journal.pbio.2005817. [DOI] [PMC free article] [PubMed] [Google Scholar]