Highlights
-
•
Emerging Infectious Diseases require novel approaches to vaccine development.
-
•
EID vaccine development typically occurs within a knowledge vacuum.
-
•
Vaccine design may occur with few studies of disease epidemiology and pathogenesis.
-
•
Vaccine development for EIDs is benefitted by a platform technology.
-
•
EID trial design must be fluid and adapt to real-time alterations in epidemiology.
-
•
Societal and cultural norms and ethical concerns require input from key stakeholders.
Keywords: Emerging infectious diseases, Zika virus, Ebola virus, MERS coronavirus, Vaccine
Abstract
The recent outbreak of Zaire Ebola virus in West Africa altered the classical paradigm of vaccine development and that for emerging infectious diseases (EIDs) in general. In this paper, the precepts of vaccine discovery and advancement through pre-clinical and clinical assessment are discussed in the context of the recent Ebola virus, Middle East Respiratory Syndrome coronavirus (MERS-CoV), and Zika virus outbreaks. Clinical trial design for diseases with high mortality rates and/or high morbidity in the face of a global perception of immediate need and the factors that drive design in the face of a changing epidemiology are presented. Vaccines for EIDs thus present a unique paradigm to standard development precepts.
1. Introduction
The recent outbreak of Zaire Ebola virus (EBOV) in West Africa altered the classical paradigm of vaccine development. The rapid pace of spread of virus through the capital cities of Sierra Leone, Liberia, and Guinea, with cases doubling almost weekly through the summer of 2014, and the potential for international dissemination, necessitated an alternative development schema. Through a massive collaborative effort between government, academia and the pharmaceutical industry, multiple vaccines and therapeutic candidates were rapidly advanced into clinical development – with two vaccines reaching advanced testing and a viral-vectored vesicular stomatitis vaccine (VSV) [1], [2] demonstrating protection of at-risk individuals [3].
Alterations to the “normal” development cycle during the Ebola outbreak included advancement into Phase II/III while Phase I studies were being completed. Second, scale up of vaccine production occurred “at-risk” and prior to large-scale safety and immunogenicity assessments. Third, and importantly was the need for novel clinical trial study designs for Phase II/III studies in the face of an ever changing epidemiology. The ring vaccination strategy used for the VSV-EBOV vaccine utilized a staged approach whereby immediate contacts of cases of Ebola virus infection were randomized to early (immediate) vs late (delayed by 21 days) inoculation [3]. While clinical trials proceeded relatively quickly, early pre-clinical development was significantly challenged by the lack of funding which necessitated moving partially tested vaccines into clinical practice during a period of increasing international panic [4]. The Ebola vaccine trials were additionally challenged by ethical concerns of whether and how to include a control arm, societal mistrust of foreign entities conducting clinical trials, and the fact that the Ebola outbreak was in decline by the time vaccine studies commenced. Vaccine developers also had concerns regarding significant costs related to vaccine development, production, and clinical trial conduct for unknown commercialization potential.
Two newly emergent infectious diseases, the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Zika virus, present additional difficult challenges for vaccine development. MERS-CoV is a Group C betacoronavirus with a clinical presentation of a rapidly progressive severe pneumonia, respiratory failure, renal failure, neurologic compromise, and cardiac arrhythmias [5], [6], [7]. MERS shares many similarities with the Severe Acute Respiratory Syndrome (SARS), a Group B betacoronavirus. However, whereas SARS was considered highly lethal with a mortality rate of ∼10%, the mortality rate for MERS is almost 40% - akin to the recent 2014–2015 Ebola epidemic. The epidemic potential for MERS-CoV was made readily apparent in the summer of 2015 when an outbreak in South Korea involving 186 individuals ensued from an index case of a businessman returning from Saudi Arabia; 20% of infected individuals died during the Korean outbreak, [8]. And finally, Zika virus, discovered in the Zika forest of Uganda in 1947, was unknown outside of equatorial Africa and tropical Southeast Asia until 2009 when it began its relentless spread Eastward across the South Pacific [9], [10], [11], and eventually landing in Brazil with reports commencing in March 2015 [12], [13]. While Zika virus infection is typically self-limited and may even be subclinical, causing a syndrome significantly milder than chikungunya or dengue, Zika’s propensity for neurologic complications such as the Guillain Barré syndrome [14], [15], as well as microcephaly and other congenital abnormalities among fetuses for women infected during pregnancy [16], [17], [18] has riveted international attention on this illness [19].
Each of these viral illnesses present distinct challenges to vaccine development and assessment (Table 1 ). Each has a different epidemiology, distinct mechanisms of transmission, and target risk groups. Clinical trial design and power calculations are intimately related to both the attack rate as well as the fraction of the population that are naïve to infection. One unique consideration with each of these illness is the fact that the epidemiology of each outbreak was changing in real time that poses significant challenges to trial design and implementation. These logistical and design concerns need to be considered within the context of societal and local mores that can affect the ability to conduct a clinical trial. In this paper, we discuss some aspects of rational trial design that focuses the Ebola, MERS-CoV, and Zika epidemic outbreaks as models for vaccine development and alternative trial design.
Table 1.
Epidemiologic and study design related characteristics of Ebola, MERS, Zika.
| Zaire Ebola virus | MERS-CoV | Zika virus | |
|---|---|---|---|
| Incubation | |||
| Typical | 7–10 days | 7–14 days | 7–10 days |
| Range | 2–21 days | 2–21 days | Unknown |
| Transmission | Body fluid exposure | Contact, droplet | Arthropod (mosquito) |
| Ease of transmission | High | Low to medium | High (in endemic region) |
| Secondary cases | All direct contacts of 1° case | Some direct contacts of 1° case | Regional (mosquito driven) |
| 2° transmission risks | Any contact with blood, vomitus, diarrhea, saliva, semen | HCW, contact with patient with high respiratory viral load | Sexual contacts, breast feeding, transfusion, droplet (?) |
| Primary at-risk groups | HCW, family, close friends | HCW, close contacts (of severely ill patients) | All residing in endemic region, sexual contacts |
| Geography of cases | West Africa; importation of ill HCW to host countries | Arabian peninsula, North Africa, South Korea | South and Central America, Caribbean basin, South Florida |
| Goal for vaccine | Prevention of infection | Prevention of infection | Prevention of infection |
| Outcome of interest | Prevention of mortality | Prevention of severe pneumonia | Prevention of microcephaly |
| Diagnosis | PCR (serum) | PCR (lower respiratory) | PCR (serum, saliva, urine), serology |
| Commercial Dx assay | Yes | Yes | No, EUA only |
| Correlates of protection | Not defined | Not defined | Not defined |
| Timing of outbreak | December 2013 - January 2016 | Ongoing with periodic outbreaks | Ongoing transmission & spread |
2. What is an Emerging Infectious Disease?
Emerging Infectious Diseases (EIDs) have varied definitions. At one end of the spectrum are infectious diseases that are well known, but either a new genetic variant emerges or a previously seen strain type gains the potential for novel or increased morbidity. Examples include influenza H1N1 and the avian H7N9 influenza with increased propensity for mortality, and the H3N2 variant outbreak that caused infection in age groups (40–59 years of age) not typically associated with more severe illness. While Ebola virus was not new, the 2014 outbreak involved a much greater geography with a much higher potential for international spread and could be technically labelled as “re-emergent”. Zika virus infection is neither new, having been discovered in 1947, nor re-emergent as it has remained endemic in Asia and Africa, but has newly recognized comorbidities and complications as well as spread into the Western Hemisphere. At the other end of the spectrum are diseases such as MERS-CoV that represent completely new pathogens, as a genetically novel coronavirus discovered in 2012 [20].
In contrast to influenza, for which live and killed vaccines and production methods have been in existence for many decades, no vaccine had advanced beyond exploratory human clinical trials for Ebola and no vaccines had even been conceived for MERS or Zika prior to the recent epidemics. The high mortality rate of Ebola and MERS and the fetal malformations related to Zika, resulted in international clamor for rapid development of vaccines and therapeutics. Among these three, Zika has a unique critical need for a vaccine as the primary means to combat disease-related complications. For both Ebola virus and MERS, therapeutic small molecules should be able to interrupt disease pathogenesis and morbidity as Ebola has a 7–21 incubation period before the onset of clinical illness and MERS patients have between 1 and 5 days prior to clinical deterioration following diagnosis that could allow an effective therapeutic to abort infection. In contrast, for pregnant women who present with Zika infection, diagnosis typically follows the onset of rash and the peak of viremia and after fetal infection has likely occurred. Thus, preventive measures for Zika are uniquely reliant on an effective vaccine.
3. The need for speed and gaps in knowledge
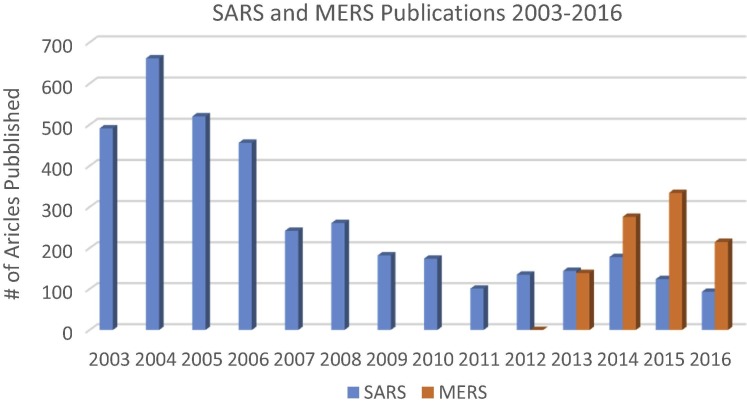
Typical for all emergent and serious infectious diseases with global potential is the desire for rapid vaccine development. However for many EIDs, there is likely only a rudimentary understanding of disease pathogenesis and epidemiology at the outset which can significantly slow vaccine development. A glaring indication of the knowledge gaps at the time of Zika and MERS emergence is evident by a review of the number of publications listed in the PubMed literature database prior to and after each disease was discovered or re-emerged (Fig. 1 ).
Fig. 1.
Number of publications listed in the PubMed database for SARS and MERS-CoV from 2003 through August 2016. Publications for SARS peaked in the two years following the epidemic and have remained relatively constant over the ensuing decade. Publications for MERS were first noted the year after discovery and peaked the following year. Data is adapted from PubMed; numbers are inclusive articles that may appear in a search for both viral diseases.
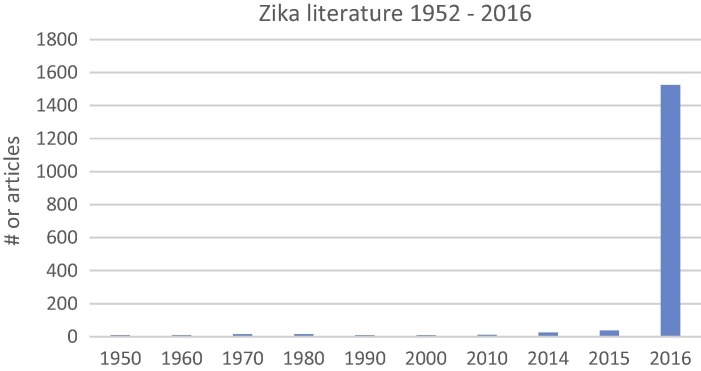
For MERS the spike in literature began in the year following discovery of the 1st cases in 2012. Notable is that the interest in SARS coronavirus never completely abated since its discovery in 2002. For MERS and SARS there have been almost 5000 publications. In contrast, for Zika, while approximately 1600 papers have been published from 1952 through 2016, greater than 90% have appeared since January 2016 (Fig. 2 ). Prior to 2016 there were a total of 0–3 articles published in a given year, with interest in Zika increasing after 2014 outbreak in French Polynesia.
Fig. 2.
Numbers of publications listed in the PubMed database for the Zika virus 1952 through to August 2016. Total citations per each decade are listed for the years 1950 through 2013. Of the approximate 1000 total articles published, greater than 90% have been published in the current year (2016). Data is adapted from PubMed, with each citation reviewed as to reference to the Zika virus.
For both MERS and SARS, there have been critical deficiencies in an understanding of the pathogenesis and epidemiology of infection. Although the pathogenesis of SARS had been well characterized in the decade since its discovery, many lessons were not applicable to MERS. SARS was known to infect cells through binding to the angiotensin converting enzyme 2 (ACE2) [21], [22]. The fact that ACE2 is expressed widely in mammalian species allowed for murine and other mammalian animal models to assess candidate vaccines. In contrast, MERS is phylogenetically restricted to primates, camelids, and bats binding to the cell surface dipeptidylpeptidase 4 (DPP4), which while universally expressed in mammals, the receptor binding domain differs between susceptible and non-susceptible species [23]. This species restriction meant that rodents were not a useful model system unless induced to express primate DPP4 either through adenoviral transfection [24] or gene exchange [25], [26]. Transfected and transgenic mice while permissive to infection have been suboptimal, whereas camels or alpacas develop infection [27], [28] but are expensive and represent difficult model systems. Primates such as macaques [29], [30] or marmosets [31] develop self-limited illness but are expensive and with multiple barriers to use.
MERS also demonstrated an epidemiology different from SARS. While MERS presented a risk to health-care workers [32] similar to SARS, other risk groups included those with exposure to camels and those with direct contact with primary cases – albeit with a lower efficiency of person-to-person spread [33], [34].
Zika posed numerable challenges at its time of “rediscovery” in 2014. In 1947, Zika was already known to be transmitted by Aedes species mosquitoes [35], [36], [37]. Study of Zika and its propensity for neurologic illness were apparent due to the ability to cause disease through direct intracranial inoculation of mice and resistance to infection by other species regardless of the route of administration [35]. In fact, neurologic consequences of disease have been known for greater than 4 decades as a study published in 1971 demonstrated histopathologic destruction of CNS tissue following infection of mice [38]. Of interest is that much of the history of Zika had been forgotten or unread as was apparent in Center of Disease Control and other health authority communiqués. “New” findings for Zika were the novel modes of transmission: via sexual contact, breast milk, direct contact, or via urine and/or saliva as either implicated or proven [39], [40], [41], [42], [43]. And novel targets for comorbid illness: fetal brains causing encephalitis, fetal tissue, and the placenta, all typically spared most maternal infections, yielding a host of congenital complications [16], [17], [44], [45], [46].
4. Immune correlates of protection
For each of the three EIDs highlighted here, the immune correlates of protection were unknown at the outset of each outbreak and are not completely understood even now.
Each of these diseases poses unique challenges in developing a comprehensive understanding of the pathogenesis and immunity of disease. For Ebola, the high mortality rate and rapid clinical course of infection posed severe limitations to specimen collection during the course of illness. The lack of early serum and peripheral blood mononuclear cell (PBMC) samples meant that immune correlates of survival could not be discerned. The availability of samples only after clinical deterioration meant that cytokine profiles and cellular findings caused by viral infection or resulting from clinical illness itself could not be differentiated. For Ebola there was the added concern of safe collection and storage of specimens because of the risk of transmission to lab personnel. MERS had a similar mortality rate and rapid course of illness that presented similar barriers to the collection of samples early in disease. Also, for both MERS and Ebola, there were societal and cultural impediments to the collection and storage of specimens.
Neutralizing antibodies are generally considered as the hallmarks for protective immunity for each of these viral illnesses. However, studies presented to date raise question as to validity of this assumption. For example, a clinical trial was completed that compared supportive therapy (standard of care, SOC) to SOC plus the use of hyperimmune, convalescent serum taken from recovered patients for the treatment of Ebola virus infection [47]. The study showed no survival benefit for those administered hyperimmune serum. One limitation of the study was that the concentration of neutralizing antibodies in donor serum was unknown. For MERS, the development of neutralizing antibodies does not predict viral clearance [48] although one small study did suggest a potential correlation between neutralization titers and outcome [49]. Importantly, indirect evidence against a role for neutralizing antibodies to protect against future disease is the observation that new infections continue to be documented for those with camel exposure (http://www.moh.gov.sa/en/CCC/PressReleases/Pages/statistics-2016–08-8–001.aspx), a group for whom repeated and ongoing exposure should be the norm and for whom immune responses against MERS are well documented [50]. While the B-cell response against coronaviruses such as SARS quickly over time [51], MERS antibodies are detected up to three years post-infection in most [52]. Whether the latter represent neutralizing antibodies was not specified. Immune correlates of Zika virus protection are still being elucidated, but protection of immunocompetent mice from viremia with binding antibodies alone was suggested by one DNA vaccine study that yielded minimal neutralization titers [53].
5. Vaccine design – the benefits of a platform technology
In contrast to traditional live and killed virus vaccines and subunit vaccines, many newer vaccine platforms are characterized by more easily alterable target antigens. Of these, DNA vaccines may be the most mutable and able to meet rapid timelines required to respond to new emerging threats. Other rapidly “tunable” systems include protein based systems that generate include virus like particles (VLPs), chimeric live viruses, and RNA vaccines. Each will be discussed briefly with regard to speed and technical barriers to adaption to new threats and are summarized in Table 2 .
Table 2.
Comparison of vaccine platforms.
| Characteristic | Attenuated virus | Inactivated virus | Vectored viral | VLP/protein | DNA | RNA |
|---|---|---|---|---|---|---|
| Platform experiencea | Classical | Classical | In development | Classical | In development | In development |
| Vaccine-associated risk of infectionb | Present | Present | Present | Low | Low | Low |
| Reliance on viral growthc | Present | Present | Present | Absent | Absent | Absent |
| Epidemic response timed | Slow | Slow | Rapid | Moderate | Rapid | TBD |
| Stability of vaccine | High | High | High | High | High | TBD |
| Adverse eventse | Moderate | Moderate | Moderate | Low | Low | TBD |
Experience with the respective platform is based on the clinical experience of FDA approved vaccines in each group. Protein vaccines such as those for pneumococcal pneumonia and the human papilloma virus (HPV) VLP vaccine would be considered as “classical”, whereas newer approaches such as nanoparticle vaccines in development for respiratory syncytial virus (RSV) are non-classical. Vectored viral vaccines as well as nucleic acid technologies have less clinical experience and/or are in clinical development.
Vaccine-associated risk of infection refers to the ability of the vaccine components to cause disease. In the case of inactivated or attenuated virus, the risk pertains to the known risk for back-mutations to a virulent state, incomplete inactivation, or contamination of an attenuated viral stock with virulent virus.
The reliance on viral growth is based on whether the replication efficiency of the vaccine strain or chimeric vaccine candidate is a factor in the speed of vaccine production.
Epidemic response time refers to the total time to bring a new vaccine into clinical trials and includes vaccine design as well as production. It is, however, noted that there has been significant improvement in protein expression systems in recent years.
Adverse events relate to known vaccine-associated reactions including viral-like infections as well as more unique reactions such as development of a lupus anticoagulant observed with adenoviral vectored vaccines.
Live viral virus vaccines include those for measles, mumps, rubella, polio (Sabin vaccine), varicella, and zoster vaccines. Killed virus vaccine examples include those for influenza and polio (Salk vaccine). Subunit vaccines include both protein based and carbohydrate based such as the pneumococcal vaccines. Challenges include limitations in growth of viral strains that may limit vaccine production (as occurred during the H1N1 influenza outbreak), assurance that live vaccines do not contain virulent back-mutants (polio), and that for live vaccines that care is taken with the ability of an attenuated vaccine strain to cause disseminated infection in those with altered host immunity (vaccinia). Subunit vaccines, primarily used for bacteria, require growth of the target organism and separation and purification of the requisite antigen which introduces requirements for adequate growth, precaution against contamination of the growth media, and assurance that bacterial or viral components such as endotoxin are not present.
Live viral recombinant vaccines offer greater development speed. Viral vectors such as the modified Ankara strain of vaccinia virus (MVA), the bovine vesicular stomatitis virus (VSV), various adenoviral (Ad) serotypes, as well as an attenuated and modified measles virus have been used to create vaccine candidates against Ebola, MERS, Zika, HIV and others. Many have the advantage of rapid immunogenicity following a single vaccine administration – which was key to the success of the VSV Ebola vaccine [3]. The respective viral vectors are modified to contain gene inserts of the target viral outer proteins allowing for rapid creation of vaccine candidates. Viral vectored vaccines are, however, associated with febrile and inflammatory responses that may be symptomatically limiting. Additionally, some viral systems have unique adverse effects that have unknown long-term consequences. For example, adenoviral vectors can induce lupus anticoagulants a complication reported for 20% of participants who received the chimpanzee Ad3 (ChAd3) Ebola vaccine [54]. Although no thrombotic events were observed, the period of follow-up was short and resolution of the autoantibody was not reported. Finally, the safety of live viral vectored vaccines in those with underlying immune diseases is unknown.
Virus like particles (VLPs), while similar to traditional subunit vaccines, are generated either through genetically modified host organisms, such as yeasts, or cellular expression systems that excrete the target antigen at high quantities. Similar to viral vectored vaccines, there can be rapid alteration in target antigens through DNA design modifications. VLPs have a well characterized safety profile. The key challenge to VLPs is to ensure appropriate post-translational protein folding to ensure exposure of appropriate conformation of immunogenic epitopes and assurance that the various proteins are not themselves allergenic. The two prophylactic vaccines for human papilloma virus (HPV) are VLPs of the viral L1 capsid protein.
Finally, nucleic acid vaccines and specifically DNA vaccines are becoming a significant area for development. The time to generate new vaccine candidates is limited only by the time to design a new DNA insert. With advances in DNA vector design, manufacture, and delivery systems having significantly advanced over the past two decades, there is great promise for this technology as the critical component of vaccine armamentarium. DNA vaccines have advanced through Phase II for the treatment of precancerous lesions due HPV [55] and have shown great promise for Ebola virus in a Phase I clinical trial [56] as well as preclinical data for Ebola [57], MERS [29], [30], West Nile virus [58], [59], [60], [61], and other EIDs. DNA vaccines have had excellent safety profiles and have not been associated with allergic reactions. The key hurdles for DNA vaccines is to ensure delivery in a manner to generate high levels of protective immunity and to overcome the psychological barrier imposed by the fact that no DNA vaccine has yet achieved licensure. RNA vaccines are still considered in their infancy with significant challenges in delivery and stability.
6. Clinical trial design amid a changing epidemiology
All three of the EIDs discussed here have experienced a changing epidemiology during their respective epidemics. This varying disease landscape critically affects clinical trial design. Since study size of Phase II and Phase III clinical trials are critically dependent upon the event rate, it requires careful consideration of how to maximize the number of infections. For EIDs (and for any other infection), it is important to be able to exclude individuals who have prior disease exposure and/or who become infected after enrollment but prior to when immunity is expected to develop. With a changing ecology and epidemiology, one needs to consider the future rather than the present dynamics of such epidemics.
For Ebola, MERS, and Zika clinical trial design was and is affected by a declining event rate. For Ebola, while the early part of the epidemic was associated with an almost geometric rise in cases, by the time vaccine trials commenced in West Africa, the outbreak was in decline and only the ring vaccination trial sponsored by the World Health Organization in cooperation with Merck that enabled the rVSV-Ebola vaccine to achieve an efficacy signal [62].
For MERS, it is postulated that a similar study design will likely be needed with two target groups: health care workers (HCWs) at hospitals for those hospitals in Saudi Arabia treating MERS cases and household contacts of primary cases. Any study among HCWs may be offset by a high baseline seropositivity rate from prior exposure, whereas a household study is limited by a low person-to-person transmission rate coupled with a poorly understood mechanism by which MERS spreads in such environments. An additional concern is that overall case numbers for MERS have declined significantly since 2013 when nosocomial outbreaks were commonplace.
Finally, Zika poses a unique challenge as rapid and widespread infection can result in significant herd immunity as was seen in Yap Island and French Polynesia with outbreaks that lasted 3 and 4 months, respectively [9], [63]. While the outbreaks in Columbia and Brazil continue, both countries have experienced a dramatic drop in new cases and both countries have announced the end of their respective epidemics. Thus, for a disease such as Zika it is imperative to conduct clinical trials at the leading edge of the epidemic – which poses logistical challenges since one needs to establish clinical sites at sites where the epidemic will be in 6–12 months from when planning is occurring.
7. Ethics of a control group
The final topic that will covered, albeit briefly, is the ethics of a control group. Assessment of vaccine efficacy is dependent upon being able to compare vaccine recipients to those receiving placebo. During the Ebola outbreak, late phase vaccine and therapeutic trials were complicated as to consensus of what constituted an adequate control arm against which to measure efficacy and whether it was ethical to even include a control arm [62]. The competing needs of therapeutic intervention versus proof of therapeutic efficacy stymied decision making. As one cannot predict efficacy a priori, the need to control for response related to vaccine is imperative. The compromise reached as part of the ring vaccination trial was to randomize primary cases into immediate versus delayed (by the 21 day incubation period for Ebola) groups. The study met study goals and was terminated early [3].
A similar ethical discussion will need to occur for MERS which also has a high mortality rate (∼40%). A potential study design would be to enroll for a HCW trial across multiple hospitals allowing for “early” versus “late” study sites. The “late” hospitals would serve as controls for “early”. However, determination of the order of when hospitals are started into the trial for the active vaccination phase will require significant local discussion and agreement.
For Zika, including a control arm for a basic protection study should be straightforward – as the disease is short-lived and without sequelae for most. The critical decision point would be how to address vaccination in pregnant women. Leaving aside any safety concerns of vaccinations during pregnancy, the ethical dilemma is whether or how to include a control group if a vaccination program is conceived for this risk group. There is a growing consensus that in pregnancy, one only needs to demonstrate safety while relying on a proof of efficacy among non-pregnant individuals. Ethical concerns still may exist for an alternative study whereby reproductive age women are targeted for primary vaccination and then randomized into booster versus no booster at the time that pregnancy is diagnosed.
8. Paradigm for vaccine development for EIDs
In this paper we delineated those considerations important for clinical trial design for a vaccine targeting EIDs. Ebola, MERS, and Zika virus were chosen as paradigms of three EIDs as current with each disease having a unique set of ethical, epidemiologic, and treatment challenges. Based on the case studies presented above, a paradigm for EID vaccine development can be constructed and is outlined below.
First and foremost is that a careful understanding and analysis of the epidemiology of an EID needs to be completed. For both the MERS and Zika virus epidemics, epidemiologic studies occurred (or are being conducted) in parallel with vaccine development. It is also key to understand the “micro-epidemiology” of disease. For example forMERS, while certain areas of Saudi Arabia are known as “hot spots” for disease, the random nature of new cases and spread within new environs raises critical logistical considerations for a ring vaccination study design. And for Zika, it is known that within cities and regions transmission and incidence rates may vary widely from neighborhood to neighborhood – again posing challenges to clinical trial design. Additionally, one needs to detail key risk groups for disease transmission and whether these groups differ from those at most risk for illness or disease complications. For example, for Zika while all are considered at risk within endemic regions, vaccination will be targeted to women of child-bearing potential and their sexual partners. And finally, it is critical to be able to determine which individuals have pre-existing immunity prior to enrollment into clinical trials.
When designing a clinical trial, it is imperative to have full stakeholder involvement in clinical trial design. This will enable discussion to mitigate against misunderstanding of outcomes, expectations, and fears of the vaccine itself. Education programs geared towards locals must be developed. And most importantly is to determine the pros and cons of a placebo control group and how the trial design can be modified based on knowledge acquired during the early phases of study implementation. The latter cannot be stressed enough since unlike “classical infectious diseases” for which the epidemiology is understood prior to study commencement, the epidemiology and knowledge base of EIDs may change during the course of the study which may require design changes in real time.
9. Conclusions
In this paper we have reviewed and discussed the key challenges and barriers to vaccine development for EIDs and have discussed some general approaches both technical and ethical as to rational vaccine and clinical trial design. EIDs will continue to be discovered and the ability of the scientific community to adapt and respond to these will continue to evolve over time.
Conflict of interest
Dr. Maslow is the Chief Medical Officer of GeneOne Life Science. Inc. and is fully employed by the company. GeneOne is developing or co-developing DNA vaccines for Ebola, MERS-CoV, and Zika virus. Dr. Maslow owns stock and stock options in GeneOne Life Science, Inc.
References
- 1.Geisbert T.W., Daddario-Dicaprio K.M., Geisbert J.B., Reed D.S., Feldmann F., Grolla A. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26(52):6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [PubMed PMID: 18930776; PubMed Central PMCID: PMC3398796] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geisbert T.W., Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011;204(Suppl 3):S1075–S1081. doi: 10.1093/infdis/jir349. [PubMed PMID: 21987744; PubMed Central PMCID: PMC3218670] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henao-Restrepo A.M., Longini I.M., Egger M., Dean N.E., Edmunds W.J., Camacho A. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. The Lancet. 2015 doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. Ebola vaccine: little and late. Science. 2014;343(6203):1441–1442. doi: 10.1126/science.345.6203.1441. [DOI] [PubMed] [Google Scholar]
- 5.Arabi Y., Arifi A., Balkhy H., Najm H., Aldawood A., Ghabashi A. Clinical course and outcomes of critically ill patients with Middle East Respiratory Syndrome coronavirus infection. Ann Int Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 6.Cha R.-H., Joh J.-S., Jeong I., Lee J.Y., Shin H.-S., Kim G. Renal complications and their prognosis in Korean patients with Middle East Respiratory Syndrome-coronavirus from the central MERS-CoV designated hospital. J Korean Med Sci. 2015;30:1807–1814. doi: 10.3346/jkms.2015.30.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. PubMed PMID: 25303830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M-d Oh., Choe P.G., Oh H.S., Park W.B., Lee M.-S., Park J. Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. J Korean Med Sci. 2015;30:1701–1705. doi: 10.3346/jkms.2015.30.11.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao-Lormeau V.M., Roche C., Teissier A., Robin E., Berry A.-L., Mallet H.P. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20(6):1086–1087. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ. 2015;351:h6983. doi: 10.1136/bmj.h6983. PubMed PMID: 26698165. [DOI] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus infection outbreak, French Polynesia, 14 February; 2014.
- 12.Campos G.S., Bandeira A.C., Sardi S.I. Zika virus outbreak, Bahia Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Am Health Organization. Epidemiological alert. Zika virus infection. Pan Am Hlth Org [Internet]. 2015, 23 Jan 2016. Available from: <http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075>.
- 14.Cao-Lormeau V.M., Blake A., Mons S., Lastere S., Roche C., Vanhomwegen J. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016 doi: 10.1016/S0140-6736(16)00562-6. Epub 29 February 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oehler E., Watrin L., Larre P., Leparc Goffart I., Lastere S., Valour F. Zika virus infection complicated by Guillain-Barre syndrome - case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9) doi: 10.2807/1560-7917.es2014.19.9.20720. (pii=20720) [DOI] [PubMed] [Google Scholar]
- 16.Brasil P, Pereira JP, Gabaglia CR, Damasceno L, Wakimoto M, Nogueira RMR, et al. Zika virus infection in pregnant women in Rio de Janeiro – Preliminary Report. N Engl J Med [Internet]; 2016 7 March 2016. http://dx.doi.org/10.1056/NEJMoa1602412.
- 17.European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barre syndrome - 10 December; 2015.
- 18.Pan Am Health Organization. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. 1 December 2015; 2015. Available from: <http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en>.
- 19.Ioos S., Mallet H.P., Leparc Goffart I., Gauthier V., Cardoso T., Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–307. doi: 10.1016/j.medmal.2014.04.008. PubMed PMID: 25001879. [DOI] [PubMed] [Google Scholar]
- 20.Corman V.M., Eckerle I., Zaki A., Landt O., Eschbach-Bludau M., van Boheemen S. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17(39) doi: 10.2807/ese.17.39.20285-en. (pII20285) [DOI] [PubMed] [Google Scholar]
- 21.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279(5):3197–4201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(69655):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlan A., Zhao J., Sarkar M.K., Li K., McCray P.B., Jr., Perlman S. Receptor variation and susceptibility to Middle East respiratory syndrome coronavirus infection. J Virol. 2014;88(9):4953–4961. doi: 10.1128/JVI.00161-14. [PubMed PMID: 24554656; PubMed Central PMCID: PMC3993797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fetta C., Zhao J. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci. 2014;111:5. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macdonald L.E., Karow M., Stevens S., Auerbach W., Poueymirou W.T., Yasenchak J. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111(14):5147–5152. doi: 10.1073/pnas.1323896111. [PubMed PMID: 24706858; PubMed Central PMCID: PMCPMC3986150] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao G., Jiang Y., Qiu H., Gao T., Zeng Y., Guo Y. Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome coronavirus. PLoS One. 2015;10(12):e0145561. doi: 10.1371/journal.pone.0145561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramer G, Durr PA, Klein R, Foord A, Yu M, Riddell S, et al. Experimental infection and response to rechallenge of alpacas with Middle East Respiratory Syndrome coronavirus. Emerg Infect Dis; 2016, vol. 22, 6 [Epub ePub ahead of print]. [DOI] [PMC free article] [PubMed]
- 28.Haagmans B.L., van den Brand J.M., Provacia L.B., Raj V.S., Stittelaar K.J., Getu S. Asymptomatic Middle East respiratory syndrome coronavirus infection in rabbits. J Virol. 2015;89(11):6131–6135. doi: 10.1128/JVI.00661-15. [PubMed PMID: 25810539; PubMed Central PMCID: PMCPMC4442453] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthumani K., Falzarano D., Reuschel E.L., Tingey C., Flingai S., Villareal D.O. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7(301):e301ra132. doi: 10.1126/scitranslmed.aac7462. [Epub 19 August 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Shi W., Joyce M.G., Modjarrad K., Zhang Y., Leung K. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. doi: 10.1038/ncomms8712. PubMed PMID: 26218507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falzarano D., de Wit E., Feldmann F., Rasmussen A.L., Okumura A., Peng X. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10(8):e1004250. doi: 10.1371/journal.ppat.1004250. [PubMed PMID: 25144235; PubMed Central PMCID: PMC4140844] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assiri A., McGeer A., Perl T.M., Price C.S., Al-Rabeeah A.A., Cummings D.A.T. Hospital outbreak of Middle East Respiratory Syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drosten C., Kellam P., Memish Z.A. Evidence of camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;371(14):1359–1360. doi: 10.1056/NEJMc1409847. PubMed PMID: 25271610. [DOI] [PubMed] [Google Scholar]
- 34.Farag E.A., Reusken C.B., Haagmans B.L., Mohran K.A., Stalin Raj V., Pas S.D. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect Ecol Epidemiol. 2015;5:28305. doi: 10.3402/iee.v5.28305. [PubMed PMID: 26183160; PubMed Central PMCID: PMCPMC4505336] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dick G.W.A. Zika virus (II). Pathogenicity and physical properties. Trans Roy Soc Trop Med Hyg. 1952;46(5):521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 36.Dick G.W.A., Kitchen S.F., Haddow A.J. Zika virus: (I). Isolations and serological specificity. Trans Roy Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 37.Haddow A.J., Williams M.C., Woodall J.P., Simpson D.I.H., Goma L.K.H. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Ugandan forest. Bull Wld Hlth Org. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 38.Bell T.M., Field R.J., Narang H.K. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35(2):183–193. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- 39.Besnard M, Lastere S, Teissier A, Cao-Lormeau VM, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill; 2014, vol. 19, 13 [pii:-20751]. [PubMed]
- 40.Foy B.D., Kobylinski K.C., Chilson Foy J.L., Blitvich B.J., Travassos da Rosa A., Haddow A.D. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882. doi: 10.3201/eid1705.101939. [PubMed PMID: 21529401; PubMed Central PMCID: PMCPMC3321795] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gourinat A.-C., O'Connor O., Calvez E., Goarant C., Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21(1):84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musso D., Roche C., Nhan T.X., Robin E., Teissier A., Cao-Lormeau V.M. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–55. doi: 10.1016/j.jcv.2015.04.021. PubMed PMID: 26071336. [DOI] [PubMed] [Google Scholar]
- 43.Musso D., Roche C., Robin E., Nhan T., Teissier A., Cao-Lormeau V.M. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21(2):359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Driggers R.W., Ho C.Y., Korhonen E.M., Kuivanen S., Jääskeläinen A.J., Smura T. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016 doi: 10.1056/NEJMoa1601824. [Epub 30 March 2016] [DOI] [PubMed] [Google Scholar]
- 45.Ministério da Saúde (Brazil). Microcefalia - Ministério da Saúde divulga boletim epidemiológico 2015, 30 Jan; 2016. Available from: <http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/20805-ministerio-da-saude-divulga-boletim-epidemiologico.#sthash.MYQuW0Gh.dpuf>.
- 46.Oliveira Melo A.S., Malinger G., Ximenes R., Szejnfeld P.O., Alves Sampaio S., Bispo de Filippis A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. doi: 10.1002/uog.15831. PubMed PMID: 26731034. [DOI] [PubMed] [Google Scholar]
- 47.van Griensven J., Edwards T., de Lamballerie X., Semple M.G., Gallian P., Baize S. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. PubMed PMID: 26735992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M. Viral shedding and antibody response in 37 patients with Middle East Respiratory Syndrome coronavirus Iinfection. Clin Infect Dis. 2016;62(4):477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park W.B., Perera R.A., Choe P.G., Lau E.H.Y., Choi S.J., Chun J.Y. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21(12):2186–2189. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15(5):559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186(12):7264–7268. doi: 10.4049/jimmunol.0903490. PubMed PMID: 21576510. [DOI] [PubMed] [Google Scholar]
- 52.Payne D.C., Iblan I., Rha B., Alqasrawi S., Haddadin A., Al Nsour M. Persistence of antibodies against Middle East Resporatory coronavirus. Emerg Infect Dis. 2016;22(10) doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larocca RA, Abbink P, Peron JPS, de A Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against Zika virus from Brazil. Nature; 2016 http://dx.doi.org/10.1038/nature18952. [DOI] [PMC free article] [PubMed]
- 54.Ledgerwood J.E., DeZure A.D., Stanley D.A., Novik L., Enama M.E., Berkowitz N.M. Chimpanzee adenovirus vector Ebola Vaccine - Preliminary Report. N Engl J Med. 2014 doi: 10.1056/NEJMoa1410863. PubMed PMID: 25426834. [DOI] [PubMed] [Google Scholar]
- 55.Trimble C.L., Morrow M.P., Kraynyak K.A., Shen X., Dallas M., Yan J. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. The Lancet. 2015;386(10008):2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sardesai N, editor. Translating DNA vaccines and immunotherapies into advanced clinical studies. World Vaccine Congress; 2016 29–31 March 2016; Washington, D.C.
- 57.Shedlock D.J., Aviles J., Talbott K.T., Wong G., Wu S.J., Villarreal D.O. Induction of broad cytotoxic T cells by protective DNA vaccination against Marburg and Ebola. Mol Ther. 2013;21(7):1432–1444. doi: 10.1038/mt.2013.61. [PubMed PMID: 23670573; PubMed Central PMCID: PMC3705942] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ledgerwood J.E., Pierson T.C., Hubka S.A., Desai N., Rucker S., Gordon I.J. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203(10):1396–1404. doi: 10.1093/infdis/jir054. [PubMed PMID: 21398392; PubMed Central PMCID: PMCPMC3080891] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin J.E., Pierson T.C., Hubka S., Rucker S., Gordon I.J., Enama M.E. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196(12):1732–1740. doi: 10.1086/523650. [PubMed PMID: 18190252; PubMed Central PMCID: PMCPMC2714735] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramanathan M.P., Kutzler M.A., Kuo Y.C., Yan J., Liu H., Shah V. Coimmunization with an optimized IL-15 plasmid adjuvant enhances humoral immunity via stimulating B cells induced by genetically engineered DNA vaccines expressing consensus JEV and WNV E DIII. Vaccine. 2009;27:4370–4380. doi: 10.1016/j.vaccine.2009.01.137. [DOI] [PubMed] [Google Scholar]
- 61.Yang J.-S., Kim J.J., Hwang D., Choo A.Y., Dang K., Maguire H. Induction of potent Th1-Type Immune Responses from a Novel DNA Vaccine for West Nile Virus New York Isolate (WNV-NY1999) J Infect Dis. 2001;184(1 October):809–816. doi: 10.1086/323395. [DOI] [PubMed] [Google Scholar]
- 62.Cohen J., Enserink M. Ebola vaccines face daunting path to approval. Science. 2015;349(6254):1272–1273. doi: 10.1126/science.349.6254.1272. [DOI] [PubMed] [Google Scholar]
- 63.Duffy M.R., Chen T.-H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]