Abstract
A new series of 3-(benzylideneamino)-2-phenylquinazoline-4(3H)-ones were prepared through Schiff base formation of 3-amino-2-phenyl quinazoline-4(3)H-one with various substituted carbonyl compounds. Their chemical structures were elucidated by spectral studies. Cytotoxicity and antiviral activity were evaluated against herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus, herpes simplex virus-1 TK- KOS ACVr, para influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus, feline corona virus (FIPV), feline herpes virus, respiratory syncytial virus, influenza A H1N1 subtype, influenza A H3N2 subtype, and influenza B virus. Compound 2a showed better antiviral activity against the entire tested virus.
Keywords: Quinazoline, Schiff bases, 3-(Benzylideneamino)-2-phenylquinazoline-4(3H)-ones, Non-nucleoside antiviral agents, HSV, FIPV, Influenza, Vaccinia virus, Vesicular stomatitis virus
Graphical abstract
In the present study, new series of 3-(benzylideneamino)-2-phenylquinazoline-4(3H)-ones were prepared and evaluated for their antiviral activity.

1. Introduction
Viral infections caused by the rapid emergence of antiviral drug resistant strains have become a serious threat globally and have fostered the search for new antiviral agents directed against unexplored drug targets. Various nucleoside analogues have been currently used as antiviral agents these derivatives often inhibit viral polymerases. However, only few non-nucleoside antiviral agents are currently marketed.
Quinazoline is an interesting molecule and its pharmacological activities are well documented. It has been reported as antimicrobial [1], [2], [3], [4], [5], [6], [7], antiviral [8], anti-HIV [9], anticonvulsant [10], [11], anti-inflammatory [12], [13], [14], antihistaminic [15], anti-tubercular [16], [17], and anticancer [18], [19] activity, etc. A huge number of quinazolines had been synthesized and evaluated for various activities however their antiviral activity was not fully investigated to a greater extent. We have synthesized a simple 3-(benzylideneamino)-2-phenyl quinazoline-4(3H)-ones and tested in vitro cytotoxicity and antiviral activity, against viruses representative of herpes simplex virus-1 (KOS),herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus, herpes simplex virus-1 TK- KOS ACVr, para influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus, feline corona virus (FIPV), feline herpes virus, respiratory syncytial virus, influenza A H1N1 subtype, influenza A H3N2 subtype, influenza B and vesicular stomatitis virus.
2. Chemistry
The synthetic approach for the title compounds were shown in Scheme 1 . We utilized the method reported by Anjani et al. for synthesis of 2-phenyl- 4H-3, 1-benzoxazin-4-one [20]. Anthranilic acid (0.02 mol) was dissolved in 30 mL of anhydrous pyridine by stirring slowly at room temperature. The solution was cooled to 0 °C and a solution of benzoyl chloride (0.02 mol) in anhydrous pyridine 30 mL was added to this solution slowly with constant stirring. When the addition was complete the reaction mixture was stirred for half an hour mechanically at room temperature and set aside for 1 h. The pasty mass obtained was diluted with water and treated with aqueous sodium bicarbonate to remove the unreacted acid. When effervescence ceased, the solid material was filtered off and washed with water to remove the inorganic materials and the adhered pyridine. The crude benzoxazine thus obtained was dried and re-crystallized from diluted ethanol.
Scheme 1.
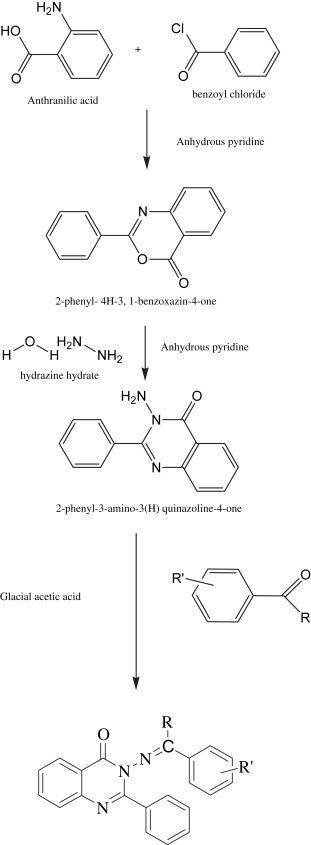
Synthetic scheme for the title compounds.
Subsequently the crystallized material was converted to 2-phenyl-3-amino-3(H) quinazoline-4-one by adding dropwise a solution of hydrazine hydrate (0.1 mol) in anhydrous pyridine (25 mL) to cold solution of 2-phenyl-4H-3, 1-benzoxazin-4-one (0.05 mol) in anhydrous pyridine (25 mL), with constant stirring [20]. When the addition was complete, the resultant reaction mixture was stirred vigorously for 30 min at room temperature and subsequently heated under reflux for 6 h under anhydrous reaction conditions. It was allowed to cool at room temperature and poured into ice cold water containing dilute hydrochloric acid on standing for 1 h, solidification occurred which was allowed to settle down. It was filtered off, washed repeatedly with water and dried in vacuum and purified by HPLC.
Title compounds were prepared through Schiff’s reaction. An equimolar quantity of 2-phenyl-3-amino-3(H)-quinazoline-4-one and aldehyde/ketone were dissolved in ethanol and its pH was adjusted to 4.0–4.5 with glacial acetic acid. The content of the mixture were refluxed for 30–150 min and poured into ice cooled water thus the solid obtained was filtered and purified by HPLC (Compound 2a–l, Table 1 ). Their structures are in agreement with elemental and spectral data.
Table 1.

| Compd. | R | R′ |
|---|---|---|
| 2a | H | 2-OH |
| 2b | H | 3-NO2 |
| 2c | H | 4-OCH3 |
| 2d | H | 4-N(CH3)2 |
| 2e | CH3 | 4-Cl |
| 2f | H | H |
| 2g | H | 4-OH |
| 2h | CH3 | H |
| 2i | CH3 | 4-OH |
| 2j | H | 4-Cl |
| 2k | H | 3-OH & 4-OCH3 |
| 2l | H | 2-OCH3 |
3. Pharmacology
Cytotoxicity and antiviral activity of all compounds were evaluated against herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus, herpes simplex virus-1 TK- KOS ACVr in HEL cell culture, vero cell culture, CRFK, Hela & MDCK cell cultures. Cytotoxic concentration, required to cause a microscopically detectable alteration of normal cell morphology, was measured. Cytopathogenicity concentration required to reduce virus-induced cytopathogenicity by 50%, was determined.
4. Results and discussion
Anthranilic acid reaction with benzoyl chloride yielded 2-phenyl-4H-3, 1-Benzoxazin-4 one by N-acylation via dehydrative cyclization mechanism. Subsequently which was converted to 2-phenyl-3-amino-3(H) quinazoline-4 one with hydrazine hydrate. A series of novel 3-(benzylideneamino)-2-phenylquinazoline-4(3H)-ones were synthesized by reaction with 2-phenyl-3-amino-3(H) quinazoline-4 one and compounds containing aldehyde and ketones to afford 2,3disubstituted quinazoline-4(3)H one derivatives (Table 1). The spectral data of synthesized compounds were consistent with the assigned structures and the compounds were obtained in 68–78% yield.
Title compounds were tested against representative members of the virus includes herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus, herpes simplex virus-1 TK- KOS ACVr (Table 2 ), para influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus (Table 3 ), feline corona virus (FIPV), feline herpes virus (Table 4 ), respiratory syncytial virus (Table 5 ) and influenza A H1N1 subtype, influenza A H3N2 subtype, influenza B (Table 6 ).
Table 2.
Cytotoxicity and antiviral activity of compounds in HEL cell cultures.
| Compd. | Minimum cytotoxic concentrationa (μg/mL) | EC50b (μg/mL) |
||||
|---|---|---|---|---|---|---|
| Herpes simplex virus-1 (KOS) | Herpes simplex virus-2 (G) | Vaccinia virus | Vesicular stomatitis virus | Herpes simplex virus-1 TK- KOS ACVr | ||
| 2a | 100 | 1 | 1 | 0.8 | 15 | 1 |
| 2b | ≥20 | >20 | >20 | >20 | >20 | >20 |
| 2c | >100 | 9 | 8 | 9 | >100 | 9 |
| 2d | 100 | >20 | >20 | >20 | >20 | >20 |
| 2e | 100 | >20 | >20 | >20 | >20 | >20 |
| 2f | >100 | 7 | 9 | 9 | >100 | 9 |
| 2g | 100 | >20 | >20 | 12 | >20 | >20 |
| 2h | >100 | >100 | >100 | >100 | >100 | >100 |
| 2i | >100 | >100 | >100 | >100 | >100 | >100 |
| 2j | 100 | >20 | >20 | 20 | >20 | >20 |
| 2k | 100 | >20 | >20 | 20 | >20 | >20 |
| 2l | 100 | >20 | >20 | >20 | >20 | >20 |
| Brivudin (μM) | >250 | 0.04 | 50 | 4 | >250 | 250 |
| Ribavirin (μM) | >250 | >250 | >250 | >250 | 146 | >250 |
| Cidofovir (μM) | >250 | 2 | 2 | 8 | >250 | 3 |
| Ganciclovir (μM) | >100 | 0.05 | 0.07 | >100 | >100 | 2 |
Data represent mean values for three independent determinations. Variation among duplicate samples was less than 15%.
Required to cause a microscopically detectable alteration of normal cell morphology.
Required to reduce virus-induced cytopathogenicity by 50%.
Table 3.
Cytotoxicity and antiviral activity of compounds in Vero cell cultures.
| Compd. | Minimum cytotoxic concentrationa (μg/mL) | EC50b (μg/mL) |
||||
|---|---|---|---|---|---|---|
| Para influenza-3 virus | Reovirus-1 | Sindbis virus | Coxsackie virus B4 | Punta Toro virus | ||
| 2a | 100 | >20 | 10 | 5 | 10 | 3 |
| 2b | 20 | >4 | >4 | >4 | >4 | >4 |
| 2c | 100 | >20 | >20 | >20 | >20 | >20 |
| 2d | 100 | >20 | >20 | >20 | >20 | >20 |
| 2e | 100 | >20 | >20 | >20 | >20 | >20 |
| 2f | 20 | >4 | >4 | >4 | >4 | >4 |
| 2g | 100 | >20 | >20 | >20 | >20 | >20 |
| 2h | 100 | >20 | >20 | >20 | >20 | >20 |
| 2i | >100 | >100 | >100 | >100 | >100 | >100 |
| 2j | 20 | >4 | >4 | >4 | >4 | >4 |
| 2k | 100 | >20 | >20 | >20 | >20 | >20 |
| 2l | 100 | >20 | >20 | >20 | >20 | >20 |
| DS-5000 | >100 | >100 | >100 | 20 | 20 | 20 |
| (S)-DHPA (μM) | >250 | >250 | >250 | >250 | >250 | >250 |
| Ribavirin (μM) | >250 | 146 | >250 | >250 | >250 | 146 |
Required to cause a microscopically detectable alteration of normal cell morphology.
Required to reduce virus-induced cytopathogenicity by 50%.
Table 4.
Anti-Feline Corona Virus (FIPV) and anti-Feline Herpes Virus activity and cytotoxicity in CRFK cell cultures.
| Compd. | CC50a (μg/mL) | EC50b (μg/mL) |
|
|---|---|---|---|
| Feline Corona Virus (FIPV) | Feline Herpes Virus | ||
| 2a | 11.2 | >4 | >4 |
| 2b | 13.8 | >4 | >4 |
| 2c | 13.7 | >4 | >4 |
| 2d | >100 | >100 | >100 |
| 2e | >100 | >100 | >100 |
| 2f | 3.7 | >0.8 | >0.8 |
| 2g | 59.5 | >20 | >20 |
| 2h | 2.6 | >0.8 | >0.8 |
| 2i | >100 | >100 | >100 |
| 2j | 42.2 | >20 | >20 |
| 2k | 42.7 | >20 | >20 |
| 2l | 14.4 | >4 | >4 |
| HHA | >100 | 2.6 | 1.6 |
| UDA | >100 | 11.7 | 53.1 |
| Ganciclovir (μM) | >100 | >100 | 0.8 |
50% Cytotoxic concentration, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay.
50% Effective concentration, or concentration producing 50% inhibition of virus-induced cytopathic effect, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay. CRFK cells: Crandell-Rees Feline Kidney cells.
Table 5.
Cytotoxicity and antiviral activity of compounds in HeLa cell cultures.
| Compd. | Minimum cytotoxic concentrationa (μg/mL) | EC50b (μg/mL) |
||
|---|---|---|---|---|
| Vesicular stomatitis virus | Coxsackie virus B4 | Respiratory syncytial virus | ||
| 2a | 4 | >0.8 | >0.8 | >0.8 |
| 2b | 20 | >4 | >4 | >4 |
| 2c | 20 | >4 | >4 | >4 |
| 2d | 100 | >20 | >20 | >20 |
| 2e | 100 | >20 | >20 | >20 |
| 2f | 20 | >4 | >4 | >4 |
| 2g | 100 | >20 | >20 | >20 |
| 2h | >100 | >100 | >100 | >100 |
| 2i | >100 | >100 | >100 | >100 |
| 2j | 20 | >4 | >4 | >4 |
| 2k | 20 | >4 | >4 | >4 |
| 2l | 20 | >4 | >4 | >4 |
| DS-5000 | >100 | >100 | 7 | 0.8 |
| (S)-DHPA (μM) | >250 | >250 | >250 | >250 |
| Ribavirin (μM) | >250 | 22 | 146 | 22 |
Required to cause a microscopically detectable alteration of normal cell morphology.
Required to reduce virus-induced cytopathogenicity by 50%.
Table 6.
Anti-influenza virus activity and cytotoxicity in MDCK cell cultures.
| Compd. | Concentration | Cytotoxicity |
Antiviral EC50c |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CC50a | Minimum cytotoxic concentrationb | Influenza A H1N1 subtype |
Influenza A H3N2 subtype |
Influenza B |
|||||
| Visual CPE score | MTS | Visual CPE score | MTS | Visual CPE score | MTS | ||||
| 2a | μg/mL | 2.6 | ≥0.8 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2b | μg/mL | >100 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2c | μg/mL | 8.2 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2d | μg/mL | >100 | 100 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2e | μg/mL | >100 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2f | μg/mL | 15.0 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2g | μg/mL | 10.2 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2h | μg/mL | >100 | 100 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2i | μg/mL | >100 | >100 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2j | μg/mL | 76.4 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2k | μg/mL | 47.4 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| 2l | μg/mL | 12.4 | 20 | N.A. | N.A. | N.A. | N.A | N.A. | N.A. |
| Oseltamivir carboxylate | μM | >100 | >100 | 0.2 | 0.1 | 4 | 3.8 | 2 | 1.5 |
| Ribavirin | μM | >100 | ≥100 | 9 | 12.9 | 9 | 7.8 | 9 | 6.0 |
| Amantadin | μM | >1000 | >1000 | 101 | 87 | 117 | 115 | N.A. | N.A. |
| Rimantadin | μM | 472 | 1000 | 68 | 58 | 40 | 53 | N.A. | N.A. |
N.A – not active at the highest concentration tested, or at subtoxic concentration.
50% Cytotoxic concentration, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay.
Minimum compound concentration that causes a microscopically detectable alteration of normal cell morphology.
50% Effective concentration, or concentration producing 50% inhibition of virus-induced cytopathic effect, as determined by visual scoring of the CPE, or by measuring the cell viability with the colorimetric formazan-based MTS assay. MDCK cells: Madin Darby canine kidney cells.
Compound 2a exhibited antiviral activity against herpes simplex virus-1 (KOS), herpes simplex virus-2(G), herpes simplex virus-1 (TK- KOS ACV) and vaccinia virus in HEL cell culture (Table 2) at selectivity index of 100, 100, 100 and 125 respectively, whereas cytotoxicity was found at 100 μg/mL. Compound 2a also inhibited the replication of reovirus, Sindbis virus, Coxsackie virus B4 and Punta Toro virus in Vero cell culture (Table 3) at selectivity index of 10, 20, 10 and 33, whereas cytotoxicity was found 100 μg/mL. It also showed inhibitory to vesicular stomatitis virus, respiratory syncytial virus in HeLa cells (Table 5) albeit at a concentration closely related to the cytotoxicity (4 μg/mL).
Compounds 2c and f were found to be active against herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, herpes simplex virus-1 TK- KOS ACVr in HEL cell culture (Table 2) at selectivity index of 11, 13, 11, 11 and 14, 11, 11, 11 respectively, where they were not cytotoxic at 100 μg/mL none of the compounds tested was active against influenza A H1N1, influenza A H3N2 and influenza B in MDCK cell Cultures.
Of the compounds tested, compound 2a clearly proved the most promising in terms of antiviral activity (potency). This lead molecule 2a could be further utilized for designing newer non-nucleoside antiviral agents especially for activity against HSV and vaccinia virus.
The synthetic procedure reported in this article allows a rapid synthesis of derivative of the title compounds to be tested for their antiviral effects.
5. Experimental
Melting points of the synthesized compounds were determined by using an open capillary tube method and are uncorrected. Microanalyses were carried out on a Carlo Erba 1106 elemental analyzer. The results of elemental analysis were within ±0.3% for C and ±0.1% for H and N of the theoretical value. 1H NMR spectra were performed on a Varian Gemini 200 (200 MHz) spectrometer using tetramethylsilane (TMS) as internal standard. IR spectra were recorded on an FTIR-JASCO 4100 Spectrophotometer. GC–MS spectra were performed with HP 6890–5973. GC parameters: injector temperature 250 °C; capillary column HP5 poly (methylphenylsiloxane) 30 m, 0.35 mm, 0.25 mm; temperature program: from 100 to 300 °C at 10 °C/min. MS parameters: mode SCAN 40–600 amu.
5.1. 3-[(2-Hydroxy-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2a)
Yield: 78%; m.p 144–146 °C; IR (KBr, in cm−1): 3200–3100 (O–H str. for –OH), 1683 (C O str.), 1604 (C N str.), 1465 (ring C C str.), 1362 (Ar C–N Str); 1H NMR (CDCl3, δ in ppm): 7.28–7.46 (m, 6H, ArH), 7.66–7.92 (m, 7H, ArH), 8.68 (s, 1H, H–C N).
5.2. 3-[(3-Nitro-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2b)
Yield: 74%; m.p 246–248 °C; IR (KBr, in cm−1): 1678 (C O str.), 1645.4 (C N str.), 1502 (ring C C str.), 1458 (Ar C–N Str), 1345 (N O str. for ArNO2); 1H NMR (CDCl3, δ in ppm): 7.25–7.52 (m, 6H, ArH), 7.62–7.80 (m, 4H, ArH), 8.0–8.21 (m, 3H, ArH), 8.67 (s, 1H, H–C N).
5.3. 3-[(4-Methoxy-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2c)
Yield: 68%; m.p 242–244 °C; IR (KBr, in cm−1): 1680 (C O str.), 1644.6 (C N str.), 1502 (ring C C str.), 1446 (Ar C–N Str), 1026 (C–O–C str. for –C–O–CH3); 1H NMR (CDCl3, δ in ppm): 3.73 (s, 3H, –OCH3), 7.25–7.49 (m, 6H, ArH), 7.58–7.80 (m, 4H, ArH), 8.10–8.22 (m, 3H, ArH), 8.60 (s, 1H, H–C N).
5.4. 3-[(4-Dimethylamino-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2d)
Yield: 72%; m.p 175–177 °C; IR (KBr, in cm−1): 1676 (C O str.), 1640 (C N str.), 1510 (ring C C str.), 1460 (Ar C–N Str); 1H NMR (CDCl3, δ in ppm): 3.12 (s, 6H, -NCH3), 7.23–7.42 (m, 6H, ArH), 7.62–7.80 (m, 7H, ArH), 8.56 (s, 1H, H–C N).
5.5. 3-[1-(4-Chloro-phenyl)-ethylideneamino-]-2-phenyl-3H-quinazolin-4one (2e)
Yield: 74%; m.p 243–245 °C; IR (KBr, in cm−1): 1670 (C O str.), 1646 (C N str.), 1515 (ring C C str.), 1455 (Ar C–N Str); 1H NMR (CDCl3, δ in ppm): 2.46 (s, 3H, -CH3), 7.20–7.48 (m, 6H, ArH), 7.57–7.78 (m, 7H, ArH).
5.6. 3-(Benzylidine-amino)-2-phenyl-3H-quinazolin-4one (2f)
Yield: 75%; m.p 194–196 °C; IR (KBr, in cm−1): 1665 (C O str.), 1642 (C N str.), 1510 (ring C C str.), 1449 (Ar C–N Str); 1H NMR (CDCl3, δ in ppm): 7.25–7.44 (m, 6H, ArH), 7.62–7.80 (m, 8H, ArH), 8.58 (s, 1H, H–C N).
5.7. 3-[(4-Hydroxy-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2g)
Yield: 78%; m.p 164–166 °C; IR (KBr, in cm−1): 3304–3212 (O–H str. for –OH), 1668 (C O str.), 1645 (C N str.), 1554 (ring C C str.), 1372 (Ar C–N Str); 1H NMR (DMSO-d 6, δ in ppm): 5.10 (s, 1H, –OH), 7.42–7.48 (m, 6H, ArH), 7.70–7.79 (m, 5H, ArH), 8.12–8.29 (m, 3H, ArH), 8.62 (s, 1H, H–C N).
5.8. 2-Phenyl-3(1-phenyl-ethylideneamino)-3H-quinazolin-4one (2h)
Yield: 73%; m.p 194–196 °C; IR (KBr, in cm−1): 1685 (C O str.), 1614 (C N str.), 1464.2 (ring C C str.), 1363.8 (Ar C–N Str); 1H NMR (CDCl3, δ in ppm): 2.44 (s, 3H, CH3), 7.28–7.46 (m, 6H, ArH), 7.66–7.92 (m, 7H, ArH).
5.9. 3-[1-(4-Hydroxy-phenyl)-ethylideneamino]-2-phenyl-3H-quinazolin-4one (2i)
Yield: 68%; m.p 166–168 °C; IR (KBr, in cm−1): 3200–3150 (O–H str. for –OH), 1686.4 (C O str.), 1614 (C N str.), 1468 (ring C C str.), 1372 (Ar C–N Str); 1H NMR (CDCl3, δ in ppm): 2.47 (s, 3H, CH3), 6.84–6.92 (m, 3H, ArH), 7.29–7.51 (m, 8H, ArH), 7.64–7.82 (m, 3H, ArH).
5.10. 3-[(4-Chloro-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2j)
Yield: 70%; m.p 160–162 °C; IR (KBr, in cm−1): 1680 (C O str.), 1587 (C N str.), 1552 (ring C C str.), 1374 (Ar C–N Str); 1H NMR (CDCl3, δ in ppm): 7.42–7.58 (m, 6H, ArH), 7.66–7.82 (m, 7H, ArH), 8.76 (s, 1H, H–C N).
5.11. 3-[(3-Hydroxy-4-methoxy-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2k)
Yield: 75%; m.p 180–182 °C; IR (KBr, in cm−1): 3206–3178 (O–H str. for –OH), 1686 (C O str.), 1624 (C N str.), 1460 (ring C C str.), 1364 (Ar C–N Str), 1025 (C–O–C str. for –C–O–CH3); 1H NMR (DMSO-d 6, δ in ppm): 3.72 (s, 3H, –OCH3), 5.02 (s, 1H, –OH), 6.64 (d, 1H, ArH), 7.27–7.46 (m, 8H, ArH), 7.62–7.75 (m, 3H, ArH), 8.72 (s, 1H, H–C N).
5.12. 3-[(2-Methoxy-benzylidine)-amino]-2-phenyl-3H-quinazolin-4one (2l)
Yield: 75%; m.p 212–214 °C; IR (KBr, in cm−1): 1680 (C O str.), 1644.6 (C N str.), 1502 (ring C C str.), 1446 (Ar C–N Str), 1024.37 (C–O–C str. for –C–O–CH3); 1H NMR (CDCl3, δ in ppm): 3.74 (s, 3H, -OCH3), 6.78–6.81 (m, 2H, ArH), 7.26–7.43 (m, 7H, ArH), 7.58–7.80 (m, 4H, ArH), 8.59 (s, 1H, H–C N).
5.13. Antiviral activity
Activity of compounds against para influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus was measured by plaque reduction assays in Vero cell monolayer. To this end, Vero cells were seeded in 24-well plates at a density of 2 × 105 cells/well and were allowed to form confluent monolayers by incubating overnight in growth medium at 37 °C in humidified CO2 (5%) atmosphere. Then, monolayers were infected with 250 μL of proper virus dilutions to give 50–100 PFU/well. Following removal of unadsorbed virus, 500 μL of Dulbecco’s modified Eagle’s medium supplemented with 1% inactivated FCS and 0.75% methyl cellulose, without or with serial dilutions of test compounds were added. Cultures were incubated at 37 °C for 2 days and then fixed with PBS containing 50% ethanol and 0.8% crystal violet, washed and air-dried. Plaques were then counted, and 50% effective concentrations (EC50) values were calculated by the linear regression technique.
Cytotoxicity and antiviral activity of compounds were also tested against vesicular stomatitis virus, Coxsackie virus B4 and respiratory syncytical virus in HeLa cell cultures. Minimum cytotoxic concentration was the concentration required to cause a microscopically detectable alteration of normal cell morphology. Effective concentration was the concentration required to reduce the virus-induced cytopathogenicity by 50%.
Compounds were also evaluated against influenza A HIN1, influenza A H3N2 and influenza B in Madin Darby canine kidney cells (MDCK) Cell cultures and 50% cytotoxic concentration, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay similarly 50% effective concentration or concentration producing 50% inhibition of virus-induced cytopathic effect, as determined by visual scoring of the CPE, or by measuring the cell viability with the colorimetric formazan-based MTS assay protocol.
Activity of compounds against feline corona virus and feline herpes virus was based on 50% effective concentration, or concentration producing 50% inhibition of virus-induced cytopathic effect as determined by measuring cell viability with the colorimetric formazan-based MTS assay in Crandell-Rees Feline Kidney cells (CRFK Cells).
Compounds which are potentially active in the earlier screening were evaluated against herpes simplex virus-1(KOS), Herpes simplex virus-2(G), vaccina virus and herpes simplex virus-1 TK- KOS ACV in HEL cell cultures and analyses for their cytotoxic concentration and effective concentration. Selective compounds were screened against para influenza-3 virus, reovirus-1, Sindbis virus B4 and Punta Toro virus in Vero cell cultures.
Acknowledgements
We thank Mrs. Leentje Persoons for excellent technical assistance with the antiviral activity assays. We also extend our thanks to Chairman Dr. Nalla G. Palanisami & Dr Thavamani D. Palanisami Trustee, Koval Medical Center Research and Educational Trust, Coimbatore for their support.
References
- 1.Rohini R., Shanker K., Reddy P.M., Ho Y.P., Ravinder V. Eur. J. Med. Chem. 2009;44:3330–3339. doi: 10.1016/j.ejmech.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Kohli D., Hashim S.R., Vishal S.R., Sharma M., Singh A.K. Inter. J. Pharm. Pharma. Sci. 2009;1:163–169. [Google Scholar]
- 3.Raghavendra N.M., Thampi P.P., Gurubasavarajaswamy P.M. E. J. Chem. 2008;5:23–33. [Google Scholar]
- 4.Alafeefy A.M. Pharma. Biol. 2008;46:751–756. [Google Scholar]
- 5.Suthakaran R., Kavimani S., Venkaiaiah P., Suganthi K. Rasayan J. Chem. 2008;1:22–29. [Google Scholar]
- 6.Siddappa K., Reddy T., Mallikarjun M., Reddy C.V. E. J. Chem. 2008;5:155–162. [Google Scholar]
- 7.Patel J.A., Mistry B.D., Desai K.R. E. J. Chem. 2006;3:97–102. [Google Scholar]
- 8.Selvam P., Babu K., Padamraj R., Persoons L., De Clercq E. Afr. J. Pharm. Pharmacol. 2008;2:110–115. [Google Scholar]
- 9.Desai N.C., Undavia N.K., Trivedi P.B., Dave D., Vyas G.D. Indian J. Exp. Biol. 1998;36:1280–1283. [PubMed] [Google Scholar]
- 10.Varsha J., Pradeep M., Sushil K., Stables J.P. Eur. J. Med. Chem. 2008;43:135–141. [Google Scholar]
- 11.Georgey H., Gawad N.A., Abbas S. Molecules. 2008;13:2557–2569. doi: 10.3390/molecules13102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rather B.A., Raj T., Reddy A., Paul M.S., Sivakumar S., Paneerselvam P. Biomed. Pharmacother. 2008;62:454–461. [Google Scholar]
- 13.Alagarsamy V., Muthukumar V., Pavalarani N., Vasanthanathan P., Revathi R. Biol. Pharm. Bull. 2003;26:557–559. doi: 10.1248/bpb.26.557. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava S.K., Kumar V., Agarwal S.K., Mukherjee R., Burman A.C. Med. Chem. 2009;9:246–275. doi: 10.2174/1871520610909030246. [DOI] [PubMed] [Google Scholar]
- 15.Alagarsamy V., Sharma H., Parthiban P., Singh J.C., Murugan S.T., Solomon V.R. Pharmazie. 2009;64:5–9. [PubMed] [Google Scholar]
- 16.Kumar P., Dhawan K.N., Vrat S., Bhargava K.P., Kishore K. Arch. Pharm. 2006;316:759–763. doi: 10.1002/ardp.19833160906. [DOI] [PubMed] [Google Scholar]
- 17.Deep O.A., Alafeefy A.M. World Appl. Sci. J. 2008;5:94–99. [Google Scholar]
- 18.Raghavendra N.M., Gurubasavarajaswamy P., Nagaranavile K.S., Parameshwaran T. Arch. Pharm. Res. 2009;32:431–436. doi: 10.1007/s12272-009-1317-8. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava M., Salahuddin M.D., Shantakumar S.M. E. J. Chem. 2009;6:1055–1062. [Google Scholar]
- 20.Anjani K.T., Vinay K.S., Aruna B., Gauri S., Sweta S., Anil K.M. Eur. J. Med. Chem. 2007;42:1234–1238. [Google Scholar]


