Abstract
Influenza is an acute respiratory disease and a major health problem worldwide. Since mucosal immunity plays a critical role in protection against influenza virus infection, mucosal immunization is considered a promising vaccination route. However, except for live-attenuated vaccines, there are no effective killed or recombinant mucosal influenza vaccines to date. Outer membrane vesicles (OMVs) are nano-sized vesicles produced by gram-negative bacteria, and contain various bacterial components capable of stimulating the immune system of the host. We generated an OMV with low endotoxicity (fmOMV) by modifying the structure of the lipid A moiety of lipopolysaccharide and investigated its effect as an intranasal vaccine adjuvant in an influenza vaccine model. In this model, fmOMV exhibited reduced toll-like receptor 4-stimulating activity and attenuated endotoxicity compared to that of native OMV. Intranasal injection of the vaccine antigen with fmOMV significantly increased systemic antibody and T cell responses, mucosal IgA levels, and the frequency of lung-resident influenza-specific T cells. In addition, the number of antigen-bearing CD103+ dendritic cells in the mediastinal lymph nodes was significantly increased after fmOMV co-administration. Notably, the mice co-immunized with fmOMV showed a significantly higher protection rate against challenge with a lethal dose of homologous or heterologous influenza viruses without adverse effects. These results show the potential of fmOMV as an effective mucosal adjuvant for intranasal vaccines.
Keywords: Outer membrane vesicles, Influenza, Intranasal, Vaccine, Adjuvant, CD103+ dendritic cells
1. Introduction
Influenza is an acute respiratory disease caused mainly by influenza A and B viruses and has been a major health problem, worldwide. Due to the segmented RNA genome structure, the viruses frequently and constantly alter their antigenic characteristics, and consequently change their infectivity and pathogenicity. To cope with these diverse strains or subtypes, influenza vaccines need to induce cross-reactive immune responses capable of covering a wide range of subtypes. The use of adjuvants, such as alum and MF59, improves the potency of the vaccine in terms of breadth and the magnitude of immune responses to the vaccine antigens [1], [2]. Therefore, efficacious adjuvants could be a breakthrough for the development of a ‘universal’ influenza vaccine.
Mucosal immunization has been considered a promising route of the vaccine delivery because it efficiently induces strong mucosal immunity, resulting in a more efficient defense against mucosal infections compared to a systemic immune response [3], [4]. Among diverse mucosal routes, intranasal delivery is particularly advantageous in eliciting the strongest respiratory immune response, which plays a critical role in the protection against respiratory infections such as influenza [5]. Two intranasal influenza vaccines, FluMist and NASOVAC, are currently available and both consist of attenuated-live viruses. In addition to these licensed vaccines, many studies have revealed the possibility of protein-based intranasal vaccination against respiratory pathogens such as Streptococcus pneumoniae and respiratory syncytial virus [6], [7]. However, no approved intranasal adjuvant, capable of enhancing the immunogenicity of protein-based or killed-virus vaccine antigens, has been developed to date.
Outer membrane vesicles (OMVs), which are naturally produced nano-sized vesicles from Gram-negative bacteria, contain various bacterial components such as lipopolysaccharide (LPS), lipoproteins, flagellin monomers, and bacterial DNA fragments [8]. Due to the nature of these components, OMVs can stimulate the host immune system through innate immune receptors, including toll-like receptors (TLRs) and NOD-like receptors (NLRs) [9]. In recent studies, intramuscular injection of OMV with irrelevant antigens enhanced antigen-specific humoral and cellular immune responses, and increased the protection rate against tumor and virus challenges [10], [11]. However, in order to use OMVs as vaccine adjuvants or delivery vehicles, the safety of this system must be addressed because LPS in OMVs may excessively provoke innate immune responses and lead to endotoxicity.
In this study, we generated a novel OMV with attenuated endotoxicity (fmOMV) by modifying the structure of the lipid A moiety of LPS and investigated the safety and efficacy of fmOMV as a mucosal vaccine adjuvant using an influenza vaccine model. fmOMV exhibited attenuated endotoxicity compared with native OMV (nOMV), and intranasal injection of vaccine antigens with fmOMV significantly enhanced both systemic and mucosal immune responses. Furthermore, co-administration of fmOMV provided protective immunity against homologous and heterologous virus challenge, suggesting the potential of fmOMV as an effective mucosal adjuvant for intranasal vaccines.
2. Methods
2.1. Modification and purification of OMVs
fmOMV was purified as described previously with slight modifications [12]. Briefly, the Escherichia coli W3110 ΔmsbB/ΔpagP strain [13] was transformed with pWSK29-LpxF plasmid, which encodes lipid A 4′-phosphatase, and cultured in LB broth at 37 °C. The culture broth was filtered using a 0.22-μm pore-sized filter (Merck, NJ) and precipitated in a 390 g/l ammonium sulfate solution. After resuspending the pellets, the suspension was centrifuged again at 16,000g. The crude fraction was further purified by performing sucrose-gradient ultracentrifugation. nOMV was similarly prepared except the transformation procedure.
2.2. Analysis of lipid A
The composition of lipid A on fmOMV was analyzed as described previously [14]. Briefly, cultured E. coli cells were incubated in the presence of 5 μCi/ml of 32Pi at 37 °C for 3 h. After collecting and washing the cells by centrifugation, the pellet was dissolved in a chloroform/methanol/water (1:2:0.8, v/v) solution. The insoluble fraction was collected and hydrolyzed in 12.5 mM sodium acetate (pH 4.5) containing 1% SDS at 100 °C for 30 min. A mixture of methanol and chloroform was added to make the ratio of chloroform/methanol/water 2:2:1.8 (v/v). The lower phase was dried and then 1000 cpm of the sample was run on a Silica Gel 60 TLC plate. The plate was visualized using an FLA-7000 image analyzer (Fujifilm, Tokyo, Japan).
2.3. TLR signaling assay
HEK-Blue™ cell lines expressing mouse TLR2, TLR4, or TLR5 (InvivoGen, San Diego, CA, USA) were cultured in RPMI1640 media (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; GE Healthcare, Little Chalfont, UK) and 1X antibiotics (Life Technologies). After resuspending 5 × 104 cells in HEK-BlueTM Detection media (Life Technologies), each cell line was treated with nOMV, fmOMV, or control reagents; Pam3Cys-Ser-(Lys)4 (Pam3; Merck Millipore, Billerica, MA, USA), LPS (InvivoGen), or flagellin (InvivoGen). After 24-h incubation, the activity of secreted alkaline phosphatase was determined.
2.4. Mice
Six- to eight-week-old C57BL/6 female mice were purchased from KOATECH (Korea) and kept in a specific pathogen-free, biosafety level-2 facility at Korea Research Institute of Bioscience and Biotechnology (KRIBB). All animals were treated in accordance with the guidelines established by the Institutional Animal Use and Care Committee of KRIBB.
2.5. Viruses
Influenza A/California/04/2009 (pandemic H1N1, pH1N1), influenza A/Puerto Rico/8/1934 (H1N1, PR8) and influenza A/aquatic bird/Korea/CN2-MA/2009 (H5N2) viruses were cultivated in the allantoic cavities of embryonated chicken eggs. Viruses were titrated by calculating the 50% egg infectious dose (EID50) and stored at −80 °C until use.
2.6. Immunization and challenge
Mice were immunized intranasally with the trivalent split influenza vaccine antigen containing A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Massachusetts/2/2012 (0.8 μg of each subtype HA/mouse, Green Cross, Korea) twice at a two-week interval. Purified fmOMV (1 to 10 μg/head) or cholera toxin (CT; List Biological Laboratories, CA) was mixed with the vaccine antigen immediately before injection. The total injection volume was adjusted to 30 μl/mouse by using PBS. Two weeks after the second injection, the mice were challenged with a 10 LD50 of pH1N1, PR8, or H5N2 influenza virus. The body weight and mortality rates were monitored for two weeks. A humane endpoint of 20% weight loss was used for this challenge study.
2.7. Antigen uptake and flow cytometry
DQ™ Ovalbumin (DQ-OVA) (40 μg/head; ThermoFisher Scientific, MA) was delivered intranasally into the lungs in the presence or absence of fmOMV (3 μg/head). After 24 h, mediastinal lymph node (mLN) cells were resuspended in FACS buffer (PBS containing 0.1% bovine serum albumin and 0.01% sodium azide) and incubated with Fc-block (anti-CD16/CD32; eBioscience, CA). After washing, the cells were stained with fluorescence dye-conjugated anti-CD11b, CD11c, Gr-1, CD80, and CD103 antibodies (eBioscience). Samples were acquired on Gallios™ (Beckman Coulter, CA) and data were analyzed using FlowJo software (Tree Star, OH).
2.8. Enzyme linked-immunosorbent assay (ELISA)
Two and four weeks after the first immunization, serum and bronchoalveolar lavage fluid (BALF) samples were analyzed for antigen (Ag)-specific IgG and IgA by enzyme linked-immunosorbent assay. ELISA plates (ThermoFisher Scientific) were coated with vaccine antigen (200 ng/well) and then incubated with the samples. After sequential incubation with peroxidase goat anti-mouse total IgG and IgA (Cell Signaling Technology, MA), 3,3′,5,5′-tetramethylbenzidine substrate (BD Bioscience, CA) was added to each well. The optical density was measured at a 450 nm wavelength by using VICTOR3™ (PerkinElmer, MA). To determine the HA stalk-specific Abs, HA419-473 from PR8 and HA379-473 from pH1N1 polypeptides were expressed in E. coli and used as coating antigens in a subsequent ELISA.
2.9. Hemagglutination inhibition assay
For the hemagglutination inhibition (HI) assay, sera were pre-treated with receptor-destroying enzyme (RDE; Denka Seiken, Japan) and diluted with PBS. The pH1N1 virus was diluted with 4-HA unit and then mixed with RDE-treated sera for 30 min in U-bottom 96-well plates (SPL Life Sciences, South Korea). A chicken red blood cell suspension (0.5%) was added to each well. The test endpoint was determined by visual inspection for an agglutination reaction.
2.10. Enzyme-linked immunospot (ELISPOT) assay
The frequency of influenza-specific IFN-γ-producing cells was determined using a mouse IFN-γ ELISPOT set (BD Biosciences). Briefly, total splenocytes (5 × 105 cells/well) were stimulated with inactivated pH1N1 virus on ELISPOT plates coated with IFN-γ capture antibody. Forty hours later, the plates were washed and sequentially incubated with biotinylated IFN-γ detection antibody and HRP-conjugated streptavidin. After adding AEC (3-amino-9-ethyl-carbazole) substrate, spots were counted using the BioSpot analyzer (Cellular Technology, OH).
2.11. Virus titration
Viral RNAs were extracted from the lung homogenate mixtures using the RNeasy Mini Kit (QIAGEN, CA) following the manufacturer’s protocol. Real time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using the QuantiTect Probe RT-PCR Kit (QIAGEN) and the Roche Lightcycler 96 system. The matrix (M) gene-specific primer (forward: 5′-GACCRATCCTGTCACCTCTGAC-3′; reverse: 5′-AGGGCATTYTGGACAAAKCGTCTA-3′) and specific probe (5′-FAM-TGCAGTCCTCGCTCACTGGGCACG-BHQ-1-3′) were used.
2.12. Statistical analysis
Statistical differences among groups were assessed using a two-tailed Student’s t-test or a log-rank test with Prism software (GraphPad Software, CA). p < 0.05 was considered to be statistically significant.
3. Results
3.1. fmOMV presents attenuated TLR4-stimulating activity and lower endotoxicity compared with nOMV
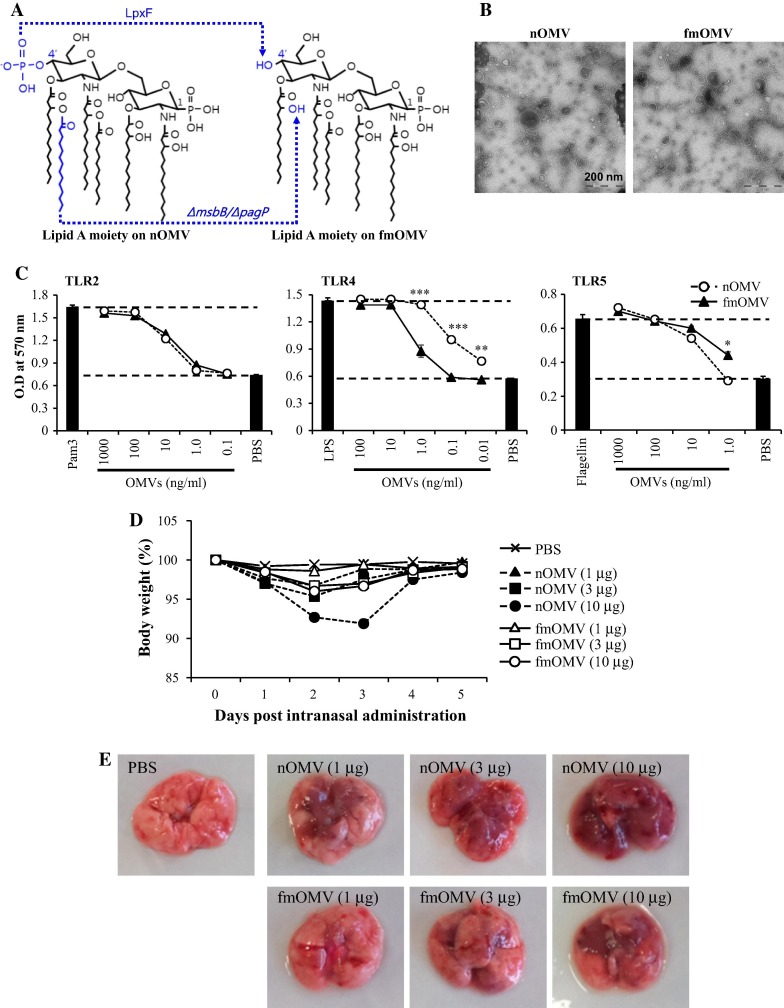
The endotoxicity of LPS is highly related to the structure of the lipid A moiety, particularly the degree of acylation [15], [16]. Previously, we generated an E. coli W3110 ΔmsbB/ΔpagP strain that produces LPS, on which lipid A is penta-acylated [13]. In order to further attenuate the endotoxicity of E. coli LPS in this study, we introduced lpxF, a lipid A 4′-phosphatase from Francisella novicida (Fig.1 A) [17], [18]. The size of purified fmOMV was similar to that of nOMV, ranging from 50 to 150 nm in diameter and its morphology was spherical (Table 1 and Fig.1B). In addition, both OMVs had similar zeta potential values of approximately −13.5 mV (Table 1). Interestingly, when fmOMV was tested for the activation of diverse TLRs in vitro, it triggered significantly reduced TLR4 signaling compared to that induced by nOMV (p < 0.001–0.005), whereas both OMVs activated TLR2 and TLR5 to a similar extent (Fig.1C). In agreement with this in vitro result, fmOMV showed significantly lower endotoxicity than nOMV following delivery into mice. While nOMV resulted in significant weight loss and red consolidation in the lung tissue 2 or 3 days after intranasal administration, fmOMV caused milder weight loss and inflammation in the lungs (Fig.1D and E). Notably, 1 μg of fmOMV did not cause body weight loss or pulmonary inflammation. These data indicate that fmOMV carrying penta-acylated and mono-phosphorylated lipid A species reduced TLR4-stimulating activity in vitro and significantly attenuated endotoxicity in vivo compared with nOMV.
Fig. 1.
Modified outer membrane vesicle (fmOMV) presents attenuated TLR4-stimulating activity and lower endotoxicity compared with native OMV (nOMV). (A) Structure of lipid A on nOMV and fmOMV. (B) Morphology of purified fmOMV evaluated by electron microscopy. HEK293 cell lines expressing TLR2, TLR4, or TLR5 were cultured with indicated amounts of OMVs for 24 h. The extent of TLR stimulation was determined by measuring secreted embryonic alkaline phosphatase (SEAP) activity. Data are presented as the mean ± standard deviation (SD) from triplicate culture wells at an optical density (OD) of 570 nm. ∗∗∗p < 0.001, ∗∗p < 0.01 (C). After the mice were injected intranasally with the indicated amount of OMVs, body weight changes were monitored for 5 days (n = 5) (D) or macroscopic lung inflammation was observed on day 3 (n = 3–4) (E).
Table 1.
Analysis of OMVs.
| Vesicle size (mean diameter; nm) | Zeta potential (mV) | |
|---|---|---|
| nOMV | 108 ± 70 | −15.8 |
| fmOMV | 119 ± 65 | −11.4 |
3.2. Co-administration of fmOMV increases systemic antibody and T cell responses
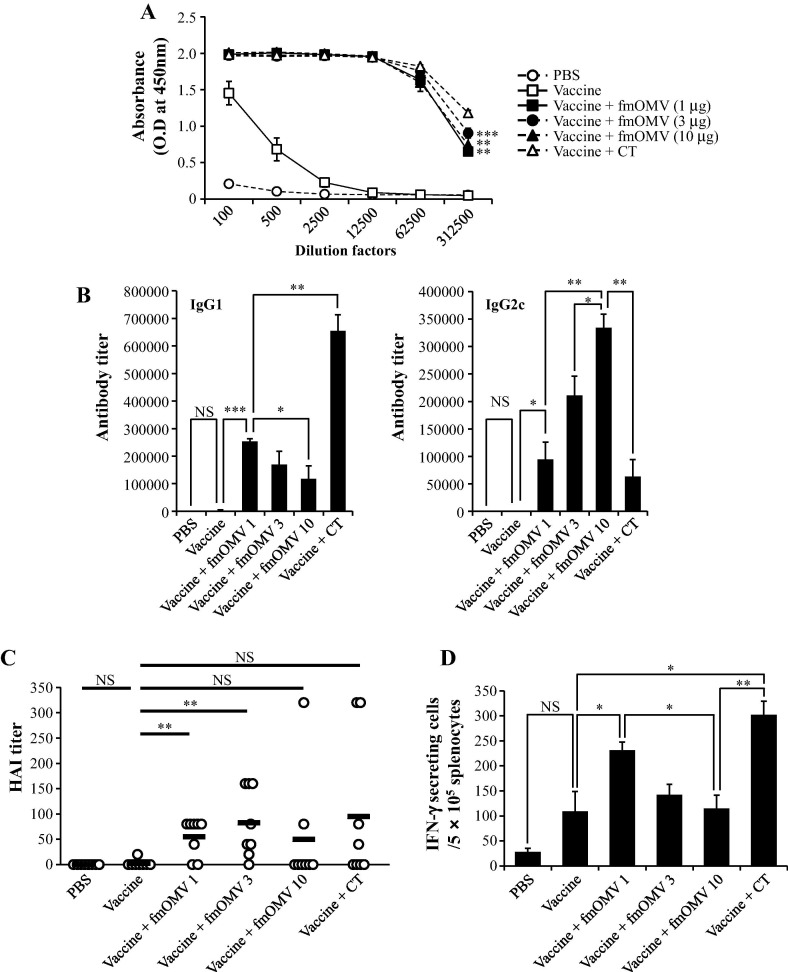
Previously, it has been shown that co-immunization of OMV increased the antigen-specific immune response to co-delivered antigens in intramuscular vaccination models [11], [13]. To investigate whether the intranasal delivery of fmOMV effectively induces strong antigen-specific immune responses, we injected influenza vaccine antigens with various doses of fmOMV (1.0–10 μg) intranasally. Two weeks after the second immunization, the influenza-specific IgG response was significantly higher in all three groups that were administered with fmOMV compared to that of the vaccine-alone group (p = 0.0023, 0.0009, and 0.0018, respectively) and was comparable to that of the CT-treated group (p = 0.674, Fig.2 A). The antibody titers of both IgG1 and IgG2c also significantly increased in mice co-injected with fmOMV and the vaccine (p < 0.001–0.05, Fig.2B). IgG1 and IgG2c titers exhibited an inverse relationship with escalating fmOMV dose; although the IgG1 titer decreased (Fig.2B, left panel), the IgG2c titer increased (Fig.2B, right panel). Consistent with the serum IgG response, HI activity against pH1N1 virus was significantly higher in fmOMV-co-immunized groups (1.0 and 3.0 μg of fmOMV) compared to that of the vaccine alone-immunized group (Fig.2C). Since cell-mediated adaptive immunity also contributes to protection against influenza infection [2], [19], we measured the frequency of the influenza-specific IFN-γ-secreting T cells. Notably, only 1.0 μg of fmOMV significantly enhanced the antigen-specific T cell response, whereas the T cell response in the 3 and 10 μg-fmOMV-injected groups were comparable to that of vaccine-alone injected group (Fig.2D). Collectively, intranasal administration of fmOMV was shown to enhance systemic humoral and cellular responses to an influenza vaccine.
Fig. 2.
Co-administration of fmOMV increases systemic antibody and T cell responses. Mice (n = 8) were immunized intranasally with a seasonal influenza vaccine in the presence or absence of OMV (1–10 μg). Two weeks after the booster injection, an IgG ELISA (A) and a titration of IgG1 and IgG2c (B) were performed against the vaccine antigen. HI assay against pH1N1 virus were performed on serum samples (C). An IFN-γ ELISPOT assay against UV-inactivated pH1N1 virus was performed with total splenocytes (D). Data are representative of three independent experiments with similar results and presented as the mean ± standard error of the mean (SEM). ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
3.3. Co-administration of fmOMV activates respiratory CD103+ DCs and increases local antibody and T cell responses in the lungs
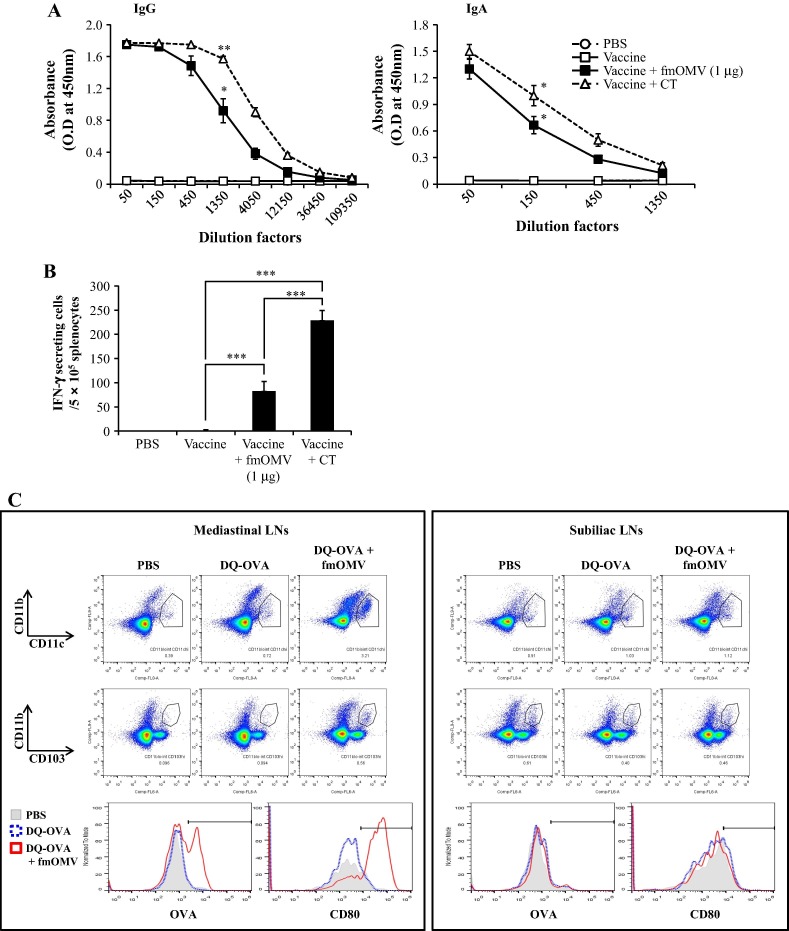
To verify that co-administration of fmOMV modulates immune responses in the respiratory mucosa, the level of influenza-specific antibodies in the BALF samples was determined. The dose of fmOMV was fixed to 1 μg because this amount of fmOMV was sufficient to increase the levels of antibody and T cell responses without resulting in toxicity in vivo (Figs. 1B–D and 2). Consistent with serum IgG levels (Fig.2A), fmOMV significantly increased antigen-specific IgG levels in the BALF (p = 0.0105, vs. vaccine alone, Fig.3 A, left panel). In addition, secretory IgA in the BALF, which plays an important role in the protection against mucosal infection [20], dramatically increased in the fmOMV-injected group compared with that of the vaccine-alone immunized group (Fig.3A, right panel). fmOMV co-administration also significantly enhanced influenza-specific T cell responses in the lungs (p = 0.0015, Fig.3B). Since fmOMVs contain diverse TLR ligands such as LPS and lipoproteins, which can stimulate antigen-presenting cells (APCs) [8], we examined the change in DCs in mLNs after treatment with fmOMV and DQ-OVA model antigen. The DC population in the mLNs dramatically increased after intranasal administration of DQ-OVA + fmOMV as compared to that of DQ-OVA treatment alone (3.21 vs. 0.72%, Fig.3C, left panel). This indicates active migration of DCs from the tissue to the draining LNs by fmOMV administration. In addition to the increase in number, the DCs in the mLNs exhibited high levels of DQ-OVA signal or antigen uptake and up-regulation of CD80 (Fig.3C, left panel). CD103+ DCs are critical for the induction of immune responses in the respiratory tract [21], [22]. The CD103+ population in CD11blo/intCD11chi lung cells increased more than 2-fold through fmOMV administration (53% and 22% in fmOMV-co-injected and DQ-OVA alone-injected groups, respectively, Fig.3C, left panel). The activated phenotype of DCs was not observed in the non-draining subiliac LNs (Fig.3C, right panel), indicating that fmOMV affected DCs at the local delivery site. These data show that intranasal vaccination with fmOMV activates CD103+ DCs in the lungs and enhances both antibody and T cell responses in the respiratory mucosa.
Fig. 3.
Co-administration of fmOMV activates respiratory CD103 + DCs and increases local antibody and T cell responses in the lungs. Mice (n = 6) were immunized intranasally with a seasonal influenza vaccine in the presence or absence of OMV (1 μg). Two weeks after the booster injection, IgG and IgA ELISAs against the vaccine antigen were performed with BALF samples (A). An IFN-γ ELISPOT assay against UV-inactivated pH1N1 virus was performed with total lung cells (B). Twenty-four hours after intranasal injection of DQ-OVA, mediastinal and subiliac LNs were harvested and the cells were analyzed for the expression of the indicated markers using flow cytometry (C). Data are representative of three independent experiments with similar results (A–C) and presented as the mean ± SEM (A and B). ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
3.4. Co-administration of fmOMV provides strong protective immunity against homologous and heterologous influenza virus challenge
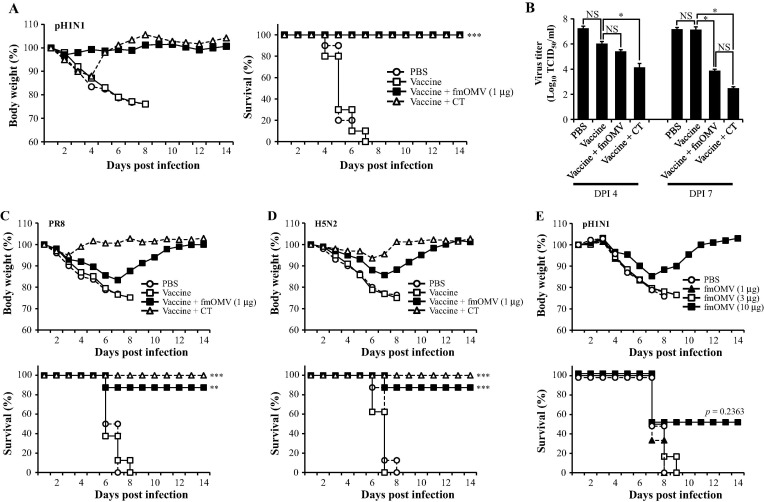
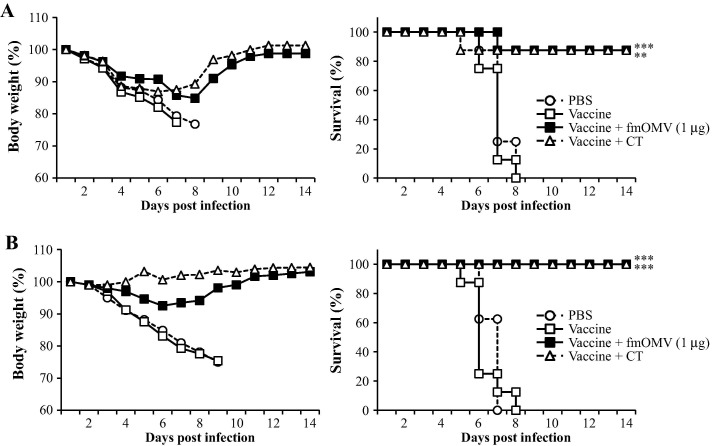
Given that fmOMV efficiently elicit both mucosal and systemic antigen-specific immune responses, we next examined the protective efficacy of vaccination with fmOMV by challenging mice with a lethal dose of influenza virus. When a 10LD50 dose of pH1N1 virus was intranasally administered, the fmOMV-co-immunized mice did not lose body weight and demonstrated a 90–100% survival rate for all three groups (p = 0.0001 and 0.0008, Fig.4 A). Lung virus titer was also significantly reduced by vaccination with fmOMV (p = 0.07084 and 0.00037 on DPI 4 and 7, respectively, Fig.4B). To verify that fmOMV-co-immunization conferred cross-protective immunity, a requirement for influenza vaccines, we challenged the mice with PR8 and H5N2 viruses and monitored the body weight loss and survival rate. Although the body weight decreased by about 15% up to day 7 after the challenge with PR8 virus, the mice showed a gradual recovery in body weight and exhibited a 87.5% survival rate (p = 0.0055, Fig.4C). Similarly, when the mice were challenged with H5N2 virus, the fmOMV-co-immunized group exhibited a significant increase in the survival rate (87.5%, p = 0.0008, Fig.4D). Even though fmOMV significantly enhanced influenza-specific adaptive immune responses and protective immunity against viral infection, it is possible that fmOMV contributed to the protective efficacy of the vaccine by inducing innate immune responses in the respiratory tract [23], [24], [25], [26]. To verify if innate immunity contributed to the observed protective efficacy, mice were injected with different doses of fmOMV alone (ranging from 1 to 10 μg) in the absence of an influenza vaccine antigen and challenged 2 weeks later with 10 LD50 of pH1N1 virus. From day 3 post infection, all mice began to lose body weight irrespective of the fmOMV dose, which resulted in most of them dying between day 7 and 8 post infection (Fig.4E). Survivors were only detected in the group given 10 μg of fmOMV for which a 50% survival rate was observed. However, the survival rate of this group was not statistically significant when compared with that of the PBS group (p = 0.2363), indicating that innate immunity is not involved in the protective efficacy observed after co-administration of fmOMV as an adjuvant. Therefore, these data, together with the previous results showing enhanced adaptive immune responses (Fig. 2, Fig. 3), demonstrate that vaccination with fmOMV provided broad protective efficacy against homologous and heterologous influenza viruses based on adaptive immune responses.
Fig. 4.
Co-administration of fmOMV provides strong protective immunity against homologous and heterologous influenza virus challenge. Two-weeks after the booster immunization, the mice were challenged with homologous (pH1N1) (n = 10, A), hetero-strain (PR8) (n = 8, C), and hetero-subtype (H5N2) (n = 8, D) viruses. Four and seven days after the pH1N1 challenge, the virus titer in the lungs was determined (n = 4 for each time point, B). Two-weeks after intranasal injection with fmOMV alone (ranging from 1 to 10 μg), mice were challenged with pH1N1 virus (n = 6, E). Data are representative of two independent experiments with similar results (A–C) and presented as the mean ± SEM (B). ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
3.5. Co-administration of fmOMV provides both immediate and long-lasting protective immunity
Since influenza viruses constantly change their antigenic characteristics, some mutants or new variants may be highly infectious and transmissible as exemplified by pH1N1 in 2009 [27], [28]. For the control of these highly infectious variants or prevention of mass infection, influenza vaccines need to provide immediate protective immunity and long-term memory immune responses. To check whether the fmOMV-adjuvanted vaccine meets these requirements, we first vaccinated the mice once, and challenged them with a lethal dose of pH1N1 virus 2 weeks later. Vaccination with fmOMV significantly increased the survival rate, whereas vaccine alone failed to protect the mice (p = 0.0004, Fig.5 A). In terms of long-term efficacy, the mice were challenged with a lethal dose of influenza virus (10LD50), 20 weeks after the booster immunization. The fmOMV-co-immunized group exhibited a similar survival rate compared to that of the CT-co-immunized group (Fig.5B); indicating that co-administration of fmOMV conferred prolonged protective immunity. These data show that intranasal vaccination with fmOMV induces both immediate and prolonged protective immunity, suggesting that fmOMV could be a useful vaccine adjuvant in a pandemic situation.
Fig. 5.
Co-administration of fmOMV provides both immediate and long-lasting protective immunity. Two weeks after the prime immunization (n = 8, A) or 20 weeks after the booster immunization (n = 8, B), the mice were challenged with pH1N1 virus and body weight changes and survival rates were monitored for 2 weeks. Data are representative of two independent experiments with similar results. ∗∗∗p < 0.001, ∗∗p < 0.01.
4. Discussion
With the constant emergence of fatal respiratory viral diseases, such as the highly pathogenic influenza and Middle East respiratory syndrome, the requirement for efficacious vaccines is increasing. Owing to its efficacy and feasibility, intranasal administration is an attractive method to deliver vaccines against respiratory viruses as exemplified by influenza vaccine. [5], [29], [30]. Therefore, it is a promising approach to develop a novel mucosal adjuvant that can potentiate the vaccine-induced immunity in the respiratory tract. In this study, we demonstrated the effect of fmOMV as an intranasal vaccine adjuvant using the influenza vaccine system.
OMVs show potential as vaccine adjuvants because of their immunostimulatory activity. In terms of safety, however, native OMVs cannot be applied for vaccination because they contain fully endotoxic LPS, which induces excessive immune activation and inflammation [31]. The endotoxicity of LPS is closely related to the degree of acylation of the lipid A moiety [15], [16], [32]. Therefore, in this study, we generated an msbB- and pagP-deletion strain (Fig.1A) lacking the acyltransferase and palmitoyltransferase required for the maximum acylation of lipid A. In addition, we introduced the lpxF gene, which encodes lipid A 4′-phosphatase, to further attenuate the endotoxicity of LPS by generating mono-phosphoryl lipid A (Fig.1A) [17]. As expected, fmOMV produced from the genetically engineered E. coli showed much greater attenuation than nOMV in terms of TLR4-stimulating activity in vitro and toxicity in vivo (Fig. 1C–E). This approach agrees well with the development of the mono-phosphoryl lipid A adjuvant for wide range of human vaccines [33].
Lung-residential DCs, in particular CD103+ DCs, play a pivotal role in the induction of mucosal and systemic immune responses against an influenza infection [21], [34], [35]. These DCs take up viral antigens and migrate to draining LNs where they initiate adaptive immune responses. In this study, we observed a substantial increase in the CD11bloCD11chiCD103+ population in the mLNs after 24 h of fmOMV treatment (Fig.3C). The DCs from fmOMV-injected mice also showed an increased level of activation markers and antigen uptake signals, suggesting that fmOMV stimulated CD103+ DCs in the lungs, leading to an enhanced adaptive immune response and consequently an increase in vaccine efficacy. The mechanism by which fmOMV modulates the function of APCs in vitro and in vivo is currently under investigation.
In this study, we observed increased protective immunity against heterologous influenza viral infection in the fmOMV-injected group compared to that in the group immunized with vaccine alone (Fig.4C and D). Many adjuvants or vaccines targeting T cell responses have been shown to induce cross-reactive T cell responses and provide protective immunity to heterologous influenza infection [36], [37], [38], [39]. In addition to T cell responses, broad-specific neutralizing antibodies are known to be cross-protective [40], [41]. Serum samples from fmOMV-co-immunized mice weakly bound to HA-stalk antigens in ELISA, suggesting that fmOMV did not efficiently induce an HA stalk-specific antibody response (data not shown). Because the IFN-γ response was significantly increased in the fmOMV-infected group (Figs. 2D and 3B), it appears that the enhanced T cell response mainly contributed to the increased survival rate in the fmOMV-co-immunized group. Even though the differences in survival rates between the groups challenged with heterologous viruses (PR8 and H5N2) and the one challenged with homologous virus (pH1N1) were not statistically significant (87.5% vs. 100%, respectively; p = 0.3173), body weight in the groups receiving a heterologous virus challenge tended to decline more during the early phase of infection when compared with the group receiving a homologous virus challenge (Fig.4C and D, upper panels vs. Fig.4A, left panel). These observations suggest that a cross-reactive T cell response, rather than a cross-reactive neutralizing antibody response, contributed to the clearance of virus presumably by removing infected cells during the late phase of infection.
The CT-injected group clearly showed a reverse correlation between IgG1 and IgG2c titers (Fig.2B), and this observation agrees well with the fact that CT is a potent Th2-biased adjuvant [42], [43], [44]. fmOMV simultaneously increased IgG2c and decreased IgG1 titers, indicating that fmOMV promotes Th1-dominant immune responses (Fig.2B). This observation can be further supported by the fact that fmOMV has strong Th1-stimulating components such as LPS, flagellin, and bacterial DNA fragments containing CpG motifs [8], [9]. Nevertheless, it was unexpected that increasing the dose of fmOMV down-regulated the antigen-specific IFN-γ response (Fig.2D). One possible explanation for this is that over-activation of APCs, especially DCs, may negatively regulate antigen-specific T cell responses by secreting IFN-γ and, in turn, induce the expression of indoleamine-2,3-dioxygenase (IDO). Although IDO is well known to suppress T cell responses by depleting tryptophan [45], [46], B cell responses appear not to be as sensitive to this mechanism as T cell responses. Rather, IDO has shown an activating role during Th2-mediated inflammatory responses [47], which correlates well with our observation that T cell responses decreased with increasing fmOMV doses whereas antibody responses remained high (Fig.2A and D). Further study is required to assess the underlying mechanism of this dose-dependent inverse correlation between fmOMV dose and T cell response.
To summarize, we generated a novel OMV harboring modified lipid A (fmOMV) by manipulating genes related to the acylation and phosphorylation of lipid A in E. coli and evaluated its safety and efficacy as an intranasal vaccine adjuvant in a murine influenza model. Co-administration of fmOMV provided broad protection against heterologous virus challenge, and conferred both immediate and prolonged immunity. These results show the potential of fmOMV as an intranasal vaccine adjuvant for the preparedness against an influenza pandemic.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by grants of the KRIBB Initiative program (KGM4691612) and by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (grant number: 2016M3A9B6918675). Work in SH Kim’s laboratory was supported by the fund for the New Professor Research Foundation Program of Gyeongsang National University, 2015.
References
- 1.Carter D., Reed S.G. Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS. 2010;5:409–413. doi: 10.1097/COH.0b013e32833d2cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Hagan D.T., Rappuoli R., De Gregorio E., Tsai T., Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011;10:447–462. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol Invest. 2010;39:303–355. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- 4.Neutra M.R., Kozlowski P.A. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 5.Carter N.J., Curran M.P. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71:1591–1622. doi: 10.2165/11206860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Briles D.E., Ades E., Paton J.C., Sampson J.S., Carlone G.M., Huebner R.C. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68:796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigter A., Widjaja I., Versantvoort H., Coenjaerts F.E., van Roosmalen M., Leenhouts K. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS ONE. 2013;8:e71072. doi: 10.1371/journal.pone.0071072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuehn M.J., Kesty N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 9.Kaparakis-Liaskos M., Ferrero R.L. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 10.Kim O.Y., Hong B.S., Park K.S., Yoon Y.J., Choi S.J., Lee W.H. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2013;190:4092–4102. doi: 10.4049/jimmunol.1200742. [DOI] [PubMed] [Google Scholar]
- 11.Lee B.J., Kwon H.I., Kim E.H., Park S.J., Lee S.H., Choi Y.K. Assessment of mOMV adjuvant efficacy in the pathogenic H1N1 influenza virus vaccine. Clin Exp Vaccine Res. 2014;3:194–201. doi: 10.7774/cevr.2014.3.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.H., Kim K.S., Lee S.R., Kim E., Kim M.S., Lee E.Y. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochim Biophys Acta. 2009;1788:2150–2159. doi: 10.1016/j.bbamem.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D.H., Kim S.H., Kang W., Choi Y.S., Lee S.H., Lee S.R. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011;29:8293–8301. doi: 10.1016/j.vaccine.2011.08.102. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z., Lin S., Cotter R.J., Raetz C.R. Lipid A modifications characteristic of Salmonella typhimurium are induced by NH4VO3 in Escherichia coli K12. Detection of 4-amino-4-deoxy-l-arabinose, phosphoethanolamine and palmitate. J Biol Chem. 1999;274:18503–18514. doi: 10.1074/jbc.274.26.18503. [DOI] [PubMed] [Google Scholar]
- 15.Ranallo R.T., Kaminski R.W., George T., Kordis A.A., Chen Q., Szabo K. Virulence, inflammatory potential, and adaptive immunity induced by Shigella flexneri msbB mutants. Infect Immun. 2010;78:400–412. doi: 10.1128/IAI.00533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teghanemt A., Zhang D., Levis E.N., Weiss J.P., Gioannini T.L. Molecular basis of reduced potency of underacylated endotoxins. J Immunol. 2005;175:4669–4676. doi: 10.4049/jimmunol.175.7.4669. [DOI] [PubMed] [Google Scholar]
- 17.Phillips N.J., Schilling B., McLendon M.K., Apicella M.A., Gibson B.W. Novel modification of lipid A of Francisella tularensis. Infect Immun. 2004;72:5340–5348. doi: 10.1128/IAI.72.9.5340-5348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., McGrath S.C., Cotter R.J., Raetz C.R. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J Biol Chem. 2006;281:9321–9330. doi: 10.1074/jbc.M600435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taliansky M., Kim S.H., Mayo M.A., Kalinina N.O., Fraser G., McGeachy K.D. Escape of a plant virus from amplicon-mediated RNA silencing is associated with biotic or abiotic stress. Plant J: Cell Mol Biol. 2004;39:194–205. doi: 10.1111/j.1365-313X.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 20.van Riet E., Ainai A., Suzuki T., Hasegawa H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine. 2012;30:5893–5900. doi: 10.1016/j.vaccine.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 21.Ho A.W., Prabhu N., Betts R.J., Ge M.Q., Dai X., Hutchinson P.E. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J Immunol. 2011;187:6011–6021. doi: 10.4049/jimmunol.1100987. [DOI] [PubMed] [Google Scholar]
- 22.Waithman J., Zanker D., Xiao K., Oveissi S., Wylie B., Ng R. Resident CD8(+) and migratory CD103(+) dendritic cells control CD8 T cell immunity during acute influenza infection. PLoS ONE. 2013;8:e66136. doi: 10.1371/journal.pone.0066136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul-Careem M.F., Firoz Mian M., Gillgrass A.E., Chenoweth M.J., Barra N.G., Chan T. FimH, a TLR4 ligand, induces innate antiviral responses in the lung leading to protection against lethal influenza infection in mice. Antiviral Res. 2011;92:346–355. doi: 10.1016/j.antiviral.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Shinya K., Ito M., Makino A., Tanaka M., Miyake K., Eisfeld A.J. The TLR4-TRIF pathway protects against H5N1 influenza virus infection. J Virol. 2012;86:19–24. doi: 10.1128/JVI.06168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinya K., Okamura T., Sueta S., Kasai N., Tanaka M., Ginting T.E. Toll-like receptor pre-stimulation protects mice against lethal infection with highly pathogenic influenza viruses. Virol J. 2011;8:97. doi: 10.1186/1743-422X-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan A.C., Mifsud E.J., Zeng W., Edenborough K., McVernon J., Brown L.E. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm. 2012;9:2710–2718. doi: 10.1021/mp300257x. [DOI] [PubMed] [Google Scholar]
- 27.Yen H.L., Liang C.H., Wu C.Y., Forrest H.L., Ferguson A., Choy K.T. Hemagglutinin-neuraminidase balance confers respiratory-droplet transmissibility of the pandemic H1N1 influenza virus in ferrets. Proc Natl Acad Sci USA. 2011;108:14264–14269. doi: 10.1073/pnas.1111000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon S.W., Chen N., Ducatez M.F., McBride R., Barman S., Fabrizio T.P. Changes to the dynamic nature of hemagglutinin and the emergence of the 2009 pandemic H1N1 influenza virus. Sci Rep. 2015;5:12828. doi: 10.1038/srep12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandtzaeg P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am J Respir Crit Care Med. 2011;183:1595–1604. doi: 10.1164/rccm.201011-1783OC. [DOI] [PubMed] [Google Scholar]
- 30.Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010;18:771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 31.Raetz C.R., Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop R.E. The lipid A palmitoyltransferase PagP: molecular mechanisms and role in bacterial pathogenesis. Mol Microbiol. 2005;57:900–912. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 33.Evans J.T., Cluff C.W., Johnson D.A., Lacy M.J., Persing D.H., Baldridge J.R. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi. 529. Expert Rev Vaccines. 2003;2:219–229. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- 34.GeurtsvanKessel C.H., Willart M.A., van Rijt L.S., Muskens F., Kool M., Baas C. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kandasamy M., Ying P.C., Ho A.W., Sumatoh H.R., Schlitzer A., Hughes T.R. Complement mediated signaling on pulmonary CD103(+) dendritic cells is critical for their migratory function in response to influenza infection. PLoS Pathog. 2013;9:e1003115. doi: 10.1371/journal.ppat.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cargnelutti D.E., Sanchez M.V., Mattion N.M., Scodeller E.A. Development of a universal CTL-based vaccine for influenza. Bioengineered. 2013;4:374–378. doi: 10.4161/bioe.23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju Y., Fan H., Liu J., Hu J., Li X., Li C. Heat shock protein gp96 adjuvant induces T cell responses and cross-protection to a split influenza vaccine. Vaccine. 2014;32:2703–2711. doi: 10.1016/j.vaccine.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Soema P.C., van Riet E., Kersten G., Amorij J.P. Development of cross-protective influenza a vaccines based on cellular responses. Front Immunol. 2015;6:237. doi: 10.3389/fimmu.2015.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Sandt C.E., Kreijtz J.H., Geelhoed-Mieras M.M., Vogelzang-van Trierum S.E., Nieuwkoop N.J., van de Vijver D.A. Novel G3/DT adjuvant promotes the induction of protective T cells responses after vaccination with a seasonal trivalent inactivated split-virion influenza vaccine. Vaccine. 2014;32:5614–5623. doi: 10.1016/j.vaccine.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Ekiert D.C., Bhabha G., Elsliger M.A., Friesen R.H., Jongeneelen M., Throsby M. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tharakaraman K., Subramanian V., Cain D., Sasisekharan V., Sasisekharan R. Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure. Cell Host Microbe. 2014;15:644–651. doi: 10.1016/j.chom.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto S., Kiyono H., Yamamoto M., Imaoka K., Fujihashi K., Van Ginkel F.W. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu-Amano J., Kiyono H., Jackson R.J., Staats H.F., Fujihashi K., Burrows P.D. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marinaro M., Staats H.F., Hiroi T., Jackson R.J., Coste M., Boyaka P.N. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 45.Mellor A.L., Munn D.H. Tryptophan catabolism and regulation of adaptive immunity. J Immunol. 2003;170:5809–5813. doi: 10.4049/jimmunol.170.12.5809. [DOI] [PubMed] [Google Scholar]
- 46.Munn D.H., Sharma M.D., Lee J.R., Jhaver K.G., Johnson T.S., Keskin D.B. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 47.Xu H., Oriss T.B., Fei M., Henry A.C., Melgert B.N., Chen L. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci USA. 2008;105:6690–6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]