Highlights
-
•
We investigated risk of acute respiratory illness post-influenza vaccination.
-
•
Post-vaccination risk of non-influenza respiratory pathogen was higher in children.
-
•
Patient perceptions of illness following influenza vaccination may be supported.
-
•
Assessments of potential mechanisms for findings are needed.
Abbreviations: ARI, acute respiratory illness; MoSAIC, Mobile Surveillance of Acute Respiratory Infections and Influenza-Like Illness in the Community; NYP, NewYork-Presbyterian; CIR, Citywide Immunization Registry
Keywords: Influenza vaccine, Influenza, Acute respiratory illness, Belief, Misperceptions
Abstract
Background
A barrier to influenza vaccination is the misperception that the inactivated vaccine can cause influenza. Previous studies have investigated the risk of acute respiratory illness (ARI) after influenza vaccination with conflicting results. We assessed whether there is an increased rate of laboratory-confirmed ARI in post-influenza vaccination periods.
Methods
We conducted a cohort sub-analysis of children and adults in the MoSAIC community surveillance study from 2013 to 2016. Influenza vaccination was confirmed through city or hospital registries. Cases of ARI were ascertained by twice-weekly text messages to household to identify members with ARI symptoms. Nasal swabs were obtained from ill participants and analyzed for respiratory pathogens using multiplex PCR. The primary outcome measure was the hazard ratio of laboratory-confirmed ARI in individuals post-vaccination compared to other time periods during three influenza seasons.
Results
Of the 999 participants, 68.8% were children, 30.2% were adults. Each study season, approximately half received influenza vaccine and one third experienced ≥1 ARI. The hazard of influenza in individuals during the 14-day post-vaccination period was similar to unvaccinated individuals during the same period (HR 0.96, 95% CI [0.60, 1.52]). The hazard of non-influenza respiratory pathogens was higher during the same period (HR 1.65, 95% CI [1.14, 2.38]); when stratified by age the hazard remained higher for children (HR 1·71, 95% CI [1.16, 2.53]) but not for adults (HR 0.88, 95% CI [0.21, 3.69]).
Conclusion
Among children there was an increase in the hazard of ARI caused by non-influenza respiratory pathogens post-influenza vaccination compared to unvaccinated children during the same period. Potential mechanisms for this association warrant further investigation. Future research could investigate whether medical decision-making surrounding influenza vaccination may be improved by acknowledging patient experiences, counseling regarding different types of ARI, and correcting the misperception that all ARI occurring after vaccination are caused by influenza.
1. Introduction
Influenza vaccination is recommended for all individuals 6 months and older who do not have contraindications [1]. However, acceptance of the influenza vaccine has many barriers including low perceived vaccine efficacy, low perceived susceptibility to influenza infection, lack of understanding of the potential for severe illness from influenza, inconvenience, and fear of adverse effects [2], [3], [4]. Parents as well as adult patients have expressed concern that acute respiratory illness (ARI) occurs after the influenza vaccine; in a national survey, 43% of adults held the belief that “the flu vaccine can give you the flu.” [2], [5] Plausible explanations for patient-perceived illness immediately following vaccination include: fever or other side effects induced by inflammatory mediators, influenza infection prior to vaccine-induced immunity, or coincidental infection with other respiratory pathogens [6], [7], [8]. Additionally, immunologic interference related to vaccination may modify the risk of ARI in potentially different ways. For example, lack of viral interference has been hypothesized to increase the risk of ARI after influenza vaccination while temporary non-specific immunity has been hypothesized to decrease the risk of ARI after influenza vaccination [8], [9], [10].
Previous studies have investigated the risk of ARI after influenza vaccination with conflicting results which may reflect variations in study design [9], [10], [11], [12]. For example, some studies included study populations recruited from cases of medically attended illness which may not reliably estimate the population incidence of ARI [11]. Use of a cohort study design comparing vaccinated to unvaccinated individuals may be better, but can also be prone to bias if groups are not well-matched groups and/or unmeasured confounders exist [10]. Additionally, previous investigations of ARI risk after vaccination, lacking a biologically plausible temporal relationship to the vaccine may not accurately estimate risk of ARI attributed to the vaccine itself [9].
The goals of this study were to assess the risk of ARI caused by (1) laboratory-confirmed influenza, (2) laboratory-confirmed non-influenza respiratory pathogens, and (3) ARI symptoms without a pathogen detected (suggestive of non-infectious etiology, such as inflammatory response) in post-vaccination risk periods compared to the other periods during the influenza season. We hypothesized that there would not be an increased risk of influenza, non-influenza respiratory pathogens, or symptoms without pathogen detected in the post-influenza vaccination risk periods. An understanding of the risk of illness in the post-vaccination period may be used to develop targeted anticipatory guidance for parents and adult patients considering influenza vaccination.
2. Methods
2.1. Study design and participants
This cohort study is part of a 5-year community-based study, Mobile Surveillance of Acute Respiratory Infections and Influenza-Like Illness in the Community (MoSAIC) [13], which follows 250 households a year for ARI surveillance. Recruitment, eligibility, and consent procedures for MoSAIC have been previously described [13]. Participants are from a primarily immigrant Latino community in northern Manhattan, New York City. The Columbia University Medical Center Institutional Review Board approved the overall study and this sub-analysis.
The observation period for this sub-analysis consisted of three influenza seasons: September 2013 – June 2014, September 2014 – May 2015, and September 2015 – May 2016. Each season start date was September 1, concurrent with typical availability of influenza vaccines. The end dates of each season varied to capture all cases of laboratory-confirmed influenza in the cohort. For this sub-analysis, children 6 months to 17 years of age were eligible if they had any vaccines recorded in the NewYork-Presbyterian (NYP) Immunization Registry and/or the New York Citywide Immunization Registry (CIR). Children who received two doses of influenza vaccine were excluded from the analysis of the season in which they received two doses due to potential challenges with interpreting events occurring during the partially vaccinated window between vaccinations. Because there is not a citywide registry for adult vaccinations, adults ≥18 years of age were eligible if they were patients at NYP, an academic health care delivery system that includes an ambulatory care network which provides primary care to New York City’s underserved communities, and had ≥1 primary care visit or hospitalization between October 1 and the end of the study season of interest. Eligibility criteria were applied in order to ensure confidence in the collection of influenza vaccine information and were applied to each individual study season. Individuals were included for the duration of their active participation in the study.
2.2. Data collection
At enrollment, a household reporter was interviewed by a member of the study team to obtain household and individual demographic characteristics including household members’ age, gender, health status, history of chronic respiratory condition, household size, and number of school-aged children per household. Influenza vaccination data for each individual were collected through NYP’s vaccination registry which captures vaccinations administered to patients of all ages at NYP-affiliated community clinics, hospitals, and emergency departments, and the New York CIR, which includes vaccinations administered to patients <19 years-old in New York City [14].
As previously described, ARI case ascertainment for the MoSAIC study occurred through twice-weekly research team-generated text messages asking the household reporters if anyone in the household had ARI symptoms [13]. Reporter-generated text messages about household illness could also be sent at any time. The research team reviewed ARI criteria with the household reported over the phone which included ≥2 of the following for participants ≥1 year old, (1) rhinorrhea or nasal congestion, (2) sore throat, (3) cough, (4) fever or feverishness, (5) muscle or body aches. For participants <1 year, ARI was defined as rhinorrhea or nasal congestion alone or as above. When an ARI case was ascertained, a member of the research team made a home visit within 72 h of symptom onset to obtain a mid-turbinate nasal swab. Samples were processed in a research laboratory via multiplex RT-PCR using the FDA-approved FilmArray® Respiratory Panel 1.7 (BioFire Diagnostics, Inc.). Detected pathogens include: adenovirus, coronavirus 229E, coronavirus HKU1, coronavirus OC43, coronavirus NL63, human metapneumovirus, human rhinovirus/enterovirus, influenza A, influenza A/H1, influenza A/H1-2009, influenza A/H3, influenza B, parainfluenza 1, parainfluenza 2, parainfluenza 3, parainfluenza 4, respiratory syncytial virus, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae.
2.3. Definition of post-vaccination risk periods
Risk periods were examined based on knowledge of post-vaccination reactogenicity events, time to vaccine effectiveness, and a priori hypothesized periods based on patient experiences. The 2-day risk period was chosen to assess the association of vaccination with inflammatory-mediated reactogenicity events which typically occur within 48 h following vaccination [15], [16], [17]. This outcome was defined as ARI symptoms without a detected pathogen that occurred on the day of vaccination to two days after vaccination. The 14-day risk period was chosen to assess the association of vaccination with events related to potential viral interference or temporary nonspecific immunity during the time of influenza-specific immunity development [17], [18]. This outcome was defined as ARI symptoms with laboratory-confirmed influenza or non-influenza respiratory pathogens that occurred on the day of vaccination to 14 days after vaccination.
2.4. Statistical methods
Multivariable Cox proportional hazards regression models were used to estimate the relative hazard of ARI associated with three vaccination exposure statuses (1) unvaccinated (starting day one of study season or day of enrollment until day of vaccination, or the entire study season for unvaccinated individuals), (2) post-vaccination as described above, and (3) vaccinated (starting after the post-vaccination risk period) [19], [20]. A time-varying variable was used for the three vaccination statuses. Because multiple ARIs can be reported by the same subject over the course of the study periods and repeat events from the same subject may be correlated, a frailty model was used. The frailty model includes a random variable in the Cox model in order to adjust for potential unmeasured confounders [19], [20]. All models were adjusted for season using a time-varying covariate (Autumn: September-November, Winter: December-February, Spring: March-May/June) and three time-independent covariates: age (categorized by years: ≤4, 5–17, 18–49, and ≥50), gender, and prior diagnosis of chronic respiratory condition (collected as reported asthma, chronic obstructive pulmonary disease, and/or chronic inhaler use). Level of significance was set at α=0.05. SAS version 9.3 statistical software (SAS Institute Inc.) was used for data analysis.
Separate models were conducted for each of the three primary outcomes of ARI based on nasal swab results as (1) influenza, (2) non-influenza respiratory pathogen, or (3) ARI symptoms without pathogen detected within the relevant post-vaccination risk periods (i.e., influenza and non-influenza pathogens in the 14-day risk periods and ARI symptoms without a detected pathogen in the 2-day risk period) (Fig. 1 ). Analyses were completed for each influenza season separately to evaluate inter-seasonal variability and with the three seasons combined to assess trends and increase power to detect differences in the rate of ARI between the three time intervals. Post-hoc analyses were completed with the three seasons combined to better understand our significant findings. This included stratification by age (i.e., children age <18 years versus adults age ≥18 years), a sensitivity analysis that included only vaccinated individuals stratified by age, a sensitivity analysis that excluded individuals who received the live attenuated influenza vaccine, and a sensitivity analysis that excluded the two days following vaccination from the 14-day risk period.
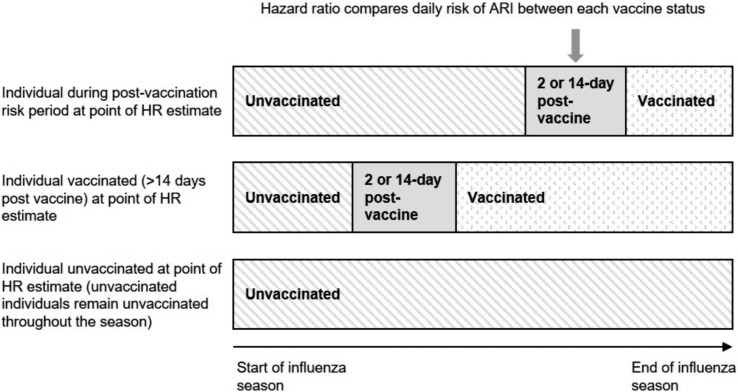
Fig. 1.
Vaccination exposure comparison groups over time. Hazard of the three outcomes of interest (laboratory-confirmed influenza, laboratory-confirmed non-influenza pathogens, and acute respiratory illness (ARI) symptoms without detectable pathogens) were compared over time between three potential exposure statuses: unvaccinated, post-vaccination risk period, and vaccinated. The arrow denotes a date during which three hypothetical individuals are in different exposure groups. Participants who were not vaccinated only contributed to the unvaccinated interval. Three separate Cox proportional hazards models were conducted, one to assess each of the three ARI outcomes.
3. Results
A total of 999 participants (697 children, 302 adults) were eligible for inclusion in this study (Fig. 2 ). Subjects were primarily children 5–17 years old, female, and approximately 12% had a chronic respiratory condition (Table 1 ). Approximately half were vaccinated for influenza each study season (Table 1); most were vaccinated in autumn (Fig. 3 ). Vaccinated and unvaccinated individuals had no difference in household size, number of school-aged children per household, gender, or chronic respiratory conditions. There was also no difference in the time to sampling after symptom onset. Rate of vaccination was higher among the youngest (range from 54.0 to 60.2% across the three study seasons) and oldest (54.0–69.1%) participants compared to those 5–17 (42.2–49.5%) and 18–49 years (45.6–52.3%). Among vaccinated individuals, 15.9%, 19.5%, and 13.3% received the live attenuated influenza vaccine during the three study seasons respectively.
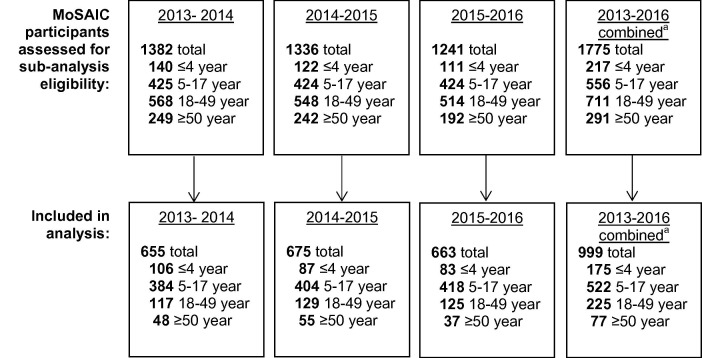
Fig. 2.
Study flow diagram. Eligible children were 6 months to 17 years, had updated vaccine records in the hospital and/or the New York Citywide Immunization Registry (CIR); children who received two doses of influenza vaccine were excluded from the analysis of the season in which they received two doses. Eligible adults were ≥18 years, were patients at the affiliated hospital, and had ≥1 primary care visit or hospitalization between October 1 and the end of the study season of interest.a Combined totals do not equal sum of years as participants may have been active in the study for more than one year. Age classification for combined years is based on age on September 1, 2013.
Table 1.
Characteristics of study participants and acute respiratory illnesses.
| 2013–2014 (n = 655) |
2014–2015 (n = 675) |
2015–2016 (n = 663) |
|
|---|---|---|---|
| Participant characteristics | |||
| Age, years, No. (%) | |||
| ≤4 | 106 (16.2) | 87 (12.9) | 83 (12.5) |
| 5–17 | 384 (58.6) | 404 (59.9) | 418 (63.1) |
| 18–49 | 117 (17.9) | 129 (19.1) | 125 (18.9) |
| ≥50 | 48 (7.3) | 55 (8.2) | 37 (5.6) |
| Female, No. (%) | 376 (57.4) | 382 (56.6) | 382 (57.6) |
| Chronic respiratory conditiona, No. (%) | 84 (12.8) | 79 (11.7) | 84 (12.7) |
| Vaccinated for influenza, No. (%) | 305 (46.6) | 352 (52.2) | 307 (46.3) |
| Experienced ≥ 1 ARI, No. (%) | 212 (32.4) | 233 (34.5) | 204 (30.8) |
| ARI characteristics | |||
| Influenza, No. (%) | |||
| Unvaccinatedb at time of ARI | 27 (67.5) | 13 (50.0) | 9 (52.9) |
| 14-day post-vaccination at time of ARI | 1 (2.5) | 0 (0) | 0 (0) |
| Vaccinatedc at time of ARI | 12 (30.0) | 13 (50.0) | 8 (47.1) |
| Non-influenza ARI, No. (%) | |||
| Unvaccinatedb at time of ARI | 117 (57.9) | 109 (60.9) | 76 (54.3) |
| 14-day post-vaccination at time of ARI | 7 (3.5) | 6 (3.4) | 6 (4.3) |
| Vaccinatedc at time of ARI | 78 (38.6) | 64 (35.8) | 58 (41.4) |
| ARI without pathogen detected, No. (%) | |||
| Unvaccinatedb at time of ARI | 72 (62.6) | 83 (50.3) | 88 (49.7) |
| 2-day post-vaccination at time of ARI | 1 (0.9) | 1 (0.6) | 1 (0.7) |
| Vaccinatedc at time of ARI | 42 (36.5) | 81 (49.1) | 53 (44.2) |
ARI: Acute respiratory illness.
Chronic respiratory condition: asthma, chronic obstructive pulmonary disease, and/or chronic inhaler use.
Unvaccinated exposure status includes events among vaccinated individuals prior to vaccination and events among those who did not receive the vaccine.
Vaccinated exposure status includes events after the relevant post-vaccination risk period (14-day or 2-day depending on the type of ARI).
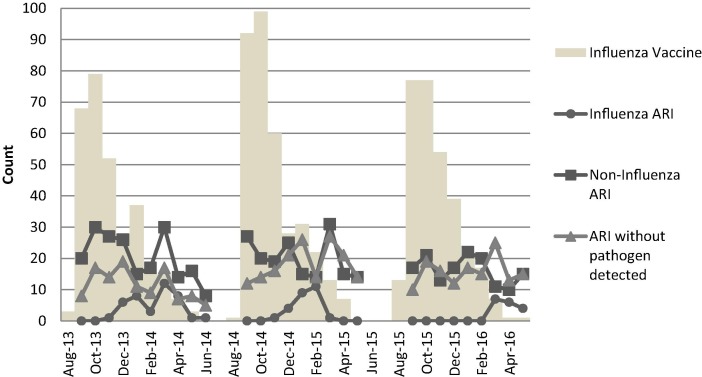
Fig. 3.
Frequency distribution of dates of influenza vaccination and onset of acute respiratory illnesses over three influenza seasons.
Approximately one third of participants experienced at least one ARI during each study season (30.8–34.5%); however, only 1.9–3.0% of vaccinated participants experienced an ARI while in the post-vaccination risk period. The frequency of ARI during each exposure status is shown in Table 1. Frequency distribution of date of onset of ARI and date of vaccination are shown in Fig. 3. In 2013–2014, the most prevalent type of influenza was influenza B, followed by influenza A H1-2009, influenza A H3. In 2014–2015, the most prevalent type of influenza was influenza A H3, followed by influenza B. In 2015–2016, the most prevalent type of influenza was influenza B, followed by influenza A H1-2009, and influenza A H3. The distributions were similar among unvaccinated and vaccinated periods. Throughout all study seasons, the most prevalent non-influenza ARI pathogens detected were rhinovirus/enterovirus, respiratory syncytial virus, and coronaviruses. The distributions were similar among vaccinated and unvaccinated periods.
The hazard of laboratory-confirmed influenza in individuals during the 14-days post-vaccination was similar to unvaccinated individuals in the same period in time. This was observed in each study season and in the three seasons combined (Table 2 ). The hazard of ARI caused by non-influenza respiratory pathogens was higher in individuals during the 14-days post-vaccination compared to unvaccinated individuals in the same period in time. This was observed in two of the three study seasons and with the three seasons combined. The hazard of ARI caused by non-influenza respiratory pathogens in vaccinated individuals (>14 days) was also higher than unvaccinated individuals in the same period, over the three study seasons. The hazard of ARI caused by non-influenza respiratory pathogens in individuals during the 14-days post-vaccination was similar to that in vaccinated individuals (>14 days) during each study season and with the three seasons combined (Table 2). The hazard of ARI symptoms without detected pathogens was similar in individuals during the 2-day post-vaccination risk period compared to unvaccinated individuals in the same period in time. This was observed during each study season and with the three seasons combined. The hazard of ARI symptoms without detected pathogens in vaccinated individuals (>2 days) was higher than unvaccinated individuals in the same period for one study season and with the three seasons combined (Table 2).
Table 2.
Adjusted Cox proportional hazards model of relative hazard of acute respiratory illness (ARI) in the post-vaccination risk period compared with unvaccinated interval and vaccinated interval during 2013–2016 influenza seasons.
| Adjusted Hazard Ratio (95% Confidence Interval) |
||||
|---|---|---|---|---|
| Time-varying exposure comparison | 2013–2014 | 2014–2015 | 2015–2016 | 2013–2016 Combined |
| Influenza | ||||
| 14-day post-vaccination vs. unvaccinateda | 0.74 (0.1, 5.48) | Estimate unreliable | 1.49 (0.19, 12.03) | 0.63 (0.16, 2.60) |
| 14-day post-vaccination vs. vaccinatedb | 0.79 (0.1, 6.29) | Estimate unreliable | 2.26 (0.25, 20.82) | 0.66 (0.16, 2.84) |
| Vaccinatedb vs. unvaccinateda | 0.94 (0.46, 1.93) | 1.34 (0.59, 3.01) | 0.66 (0.26, 1.69) | 0.96 (0.6, 1.52) |
| Non-influenza respiratory pathogen | ||||
| 14-day post-vaccination vs. unvaccinateda | 1.03 (0.49, 2.21) | 1.94 (1.08, 3.48) | 2.09 (1.12, 3.88) | 1.65 (1.14, 2.38) |
| 14-day post-vaccination vs. vaccinatedb | 0.53 (0.24, 1.14) | 1.72 (0.91, 3.27) | 1.66 (0.85, 3.23) | 1.13 (0.77, 1.68) |
| Vaccinatedb vs. unvaccinateda | 1.97 (1.39, 2.8) | 1.12 (0.78, 1.62) | 1.26 (0.86, 1.87) | 1.44 (1.17, 1.77) |
| Symptoms without pathogen detected | ||||
| 2-day post-vaccination vs. unvaccinateda | 1.8 (0.24, 13.51) | 2.44 (0.6, 10.02) | 0.17 (0.01, 32.41) | 1.3 (0.42, 4.08) |
| 2-day post-vaccination vs. vaccinatedb | 0.82 (0.1, 7.43) | 1.56 (0.38, 6.47) | 0.13 (0.01, 48.53) | 1.03 (0.33, 3.24) |
| Vaccinatedb vs. unvaccinateda | 1.23 (0.79, 1.92) | 1.56 (1.12, 2.17) | 1.02 (0.71, 1.48) | 1.27 (1.02, 1.59) |
All models adjusted for age, gender, chronic respiratory condition, and autumn, winter, spring season.
Statistically significant estimates are in bold text.
Unvaccinated exposure status includes individuals prior to vaccination and those who did not receive the vaccine.
Vaccinated exposure status includes individuals after the relevant post-vaccination risk period (14-day or 2-day depending on the model).
After stratification for age, for children <18 years, the hazard of ARI caused by non-influenza respiratory pathogens remained higher in children during the 14-day post-vaccination risk period compared to unvaccinated children in the same period (aHR 1.71, 95% CI [1.16, 2.53] in the three seasons combined); the hazard was also higher in vaccinated children compared to unvaccinated children in the same period (aHR 1.55, 95% CI [1.22, 1.96] in the three seasons combined). However, for adults ≥18 years the hazard in adults during the post-vaccination risk period was similar to unvaccinated adults (aHR 0.88, 95% CI [0.21, 3.69] in the three seasons combined); the hazard was also similar in vaccinated adults compared to unvaccinated adults in the same period (aHR 0.95, 95% CI [0.53, 1.71] in the three seasons combined). A sensitivity analysis performed including only children who ultimately received the influenza vaccine also revealed an increased hazard of ARI caused by non-influenza respiratory pathogens in the 14-day post-vaccination risk period compared to children who were not yet vaccinated in the same period (aHR 2.02, 95% CI [1.31, 3.12] in the three seasons combined); the hazard was also higher in vaccinated children compared to not yet vaccinated children in the same period (aHR 1.73, 95% CI [1.22, 2.47] in the three seasons combined). Among only adults who received the influenza vaccine there were no significant differences in the hazard of ARI caused by non-influenza respiratory pathogens. In a sensitivity analysis performed excluding individuals who received the intranasal live attenuated influenza vaccine, hazard ratio estimates were similar to those in the analysis including all individuals. There were no episodes of influenza ARI associated with the live attenuated influenza vaccine. After excluding the two days following vaccination from the 14-day risk period there were no significant differences in the hazard of ARI caused by influenza or non-influenza respiratory pathogens.
Potential associations between participant characteristics and seasonality and ARI outcomes were also evaluated (Table 3 ). Children ≤4 years and children 5–17 years had higher hazards of non-influenza respiratory pathogens throughout the study seasons compared to adults ≥50 years old. Those with chronic respiratory conditions had higher hazard of non-influenza pathogens and higher hazard of ARI symptoms without detectable pathogens compared to those without chronic respiratory conditions. Winter and spring were associated with higher hazard of influenza when compared to autumn. The hazard of non-influenza pathogens was similar among the seasons and the hazard of symptoms without a detectable pathogen was higher in spring compared to autumn and winter.
Table 3.
Participant characteristic and seasonal associations with ARI during 2013–2016 influenza seasons: Adjusted Cox proportional hazards model.
| Adjusted Hazard Ratio (95% Confidence Interval) |
|||
|---|---|---|---|
| Variablea | Influenzab | Non-influenza respiratory pathogenb | Symptoms without pathogen detectedb |
| Age, years | |||
| ≤4 | 1.68 (0.62, 4.59) | 4.8 (2.88, 7.99) | 1.27 (0.81, 1.99) |
| 5–17 | 1.12 (0.44, 2.86) | 1.61 (0.98, 2.66) | 0.68 (0.45, 1.02) |
| 18–49 | 0.9 (0.32, 2.60) | 0.99 (0.56, 1.74) | 0.71 (0.45, 1.12) |
| ≥50 | – | – | – |
| Male | 1.03 (0.67, 1.60) | 0.97 (0.8, 1.17) | 0.87 (0.69, 1.10) |
| Chronic respiratory condition | 0.87 (0.45, 1.70) | 1.46 (1.14, 1.87) | 1.55 (1.16, 2.09) |
| Season | |||
| Autumn | – | – | – |
| Winter | 8.83 (3.75, 20.80) | 1.06 (0.82, 1.38) | 1.23 (0.9, 1.67) |
| Spring | 9.33 (3.91, 22.27) | 1.18 (0.91, 1.55) | 1.5 (1.1, 2.03) |
Statistically significant estimates are in bold text.
Reference categories: age ≥50, female gender, no chronic respiratory condition, autumn season.
Estimates for influenza and non-influenza respiratory pathogen used model with the 14-day risk period; for symptoms without pathogen detected used model with the 2-day risk period.
4. Discussion
In this cohort study assessing the risk of ARI in post-vaccination risk periods during three influenza seasons, the hazard of ARIs caused by influenza was not higher in individuals during the immediate 14-day post-vaccination periods compared to individuals unvaccinated. This finding is consistent with the implausibility of acquiring influenza from inactivated vaccines. Similarly, consistent with randomized controlled trials, ARI symptoms without a detected pathogen was not higher in individuals during the immediate 2 days following vaccination compared with individuals unvaccinated [17], [21], [22]. Unexpectedly, among children <18 years, but not adults, there was a higher hazard of ARIs caused by non-influenza pathogens during the 14-days post-vaccination and other vaccinated days compared to unvaccinated children during the same time periods. Despite a higher hazard, the absolute number of vaccinated individuals who experienced ARI in the post-vaccination risk period was low (≤3%). Potential explanations postulated include bias in reporting among vaccinated individuals and a nonspecific decrease in immunity to other respiratory pathogens among vaccinated individuals [9], [10]. While the latter hypothesis is biologically plausible, it should be validated in future studies.
Patients’ experiences of increased respiratory illness after influenza vaccination may be supported by our finding of an increased rate of laboratory-confirmed non-influenza respiratory infections in the 14 days following vaccination. However, the results of the current study should not be interpreted as a reason to change the recommendation for universal vaccination for prevention of influenza, a potentially severe illness with complications such as pneumonia, bacterial infections, hospitalizations, and death [1], [12], [21]. The influenza vaccine has demonstrated reduction in morbidity and mortality across age groups and baseline health statuses [21], [23]. Additionally, others have also suggested that interference can occur between respiratory viruses that may offer protection, including against influenza infection [6], [24]. For example, epidemiologic studies have shown that when peak incidence of one major virus occurs, other major viruses are absent [25], [26]. During the 2009 H1N1 epidemic, high circulation of rhinovirus was associated with reduced likelihood of H1N1 in France and Sweden [18], [27].
Prior studies have shown conflicting results in the post-influenza vaccination risk of ARI. Similar to our findings, two studies found an increased risk of laboratory-confirmed non-influenza infections in vaccinated children compared to unvaccinated children in the months following vaccination [9], [10]. However, in two large vaccine safety studies researchers did not find an increased risk of medically attended illness in the two weeks following vaccination compared with control periods before and after vaccination [28], [29]. The limited periods evaluated in some of these studies and the outcomes of medically attended illness may not capture the true incidence of ARI post-vaccination [13]. Studies conducted during a single influenza season may have limited generalizability because of heterogeneity among circulating respiratory viruses and influenza vaccine-virus antigenic matches in different influenza seasons [9], [10], [11]. Our study attempted to overcome some of these limitations as it was conducted over three consecutive influenza seasons, included non-medically attended events, and examined an extended post-vaccination period. Our study design also minimized recall bias as ARI outcomes were collected through year-round surveillance using text-messaging and at-home nasal swab collection independent of exposure to the influenza vaccine. In support of our study design, we found expected results for the association of participant characteristics and seasonality with laboratory confirmed ARI. For example, children had a greater hazard than adults for non-influenza ARI throughout the study seasons [30]. The associations between chronic respiratory conditions and Spring with ARI without detectable pathogens may be suggestive of seasonal allergies [31], [32].
We did not observe that influenza vaccination significantly reduced overall hazard of influenza in our population, though numbers of influenza cases were small. The predominant influenza type and subtype detected among vaccinated individuals for each season were consistent with those found to have lower vaccine effectiveness estimates in national studies. For example, the 2014–2015 vaccines had low effectiveness against the predominant influenza A/H3, but were effective against influenza B [33]. In 2015–2016, when the trivalent vaccines did not include influenza B/Victoria; we saw that vaccinated individuals in our population predominantly were affected by influenza B [34].
This study had limitations, including an observational design limiting causal inference between influenza vaccination and ARI. We adjusted for important covariates in our analytic model; however, there is a potential for unmeasured confounding. Because our study population was predominantly uniform for characteristics such as ethnicity, multigenerational household size, urban neighborhood, and low socioeconomic status, we did not adjust for these variables which may affect rates of ARI and limit external validity. Our inclusion criteria were designed to minimize misclassification of vaccination status. Because there is no city registry for adult immunizations, it is possible that some adults included in the study had missing vaccination data if they were vaccinated outside of the hospital system. However, in our sensitivity analysis including only vaccinated adults we did not find a difference in the relative hazards of ARI compared to the estimates including all adults. In our statistical model, we did not adjust for co-administration of other vaccines which may affect the risk of systemic side effects after vaccination.
5. Conclusions
Vaccinated individuals were no more likely to get influenza after influenza vaccination; however, patients’ experiences of illness after vaccination may be validated by these results which suggest increased risk of ARI caused by non-influenza respiratory pathogens following influenza vaccination among children <18 years. The mechanism by which this relative increase may occur warrants further investigation. Future research could investigate whether medical decision-making surrounding influenza vaccination may be improved by acknowledging patient experiences, counseling regarding different types of respiratory viruses, and correcting the misperception that all acute respiratory illnesses occurring after vaccination are caused by influenza, while emphasizing the importance of influenza vaccination.
Acknowledgments
Acknowledgements
This study was supported by a grant from the Centers for Disease for Control and Prevention, [grant number U01 IP000618-MOSAIC Mobile Surveillance for ARI/ILI in the Community]. CDC investigators took part in the design and conduct of the study, the analysis and interpretation of the data, and the review and approval of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. S.R. was supported by Health Resources and Services Administration (HRSA) [grant number T0BHP293020100].
Conflict of interest disclosure
M.S.S. was an unremunerated coinvestigator for an unrelated, investigator-initiated grant from the Pfizer Medical Education Group.
Author contributions
Dr. Stockwell and Dr. Rikin had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Jia and Dr. Rikin conducted and are responsible for the data analysis.
References
- 1.Grohskopf L.A., Sokolow L.Z., Olsen S.J., Bresee J.S., Broder K.R., Karron R.A. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015–16 influenza season. MMWR. 2015;64(30):818–825. doi: 10.15585/mmwr.mm6430a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat-Schelbert K., Lin C.J., Matambanadzo A., Hannibal K., Nowalk M.P., Zimmerman R.K. Barriers to and facilitators of child influenza vaccine – perspectives from parents, teens, marketing and healthcare professionals. Vaccine. 2012;30(14):2448–2452. doi: 10.1016/j.vaccine.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Naleway A.L., Henkle E.M., Ball S. Barriers and facilitators to influenza vaccination and vaccine coverage in a cohort of health care personnel. Am J Infect Control. 2014;42(4):371–375. doi: 10.1016/j.ajic.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Hollmeyer H.G., Hayden F., Poland G., Buchholz U. Influenza vaccination of health care workers in hospitals—a review of studies on attitudes and predictors. Vaccine. 2009;27(30):3935–3944. doi: 10.1016/j.vaccine.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Nyhan B., Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33(3):459–464. doi: 10.1016/j.vaccine.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Hayden F.G., Fritz R.S., Lobo M.C., Alvord W.G., Strober W., Straus S.E. Local and systemic cytokine responses during experimental human influenza A virus infection – relation to symptom formation and host defense. J Clin Invest. 1998;101(3):643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaitov M.R., Laza-Stanca V., Edwards M.R. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64(3):375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 8.McGill J., Heusel J.W., Legge K.L. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009;86(4):803–812. doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling B.J., Fang V.J., Nishiura H. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis. 2012;54(12):1778–1783. doi: 10.1093/cid/cis307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dierig A., Heron L.G., Lambert S.B. Epidemiology of respiratory viral infections in children enrolled in a study of influenza vaccine effectiveness. Influenza Other Respir Viruses. 2014;8(3):293–301. doi: 10.1111/irv.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundaram M.E., McClure D.L., VanWormer J.J., Friedrich T.C., Meece J.K., Belongia E.A. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis. 2013;57(6):789–793. doi: 10.1093/cid/cit379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridges C., Thompson W.W., Meltzer M.I. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA. 2000;284(13):1655–1663. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 13.Stockwell M.S., Reed C., Vargas C.Y. MoSAIC: mobile surveillance for acute respiratory infections and influenza-like illness in the community. Am J Epidemiol. 2014;180(12):1196–1201. doi: 10.1093/aje/kwu303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vawdrey D.K., Natarajan K., Kanter A.S., Hripcsak G., Kuperman G.J., Stockwell M.S. Informatics lessons from using a novel immunization information system. Stud Health Technol Inform. 2013;192:589–593. [PubMed] [Google Scholar]
- 15.Institute of Medicine . The National Academies Press; Washington, DC: 2012. Adverse effects of vaccines: evidence and causality. [PubMed] [Google Scholar]
- 16.Stockwell M.S., Broder K., LaRussa P. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr. 2014;168(3):211–219. doi: 10.1001/jamapediatrics.2013.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry D.W., Mayner R.E., Hochstein H.D. Comparative trial of influenza vaccines. II. Adverse reactions in children and adults. Am J Epidemiol. 1976;104(1):47–59. doi: 10.1093/oxfordjournals.aje.a112273. [DOI] [PubMed] [Google Scholar]
- 18.Casalegno J.S., Ottmann M., Bouscambert Duchamp M. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin Microbiol Infect. 2010;16(4):326–329. doi: 10.1111/j.1469-0691.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 19.Therneau Terry M., Grambsch M. Patricia. Springer Science & Business Media; 2013. Modeling, survival data: extending the Cox model. [Google Scholar]
- 20.Klein J.P. Semiparametric estimation of random effects using the cox model based on the EM algorithm. Biometrics. 1992;48(3):795–806. [PubMed] [Google Scholar]
- 21.Nichol K.L., Lind A., Margolis K.L. The effectiveness of vaccination against influenza in healthy, Working Adults. N Engl J Med. 1995;333(14):889–893. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- 22.Margolis K.L., Nichol K.L., Poland G.A., Pluhar R.E. Frequency of adverse reactions to influenza vaccine in the elderly: a randomized, placebo-controlled trial. JAMA. 1990;264(9):1139–1141. [PubMed] [Google Scholar]
- 23.Flannery B., Reynolds S.B., Blanton L. Influenza vaccine effectiveness against pediatric deaths: 2010–2014. Pediatrics. 2017 doi: 10.1542/peds.2016-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henle W., Henle G. Interference of inactive virus with the propagation of virus of influenza. Science. 1943;98:87–89. doi: 10.1126/science.98.2534.87. [DOI] [PubMed] [Google Scholar]
- 25.Glezen W.P., Denny F.W. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288(10):498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 26.Mak G.C., Wong A.H., Ho W.Y., Lim W. The impact of pandemic influenza A (H1N1) 2009 on the circulation of respiratory viruses 2009–2011. Influenza Other Respir Viruses. 2012;6(3):e6–10. doi: 10.1111/j.1750-2659.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linde A., Rotzen-Ostlund M., Zweygberg-Wirgart B., Rubinova S., Brytting M. Does viral interference affect spread of influenza? Euro Surveill. 2009;14(40) [PubMed] [Google Scholar]
- 28.France E.K., Glanz J.M., Xu S. Safety of the trivalent inactivated influenza vaccine among children: a population-based study. Arch Pediatr Adolesc Med. 2004;158(11):1031–1036. doi: 10.1001/archpedi.158.11.1031. [DOI] [PubMed] [Google Scholar]
- 29.Hambidge S.J., Glanz J.M., France E.K. Safety of trivalent inactivated influenza vaccine in children 6–23 months old. JAMA. 2006;296(16):1990–1997. doi: 10.1001/jama.296.16.1990. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Common Colds: Protect Yourself and Others. Centers for Disease Control and Prevention. https://www.cdc.gov/features/rhinoviruses/Published; February 6, 2017 [accessed 01.05.17].
- 31.Gerhardsson de Verdier M., Gustafson P., McCrae C., Edsbäcker S., Johnston N. Seasonal and geographic variations in the incidence of asthma exacerbations in the United States. J Asthma. 2017:1–7. doi: 10.1080/02770903.2016.1277538. [DOI] [PubMed] [Google Scholar]
- 32.Takemura M., Inoue D., Takamatsu K. Co-existence and seasonal variation in rhinitis and asthma symptoms in patients with asthma. Respir Investig. 2016;54(5):320–326. doi: 10.1016/j.resinv.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman R.K., Nowalk M.P., Chung J. 2014–2015 Influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63(12):1564–1573. doi: 10.1093/cid/ciw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson M.L., Chung J.R., Jackson L.A. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med. 2017;377(6):534–543. doi: 10.1056/NEJMoa1700153. [DOI] [PMC free article] [PubMed] [Google Scholar]