Abstract
Cu(I)Cl promoted synthesis of N-propargylated-isatin Mannich mono- and bis-adducts with an extension towards the synthesis of N-propargylated-isatin-7-chloroquinoline conjugates was described. The synthesized scaffolds were evaluated for their in vitro activity against the veterinary protozoal pathogen Tritrichomonas foetus and cytotoxicity against human prostate (PC-3) cancer cell line. The preliminary evaluation data revealed the enhancement in the activity profiles with the introduction of 7-chloroquinoline ring with the most active conjugates 7a, 7c and 7d exhibiting an IC50 of 22.2, 11.3 and 24.5 μM respectively against T. foetus and minimal toxicity against human prostate (PC-3) cell lines.
Keywords: N-Propargylated-isatin Mannich adducts, N-Propargylated-isatin-7-chloroquinoline conjugates, Tritrichomonas foetus, Cytotoxicity
Graphical abstract
Synthesis of N-propargylated isatin-Mannich mono-/bis-adducts; isatin-7-chloroquinoline conjugates, their in vitro evaluation against Tritrichomonas foetus and cytotoxic studies.
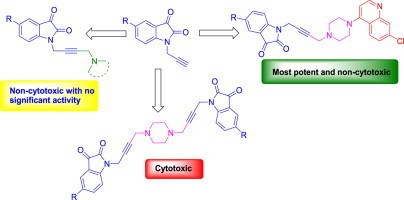
Highlights
-
•
Cu(I)Cl promoted synthesis of N-propargylated-isatin Mannich mono- and bis-adducts.
-
•
Introduction of 7-chloro-quinoline moiety remarkably enhanced the activity against Tritrichomonas foetus.
-
•
The most active and non-cytotoxic compound exhibited an IC50 value 11.3 μM.
1. Introduction
Bovine trichomoniasis is a veneral disease caused by the protozoal pathogen Tritrichomonas foetus and is considered to be one of the most important infectious causes of reproductive failure in beef cattle drastically reducing their breeding efficiency [1], [2], [3], [4]. The infection in bulls is primarily acquired through direct sexual contact and is asymptomatic in nature affecting neither the semen quality nor the sexual behaviour although in some cases, early death of developing foetus has also been observed [5], [6], [7]. Literature rationale has also revealed that the parasite provoked cell death to bovine oviduct epithelial cells [8] and extracellular products secreted by T. foetus were cytotoxic to bovine sperm [9]. Although the use of artificial insemination in breeding cattle has led to the virtual eradication of this organism from the cattle population in many countries, bovine trichomonad infection still is responsible for significant economic losses in the free-ranging cattle industry around the world. In light of this current problem, the identification of new chemotherapies for this pathogen is still warranted.
Isatin is a versatile scaffold with wide possibility of chemical modification and display a diverse array of biological and pharmacological activities which include anti-HIV [10], antiviral [11], anti-tumour [12], [13], [14], antifungal [15], [16], anti-angiogenic [17], anticonvulsants [18], anti-Parkinson's disease therapeutic [19], and effective SARS coronavirus 3CL protease inhibitor [20]. An extensive investigation on the synthetic versatility of isatin has disclosed many interesting aspects of organic synthesis allowing the generation of a large number of structurally diverse analogues derived from electrophilic aromatic substitution at positions C-5 and C-7 of the phenyl ring, nucleophilic additions onto the C-3 carbonyl group, chemoselective reductions, oxidations, ring-expansions, and spiro-annulations [21]. However there are only few reports regarding the antineoplastic activities of N-alkyl and N-aryl isatins. Vine and co-workers have reported the apoptosis behaviour of N-alkyl/aryl isatins through introduction of various hydrophobic substituents like N-phenethyl, N-benzyl and N-2-naphthylmethyl on the parent nucleus that significantly increased their cytotoxicity towards lymphoma cells and against a wide range of human cancer cell lines, including MDA-MB-231 metastatic breast adenocarcinoma cells and U937 human monocyte-like histolytic lymphoma cells (c), [22], (a), (b). Recent report from our lab has shown the synthesis of N-alkyl-isatin along with their cytotoxic profiles against a panel of cancer cell lines [23]. The approach has been further extended towards the synthesis of 1H-1,2,3-triazole-tethered isatin conjugates with four of the synthesized scaffolds being twice as potent as 5-fluorouracil on THP-1 cell line [24].
In continuation with our interest towards the synthesis of novel molecular frameworks having biological relevance (b), (c), (d), [25], (a), we recently disclosed the synthesis of 1H-1,2,3-triazole-tethered isatin-β-lactam conjugates along with the preliminary analysis of their in vitro activity against Trichomonas vaginalis at 10 and 100 μM [26]. Eighteen of the synthesized hybrids have shown 100% growth inhibition at 100 μM with the most potent and non-cytotoxic compound showing and IC50 of 7.69 μM. The methodology was further extended towards the synthesis of β-amino alcohol based β-lactam–isatin conjugates and their evaluation against T. vaginalis at 50 μM with the most active chimeric scaffold among the test series exhibited an IC50 of 9.73 μM along with low cytotoxicity [27]. As an extension of this work, we now describes the Cu(I)Cl-facilitated synthesis of N-propargylated-isatin Mannich adducts along with their preliminary in vitro evaluation studies against the veterinary protozoal pathogen T. foetus and cytotoxicity against human prostate (PC-3) cancer cell line.
2. Results and discussion
2.1. Synthetic chemistry
The synthetic approach devised for the synthesis of desired hybrids involved sodium hydride promoted initial propagylation of C-5 substituted isatins 1 with propargyl bromide in dry DMF to yield 2. This upon refluxing at 110 °C in dry dioxane with secondary amines and para-formaldehyde in the presence of catalytic amount of Cu(I)Cl resulted in the isolation of desired N-propargylated-isatin Mannich adducts. Mechanistically, the reaction is thought to proceed by an initial formation of Cu-acetylide I which undergoes Mannich addition as shown in Scheme 1 . Interestingly, the reaction employing piperazine as the secondary amine unit resulted in the isolation of bis-adduct formed via an initial condensation reaction of piperazine with para-formaldehyde at 1st and 4th position to result in the corresponding bis-iminium ion with subsequent Mannich addition.
Scheme 1.
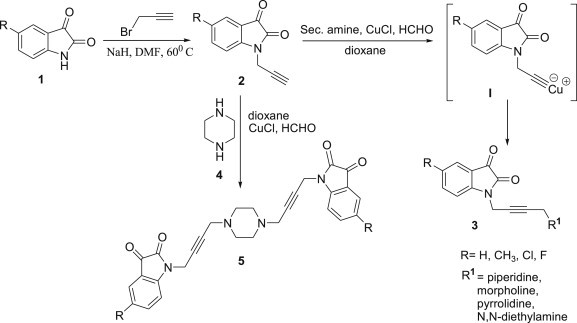
Cu(I)Cl promoted N-propargylated isatin Mannich adducts.
The developed methodology has been further extended towards the synthesis of N-propargylated isatin-7-chloroquinoline hybrids via Cu-mediated Mannich reaction of C-5 substituted N-propargylated isatins with 7-chloro-4-piperazin-1-yl-quinoline 6, prepared by heating 4,7-dichloroquinoline with an excess of piperazine and triethylamine at 120 °C overnight Scheme 2 . The inclusion of 7-chloroquinoline nucleus in the synthesized conjugates is based on the recent disclosure by Dwivedi and co-workers on the promising activity of the quinoline based compounds against different strains of T. vaginalis [28].
Scheme 2.
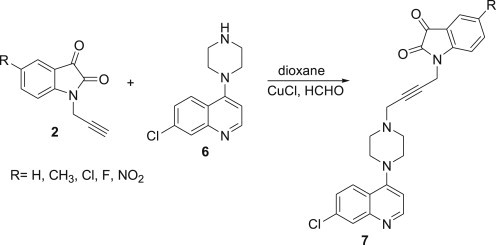
Cu(I)Cl promoted N-propargylated isatin-quinoline Mannich adducts.
The structure to the synthesized Mannich adducts were assigned on the basis of spectral data and analytical evidences. The compound 3d, for example was characterized as 1-(4-Morpholine-4-yl-but-2-ynyl)-1H-2-indole-2,3-dione analysed for C16H16N2O3 and showed molecular ion peak at m/z 284.11 (M+) in its mass spectrum. The salient features of its 1H NMR spectrum include characteristic peaks at δ 2.49(J = 4.4 Hz, 4H) and 3.69 (J = 4.4 Hz, 4H) corresponding to the morpholine group along with two singlets corresponding to two methylenes at δ 3.25 and 4.55 respectively. 13C NMR spectrum of compound 3d exhibited the appearance of a characteristic peak at δ 79.5 corresponding to two acetylenic carbons along with peaks at δ 157.0 and 182.58 assigned to the isatin ring carbonyls further corroborating the assigned structure.
2.2. In vitro evaluation against T. foetus and cytotoxicity against PC-3 cell lines
The synthesized compounds were evaluated for their anti-trichomonas activity against the veterinary protozoal pathogen T. foetus at 50 μM and the results are provided in Table 1 . As evident from Table 1, the anti-trichomonas activity of the test compounds were found to be dependent upon the nature of substituent at C-5 position as well as the presence of nature of secondary amine. The presence of a –CH3 substituent at C-5 position of isatin ring of the synthesized Mannich adducts considerably improved the activity profile as evident by compounds 3a–3p and 5a–5d. Further, the introduction of 7-chloro-4-piperazin-1-yl-quinoline moiety remarkably improved the anti-trichomonas activity with a preference for halogen substituent at C-5 position of isatin ring. The most potent of the synthesized hybrids 7b and 7c showing 100% growth inhibition at 50 μM. The active compounds viz. 7a, 7b and 7c from the preliminary inhibition data were chosen in order to determine their IC50 which is the minimum concentration required for 50% growth inhibition and the results are tabulated in Table 2 . As evident from Table 2, although the synthesized conjugates are not as active as the standard drug metronidazole, the compounds showed potent activity against the veterinary protozoal pathogen T. foetus. The compound 7c having a chloro-substituent at C-5 position of the isatin ring proved to be the most potent of the synthesized scaffolds with an IC50 of 11.3 μM. The synthesized compounds were further evaluated for their cytotoxicity against human prostate (PC-3) cancer cell lines in order to confirm if the observed activity profiles are because of their intrinsic anti-trichomonas activity or the cytotoxicity. The cytotoxic evaluation results of the test compounds at 0.2, 2.0 and 20 μM are enlisted in Table 1. As evident, virtually all the synthesized Mannich adducts are devoid of cytotoxicity except 5a and 5f, showing that the bis-adducts were cytotoxic against PC-3 cell lines compared to the mono-adducts. The most potent anti-trichomonas scaffolds viz. 7a, 7b, 7c and 7d showed no cytotoxicity against PC-3 cell line and can be considered as a good hit.
Table 1.
Inhibitory activity of compound library Tritrichomonas foetus and cytotoxicity against human prostate (PC-3) cancer cell line.
| Compound | R | R1 | Average % inhibition at 50 μM (Tritrichomonas foetus) | Cytotoxicity% inhibition |
||
|---|---|---|---|---|---|---|
| 0.2 μM | 2 μM | 20 μM | ||||
| 3a | H | Piperidine | 18.2 | 6.0 | 4.0 | 7.0 |
| 3b | H | Pyrrolidine | 10.6 | 7.0 | 7.0 | 3.0 |
| 3c | H | Morpholine | 9.1 | 13.4 | 11.6 | 8.7 |
| 3d | H | N,N-Diethylamine | 22.7 | 8.0 | 4.6 | 8.0 |
| 3e | Cl | Piperidine | 3.0 | 11.1 | 10.7 | 18.7 |
| 3f | Cl | Pyrrolidine | 15.2 | 4.6 | 4.3 | 9.6 |
| 3g | Cl | Morpholine | 22.7 | 0.8 | 4.3 | 3.4 |
| 3h | Cl | N,N-Diethylamine | 42.4 | 4.8 | 5.6 6.4 | |
| 3i | F | Piperidine | 40.9 | −4.7 | −6.0 5.1 | |
| 3j | F | Pyrrolidine | 33.3 | 5.0 | 4.3 | −2.7 |
| 3k | F | Morpholine | 56.1 | 3.8 | 0.7 | 3.2 |
| 3l | F | N,N-Diethylamine | 18.2 | 11.6 | 14.5 | 7.9 |
| 3m | CH3 | Piperidine | 31.8 | 6.8 | 10.5 | 2.4 |
| 3n | CH3 | Pyrrolidine | 65.2 | 1.9 | 4.7 | 6.1 |
| 3o | CH3 | Morpholine | 57.6 | 8.0 | 7.8 | 3.5 |
| 3p | CH3 | N,N-Diethylamine | 51.5 | 11.5 | 10.2 | 11.2 |
| 5a | H | – | 3.0 | 6.5 | 10.3 | 45.8 |
| 5b | Cl | – | 7.6 | 24.4 | 28.9 | 24.8 |
| 5c | F | – | 9.1 | −0.6 | −3.6 | 7.4 |
| 5d | CH3 | – | 71.2 | −1.0 | 6.7 | 7.1 |
| 7a | H | – | 98.5 | 9.0 | 10.1 | −3.2 |
| 7b | F | 100.0 | 2.1 | 5.0 | 3.0 | |
| 7c | Cl | – | 100.0 | 6.1 | 1.2 | 1.4 |
| 7d | CH3 | – | 59.1 | 1.3 | 8.1 | 8.2 |
| 7e | NO2 | – | 60.6 | 0.5 | 3.2 | 4.8 |
| GDC-0941 | 30.2 | 56.8 | 72.2 | |||
Table 2.
IC50 of the synthesized hybrids.
| Compound | IC50 (μM) on protozoal pathogen Tritrichomonas foetus |
|---|---|
| 7a | 22.2 |
| 7c | 11.3 |
| 7d | 24.5 |
| Metronidazolea | 0.72 |
Current FDA approved treatment for T. vaginalis infections.
In conclusion, the present manuscript describes the Cu(I)Cl promoted N-propargylated-isatin-Mannich mono- as well as bis-adducts with an extension towards the synthesis of N-propargylated-isatin-7-chloroquinoline conjugates. The synthesized scaffolds were evaluated for their activity against veterinary protozoal pathogen T. foetus. The preliminary evaluation data revealed that the introduction of 7-chloroquinoline ring markedly enhanced the activity profiles with the most active compound 7c exhibiting an IC50 of 11.3 μM. The cytotoxic studies against human prostate (PC-3) cell lines showed that the synthesized Mannich adducts are virtually devoid of any cytotoxicity and the isatin-7-chloroquinoline framework can act as an ideal starting point for the synthesis of new pharmacological templates against T. foetus.
3. Experimental section
Melting points were determined by open capillary using Veego Precision Digital Melting Point apparatus (MP-D) and are uncorrected. 1H NMR spectra were recorded in deuterochloroform and DMSO-d 6 with Jeol 300 (300 MHz) spectrometers using TMS as internal standard. Chemical shift values are expressed as parts per million downfield from TMS and J values are in hertz. Splitting patterns are indicated as s: singlet, d: doublet, t: triplet, m: multiplet, dd: double doublet, ddd: doublet of a doublet of a doublet, and br: broad peak. 13C NMR spectra were recorded on Jeol 300 (75 MHz) spectrometers in deuterochloroform and DMSO-d 6 using TMS as internal standard. High resolution mass spectra were recorded on Bruker-micrOTOF-Q II spectrometer. Column chromatography was performed on a silica gel (60–120 mesh).
3.1. Procedure for the synthesis of C-5 substituted N-propargylated isatins 2
To a stirred suspension of sodium hydride (1.5 mmol) in dry DMF (10 mL) was added isatin (1 mmol), resulting in the formation of purple coloured anion. The solution was stirred at room temperature till the evolution of hydrogen ceases. To this reaction mixture was added dropwise a solution of propargyl bromide (1.1 mmol) in dry DMF. The reaction mixture was heated to 60 °C with constant stirring for about 6 h. After the completion of reaction, as evident from TLC, it was quenched by dropwise addition of water (20 mL) and subsequently extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine solution, dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification of the reaction mixture via column chromatography using hexane: ethyl acetate (4:1) mixture furnished the desired N-propargylated isatin derivatives in good yields.
3.2. Procedure for the synthesis of 7-chloro-4-piperazin-1-yl-quinoline 6
To a well stirred solution of 4,7-dichloroquinoline (1 mmol) and triethylamine (1.2 mmol), piperazine (1 mmol) was added and the reaction mixture was allowed to stir at 110 °C for 8 h. The progress of the reaction mixture was monitored with TLC and on completion; it was washed with 2 N NaOH solution with subsequent extraction with ethy lacetate (3 × 30 mL). The combined organic layers were washed with brine solution, dried over anhydrous Na2SO4 and concentrated under vacuum resulting in the desired product.
3.3. Typical procedure for the synthesis of N-propargylated-isatin-Mannich adducts 3a–3p
To a well stirred solution of N-propargylated isatin (1 mmol) in dry dioxane, catalytic amount of copper chloride and p-formaldehyde was added in succession. The reaction mixture was stirred for 10 min with subsequent addition of secondary amine (2 mmol) and refluxed at 120 °C for 8 h. After the completion of reaction as evident from TLC, the reaction mixture was filtered and subsequently extracted with ethyl acetate (3 × 30 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure to result in the desired Mannich adducts in good yields.
3.3.1. 1-(4-Piperadin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3a)
Orange solid, yield 87%, m.p. 149 °C: 1H NMR (300 MHz, CDCl3): δ 1.14–1.74 (m, 6H, –CH2–CH2–CH2–); 2.40 (s, 4H, –CH2–N–CH2–); 3.21 (s, 2H, –CH2); 4.54 (s, 2H, –CH2); 7.10–7.17 (m, 2H Ar–H); 7.58–7.63 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz): 23.6, 25.6, 29.6, 47.5, 53.2, 80.7, 111.2, 117.4, 123.9, 125.1, 138.1, 149.7, 157.0, 182.6, HRMS calculated for C17H18N2O2 282.1368 [M+] found 282.1375; Analysis calculated for C17H18N2O2 C, 72.32; H, 6.43; N, 9.92; found C, 72.39; H, 6.37; N, 9.97.
3.3.2. 1-(4-Pyrrolidin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3b)
Orange solid, yield 86%, m.p. 136 °C: 1H NMR (300 MHz, CDCl3): δ 1.79–1.83 (m, 4H, –CH2–CH2); 2.56–2.64 (m, 4H, –CH2–N–CH–); 3.42 (s, 2H, –CH2); 4.59 (s, 2H, –CH2); 7.15–7.24 (m, 2H, Ar–H); 7.62–7.69 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz) 23.6, 29.8, 43.1, 52.7, 81.6, 111.2, 117.4, 123.8, 125.1, 138.1, 149.6, 149.8, 151.6, 182.6, HRMS calculated for C16H16N2O2 268.1212 [M+] found 268.1219; Analysis calculated for C16H16N2O2: C, 71.62; H, 6.01; N, 10.44; found C, 71.58; H, 6.08; N, 10.39.
3.3.3. 1-(4-Morpholin-4-yl-but-2-ynyl)-1H-indole-2,3-dione (3c)
Orange solid, yield 88%, m.p. 158 °C: 1H NMR (300 MHz, CDCl3): δ 2.49 (t, J = 4.4 Hz, 4H, –CH2–N–CH2–); 3.25 (s, 2H, –CH); 3.69 (t, J = 4.4 Hz, 4H, –CH2–O–CH2–); 4.55 (s, 2H, –CH); 7.07–7.18 (m, 2H, Ar–H); 7.60–7.65 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz): 29.4, 47.4, 52.6, 66.5, 79.7, 110.9, 117.4, 124.0, 125.2, 138.1, 149.6, 157.0, 182.5, HRMS calculated for C16H16N2O3 284.1161 [M+] found 284.1169; Analysis calculated for C16H16N2O3: C, 67.59; H, 5.67; N, 9.85; found C, 67.65; H, 5.72, N, 9.78.
3.3.4. 1-(4-Diethylamino-but-2-ynyl)-1H-indole-2,3-dione (3d)
Viscous liquid, yield 76%, 1H NMR (300 MHz, CDCl3): δ 1.05 (t, J = 7.1 Hz, 6H, CH3); 2.52–2.60 (m, 4H, –CH2–N–CH2–); 3.25 (s, 2H, –CH2); 4.54 (s, 2H, –CH2); 7.07–7.16 (m, 2H, Ar–H); 7.56–7.65 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz): 14.8, 29.5, 46.7, 47.4, 80.1, 110.9, 117.7, 124.1, 125.3, 138.2, 149.7, 157.1, 182.5, HRMS calculated for C16H16N2O2 270.1368 [M+] found 270.1359; Analysis calculated for C16H16N2O2: C, 71.09; H, 6.71; N, 10.36 found C, 71.01; H, 6.83; N, 10.47.
3.3.5. 5-Chloro-1-(4-piperidin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3e)
Orange solid, yield 83%, m.p. 147 °C: 1H NMR (300 MHz, CDCl3): δ 1.17–1.56 (m, 6H, –CH2–CH2–CH2–); 2.41 (s, 4H, –CH2–N–CH–); 3.23 (s, 2H, –CH2); 4.54 (s, 2H, –CH2); 7.08–7.11 (m, 1H, Ar–H); 7.57–7.60 (m, 2H, Ar–H): 13C NMR (CDCl3, 75 MHz): 23.6, 25.6, 29.6, 47.5, 53.2, 80.1, 112.4, 118.4, 125.1, 129.8, 137.6, 148.0, 151.4, 181.6, HRMS calculated for C17H17ClN2O2 316.0979 [M+] found 316.0987; Analysis calculated for C17H17ClN2O2: C, 64.46; H, 5.41; N, 9.84 found C, 64.51; H, 5.49; N, 9.78.
3.3.6. 5-Chloro-1-(4-pyrrolidin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3f)
Orange solid, yield 87%, m.p. 139 °C: 1H NMR (300 MHz, CDCl3): δ 1.75–1.77 (m, 4H, –CH2–CH2–); 2.51–2.55 (m, 4H, –CH2–N–CH–); 3.36 (s, 2H, –CH2); 4.54 (s, 2H, –CH2); 7.07–7.10 (m, 1H, Ar–H); 7.56–7.59 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz): 23.6, 29.9, 43.2, 52.8, 81.6, 112.48, 118.5, 125.2, 129.9, 137.6, 148.1, 156.5, 181.8, HRMS calculated for C16H15ClN2O2 302.0822 [M+] found 302.0831; Analysis calculated for C16H15ClN2O2: C, 63.47; H, 4.99; N, 9.25 found C, 63.52; H, 4.91; N, 9.31.
3.3.7. 5-Chloro-1-(4-morpholin-4-yl-but-2-ynyl)-1H-indole-2,3-dione (3g)
Orange solid, yield 85%, m.p. 160 °C: 1H NMR (300 MHz, CDCl3): δ 2.50 (t, J = 4.5 Hz, 4H, –CH2–N–CH2–); 3.26 (t, J = 2.0 Hz, 2H, –CH); 3.71 (t, J = 4.5 Hz, 4H, –CH2–O–CH2–); 4.56 (t, J = 2.0 Hz, 2H, –CH2); 7.07–7.10 (m, 1H, Ar–H); 7.58–7.61 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz) 29.8, 47.2, 52.3, 66.7, 80.3, 112.3, 118.4, 125.3, 130.0, 137.6, 147.9, 156.5, 181.6, HRMS calculated for C16H15ClN2O3 318.0771 [M+] found 318.0765; Analysis calculated for C16H15ClN2O3: C, 60.29; H, 4.74; N, 8.79 found C, 60.35; H, 4.79; N 8.65.
3.3.8. 5-Chloro-1-(4-diethylamino-but-2-ynyl)-1H-indole-2,3-dione (3h)
Viscous liquid, yield 78%, 1H NMR (300 MHz, CDCl3): δ 1.07 (t, J = 7.1 Hz, 6H, CH3); 2.51–2.59 (m, 4H, –CH2–N–CH2–); 3.6 (s, 2H, –CH2); 4.54 (s, 2H, –CH2); 7.07–7.10 (m, 1H, Ar–H); 7.56–7.63 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz): 14.8, 29.5, 46.7, 47.4, 81.6, 110.9, 117.7, 124.1, 125.3, 138.2, 149.7, 157.1, 181.7, HRMS calculated for C16H17ClN2O2 304.0979 [M+] found 304.0986; Analysis calculated for C16H17ClN2O2: C, 63.05; H, 5.62; N, 9.19; found C, 63.13; H, 5.75; N, 9.07.
3.3.9. 5-Fluoro-1-(4-piperidin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3i)
Orange solid, yield 85%, m.p. 149 °C: 1H NMR (300 MHz, CDCl3): δ 1.41–1.72 (m, 6H, –CH2–CH2–CH2–); 2.42 (s, 4H, –CH2–N–CH–); 3.22 (t, J = 2.0 Hz, 2H, –CH2); 4.55 (t, J = 2.0 Hz, 2H, –CH2); 7.09–7.14 (m, 1H, Ar–H); 7.31–7.38 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz) 23.7, 25.7, 29.9, 47.7, 53.4, 81.2, 112.2, 118.4, 124.4, 124.7, 138.3, 149.6, 156.6, 181.6, HRMS calculated for C17H17FN2O2 300.1274 [M+] found 300.1268; Analysis calculated for C17H17FN2O2: C, 67.99; H, 5.71; N, 9.33 found C, 67.92; H, 5.79; N, 9.41.
3.3.10. 5-Fluoro-1-(4-pyrrolidin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3j)
Orange solid, yield 81%, m.p. 141 °C: 1H NMR (300 MHz, CDCl3): δ 1.74–1.77 (m, 4H, –CH2–CH2–); 2.52–2.55 (m, 4H, –CH2–N–CH–); 3.36 (s, 2H, –CH2); 4.53 (s, 2H, –CH2); 7.07–7.10 (m, 1H, Ar–H); 7.57–7.59 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz): 23.6, 29.9, 43.2, 52.8, 81.6, 112.5, 118.5, 125.2, 129.9, 137.6, 148.1, 156.5, 181.8, HRMS calculated for C16H15ClN2O2 302.0822 [M+] found 302.0815; Analysis calculated for C16H15ClN2O2: C, 63.47; H, 4.99; N, 9.25 found C, 63.53; H, 4.85; N, 9.33.
3.3.11. 5-Fluoro-1-(4-morpholin-4-yl-but-2-ynyl)-1H-indole-2,3-dione (3k)
Orange solid, yield 85%, m.p. 157 °C 1H NMR (300 MHz, CDCl3): δ 2.50 (t, J = 4.5 Hz, 4H, –CH2–N–CH2–); 3.27 (t, J = 2.0 Hz, 2H, –CH); 3.71 (t, J = 4.5 Hz, 4H, –CH2–O–CH2–); 4.56 (t, J = 2.0 Hz, 2H, –CH2); 7.07–7.10 (m, 1H, Ar–H); 7.34–7.39 (m, 2H, Ar–H): 13C NMR (CDCl3, 75 MHz): 29.8, 47.3, 52.3, 66.7, 80.2, 112.3, 118.5, 125.3, 130.0, 137.6, 148.0, 156.5, 181.7, HRMS calculated for C16H15FN2O3 302.1067 [M+] found 302.1074; Analysis calculated for C16H15ClN2O3: C, 63.57; H, 5.00; N, 9.27 found C, 63.66; H, 5.09; N, 9.36.
3.3.12. 1-(4-Diethylamino-but-2-ynyl)-5-fluoro-1H-indole-2,3-dione (3l)
Viscous liquid, yield 78%, 1H NMR (300 MHz, CDCl3): δ 1.05 (t, J = 7.1 Hz, 6H, CH3); 2.53–2.62 (m, 4H, –CH2–N–CH2); 3.62 (s, 2H, –CH2); 4.55 (s, 2H, –CH2); 7.07–7.11 (m, 1H, Ar–H); 7.56–7.65 (m, 2H, Ar–H):13C NMR (CDCl3, 75 MHz): 14.8, 29.4, 46.7, 47.3, 80.3, 112.5, 118.7, 125.3, 130.3, 138.2, 148.2, 157.1, 181.7, HRMS calculated for C16H17FN2O2 288.3168 [M+] found 288.3157; Analysis calculated for C16H17ClN2O2: C, 66.65; H, 5.94; N, 9.72; found C, 66.73; H, 5.85, N, 9.79.
3.3.13. 5-Methyl-1-(4-pyrrolidin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3m)
Orange solid, yield 82%, m.p. 148 °C: 1H NMR (300 MHz, CDCl3): δ 1.17–1.57 (m, 6H, –CH2–CH2–CH2–); 2.29 (s, 3H, CH3); 2.45 (s, 4H, –CH2–N–CH–); 3.25 (s, 2H, –CH2); 4.54 (s, 2H, –CH2); 7.05–7.10 (m, 1H, Ar–H); 7.55–7.60 (m, 2H, Ar–H): 13C NMR (CDCl3, 75 MHz): 20.9, 23.6, 25.7, 29.6, 47.7, 53.2, 81.0, 112.4, 118.4, 125.1, 129.8, 137.6, 148.0, 149.7, 157.2, 182.6, HRMS calculated for C18H20N2O2 296.1525 [M+] found 296.1517; Analysis calculated for C18H20N2O2: C, 72.95; H, 6.80; N, 9.45 found C, 72.82; H, 6.89; N, 9.37.
3.3.14. 5-Methyl-1-(4-pyrrolidin-1-yl-but-2-ynyl)-1H-indole-2,3-dione (3n)
Orange solid, yield 84%, m.p. 139 °C: 1H NMR (300 MHz, CDCl3): δ 1.73–1.77 (m, 4H, –CH2–CH2–); 2.29 (s, 3H, CH3); 2.52–2.57 (m, 4H, –CH2–N–CH–); 3.36 (s, 2H, –CH2); 4.55 (s, 2H, –CH2); 7.07–7.12 (m, 1H, Ar–H); 7.57–7.59 (m, 2H, Ar–H) 13C NMR (CDCl3, 75 MHz): 20.9, 23.6, 29.8, 43.2, 52.8, 81.6, 112.4, 118.5, 125.2, 129.9, 137.5, 148.0, 149.9, 156.56, 181.7, HRMS calculated for C17H18N2O2 282.1368 [M+] found 282.1374; Analysis calculated for C17H18N2O2: C, 72.32; H, 6.43; N, 9.92 found C, 72.39; H, 6.37; N, 9.86.
3.3.15. 5-Methyl-1-(4-morpholin-4yl-but-2-ynyl)-1H-indole-2,3-dione (3o)
Orange solid, yield 87%, m.p. 159 °C 1H NMR (300 MHz, CDCl3): δ 2.29 (s, 3H, CH3); 2.51 (t, J = 4.5 Hz, 4H, –CH2–N–CH2–); 3.32 (s, 2H, –CH); 3.75 (t, J = 4.5 Hz, 4H, –CH2–O–CH2–); 4.53 (s, 2H, –CH2); 7.07–7.10 (m, 1H, Ar–H); 7.32–7.39 (m, 2H, Ar–H): 13C NMR (CDCl3, 75 MHz): 20.9, 29.8, 47.2, 52.3, 66.7, 81.6, 112.3, 118.4, 125.3, 129.9, 137.6, 147.9, 149.7, 156.5, 181.6, HRMS calculated for C17H18N2O3 298.1317 [M+] found 298.1328; Analysis calculated for C17H18N2O3: C, 68.44; H, 6.08; N, 9.39 found C, 68.53; H, 6.14, N, 9.45.
3.3.16. 1-(4-Diethylamino-but-2-ynyl)-5-methyl-1H-indole-2,3-dione (3p)
Viscous liquid, yield 78%, 1H NMR (300 MHz, CDCl3): δ 1.07 (t, J = 7.1 Hz, 6H, CH3); 2.27 (s, 3H, CH3); 2.52–2.60 (m, 4H, –CH2–N–CH2); 3.62 (s, 2H, –CH2); 4.55 (s, 2H, –CH2); 7.06–7.10 (m, 1H, Ar–H); 7.56–7.65 (m, 2H, Ar–H): 13C NMR (CDCl3, 75 MHz): 14.8, 20.9, 29.4, 46.7, 47.3, 80.3, 112.5, 118.7, 125.3, 130.3, 138.2, 148.2, 157.1, 181.7, HRMS calculated for C17H20N2O2 288.3168 [M+] found 288.3159; Analysis calculated for C17H20N2O2: C, 71.81; H, 7.09; N, 9.85; found C, 71.95; H, 7.17, N, 9.77.
3.4. Typical procedure for the synthesis of N-propargylated-isatin-Mannich-bis-adducts 5a–5d
To a well stirred solution of N-propargylated isatin (1 mmol) in dry dioxane, catalytic amount of copper chloride and p-formaldehyde was added in succession. The reaction mixture was stirred for 10 min with subsequent addition of piperazine (0.5 mmol) and refluxed at 120 °C for 8 h. After the completion of reaction as evident from TLC, the reaction mixture was filtered and subsequently extracted with ethyl acetate (3 × 30 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure to result in the desired bis-Mannich adducts in good yields.
3.4.1. Bis-{1-(yl-but-2-ynyl)-1H-indole-2,3-dione} piperazine (5a)
Orange solid, yield 67%, m.p. >240 °C: 1H NMR (300 MHz, CDCl3): δ 2.55 (s, 8H, piperazine); 3.26 (s, 4H, –CH2); 4.54 (s, 4H, CH2); 7.11–7.20 (m, 4H, Ar–H); 7.60–7.64 (m, 4H, Ar–H) 13C NMR (CDCl3, 75 MHz): 29.7, 46.8, 51.8, 80.0, 111.0, 117.6, 124.1, 125.4, 138.3, 149.7, 157.1, 182.7, HRMS calculated for C28H24N4O4 480.1798 [M+] found 480.1805; Analysis calculated for C28H24N4O4 C, 69.99; H, 5.03; N, 11.66; found C,69.82; H, 5.09; N, 11.73.
3.4.2. Bis-{5-chloro-1-(yl-but-2-ynyl)-1H-indole-2,3-dione} piperazine (5b)
Orange solid, yield 70%, m.p. >240 °C: 1H NMR (300 MHz, CDCl3): δ 2.56 (s, 8H, piperazine); 3.30 (s, 4H, –CH2); 4.55 (s, 4H, –CH2); 7.07–7.11 (m, 2H, Ar–H); 7.59–7.61 (m, 4H, Ar–H): 13C NMR (CDCl3, 75 MHz): 29.7, 46.8, 51.7, 80.2, 112.5, 118.6, 125.1, 126.4, 138.3, 149.7, 157.2, 182.7, HRMS calculated for C28H22Cl2N4O4 548.1018 [M+] found 548.1025; Analysis calculated for C28H22Cl2N4O4: C, 61.21; H, 4.04; N, 10.20; found C,61.30; H, 4.13; N, 10.15.
3.4.3. Bis-{5-fluoro-1-(yl-but-2-ynyl)-1H-indole-2,3-dione} piperazine (5c)
Orange solid, yield 65%, m.p. >240 °C: 1H NMR (300 MHz, CDCl3): δ 2.55 (s, 8H, piperazine); 3.35 (s, 4H, –CH2); 4.54 (s, 4H, –CH2); 7.06–7.11 (m, 2H, Ar–H); 7.58–7.62 (m, 4H, Ar–H) 13C NMR (CDCl3, 75 MHz): 29.7, 46.8, 51.8, 80.2, 111.0, 117.6, 124.1, 125.4, 138.3, 149.7, 157.1, 181.6, HRMS calculated for C28H22F2N4O4 516.1609 [M+] found 516.1615; Analysis calculated for C28H22F2N4O4: C, 65.11; H, 4.29; N, 10.85; found C, 65.23; H, 4.35; N, 10.77.
3.4.4. Bis-{5-methyl-1-(yl-but-2-ynyl)-1H-indole-2,3-dione} piperazine (5d)
Orange solid, yield 60%, m.p. >240 °C: 1H NMR (300 MHz, CDCl3): δ 2.56 (s, 8H, piperazine); 2.29 (s, 6H, CH3); 3.31 (s, 4H, –CH2); 4.55 (s, 4H, –CH2); 7.07–7.11 (m, 2H, Ar–H); 7.59–7.61 (m, 4H, Ar–H) 13C NMR (CDCl3, 75 MHz): 20.9, 29.5, 46.8, 51.8, 80.2, 111.0, 117.6, 124.1, 125.5, 138.3, 149.7, 157.1, 182.7, HRMS calculated for C30H28N4O4 508.2111 [M+] found 508.2122; Analysis calculated for C30H28N4O4 C, 70.85; H, 5.55; N, 11.02; found C, 70.79; H, 5.62; N, 11.14.
3.5. Typical procedure for the synthesis of Mannich adducts 7a–7e
A mixture of N-propargylated isatin 2 (1 mmol), paraformaldehyde (2.5 mmol), 7-chloro-4-piperazin-1-yl-quinoline 7 (1 mmol) and anhydrous cupric chloride (0.5 mmol) in dry 1,4-dioxane was heated at 100 °C for 3 h. After completion of reaction as evidenced by TLC, the reaction mixture was filtered and the filtrate was treated with brine solution and extracted with ethyl acetate (3 × 60 mL). Combined organic layers were dried over Na2SO4 and the solvent was evaporated under reduced pressure resulting in a crude product which was purified by column chromatography using a chloroform:methanol (95:5) mixture.
3.5.1. 1-{4-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-but-2-ynyl}-1H-indole-2,3-dione (7a)
Red solid; yield 88%; m.p. 115–116 °C: 1H NMR (CDCl3, 300 MHz): δ 2.79 (s, 4H, 2x-CH2–); 3.24 (s, 4H, 2x-CH2–); 3.42 (s, 2H, –N–CH2–); 4.60 (s, 2H, –N–CH2–); 6.82 (d, J = 5.1 Hz, 1H, H2); 7.15 (t, J = 7.8 Hz, 1H, ArH); 7.41 (dd, J = 2.1, 9.0 Hz, 1H, H4); 7.59–7.65 (m, 3H, ArH); 7.89 (d, J = 8.7 Hz, 1H, H3); 8.03 (d, J = 1.8 Hz, 1H, H5); 8.70 (d, J = 5.1 Hz, 1H, H1); 13C NMR (DMSO-d 6, 100 MHz): δ ppm = 29.3, 45.9, 50.9, 51.4, 78.3, 79.2, 109.3, 111.6, 112.6, 121.2, 123.8, 124.0, 125.7, 125.9, 127.8, 133.5, 145.7, 149.4, 151.9, 156.1, 157.3, 159.8, 182.0, HRMS Calculated for C25H21ClN4O2 [M]+444.1353 found 444.1345; Analysis calculated for C25H21ClN4O2: C, 67.49; H, 4.76; N, 12.59, found: C, 67.57; H, 4.66; N, 12.52.
3.5.2. 1-{4-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-but-2-ynyl}-5-fluoro-1H-indole-2,3-dione (7b)
Red Solid; Yield: 90%; m.p. 135–136 °C: 1H NMR (CDCl3, 300 MHz): δ 2.78 (s, 4H, 2x-CH2–); 3.20 (s, 4H, 2x-CH2–); 3.42 (s, 2H, –N–CH2–); 4.57 (s, 2H, –N–CH2–); 6.73 (d, J = 5.1 Hz, 1H, H2); 6.86–6.89 (m, 1H, ArH); 7.16–7.23 (m, 2H, ArH); 7.39 (d, J = 9.0 Hz, 1H, H4); 7.88 (d, J = 9.0 Hz, 1H, H3); 7.99 (s, 1H, H5); 8.66 (d, J = 5.1 Hz, 1H, H1); 13C NMR (CDCl3, 75 MHz): δ ppm = 29.9, 45.6, 51.2, 52.5, 78.4, 79.3, 108.9, 111.3, 112.4, 118.1, 121.7, 124.2, 124.9, 125.2, 126.6, 128.7, 134.8, 146.7, 150.0, 151.6, 156.4, 157.9, 181.9, HRMS Calculated for C25H20ClFN4O2 [M]+462.1259 found 462.1252; Analysis calculated for C25H20ClFN4O2: C, 64.87; H, 4.35; N, 12.10, found: C, 64.80; H, 4.41; N, 12.01.
3.5.3. 5-Chloro-1-{4-[4-(7-chloro-quinolin-4-yl)-piperazin-1-yl]-but-2-ynyl}-1H-indole-2,3-dione (7c)
Red solid; yield: 92%; m.p. 124–125 °C. 1H NMR (CDCl3, 300 MHz): δ 2.80 (s, 4H, 2x-CH2–); 3.21 (s, 4H, 2x-CH2–); 3.40 (s, 2H, –N–CH2–); 4.58 (s, 2H, –N–CH2–); 6.74 (d, J = 5.1 Hz, 1H, H2); 6.85–6.88 (m, 1H, ArH); 7.18–7.25 (m, 2H, ArH); 7.39 (d, J = 9.0 Hz, 1H, H4); 7.81 (d, J = 9.0 Hz, 1H, H3); 8.00 (s, 1H, H5); 8.67 (d, J = 5.1 Hz, 1H, H1); 13C NMR (CDCl3, 75 MHz): δ ppm = 29.5, 45.4, 51.0, 52.2, 78.3, 79.4, 108.8, 111.2, 112.6, 118.2, 121.6, 124.3, 124.8, 125.1, 126.7, 128.5, 134.6, 146.4, 150.1, 151.9, 156.2, 157.7, 182.1, HRMS Calculated for C25H20Cl2N4O2 [M]+478.0963 found 478.0971; Analysis calculated for Anal. C25H20Cl2N4O2: C, 62.64; H, 4.21; N, 11.69, found: C, 62.54; H, 4.31; N, 11.76.
3.5.4. 1-{4-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-but-2-ynyl}-5-methyl-1H-indole-2,3-dione (7d)
Red solid; yield: 82%; m.p. 114–115 °C: 1H NMR (CDCl3, 300 MHz): δ 2.23 (s, 3H, –CH3); 2.77 (s, 4H, 2x-CH2–); 3.18 (s, 4H, 2x-CH2–); 3.38 (s, 2H, –N–CH2–); 4.55 (s, 2H, –N–CH2–); 6.69 (d, J = 5.1 Hz, 1H, H2); 6.82–6.85 (m, 1H, ArH); 7.17–7.24 (m, 2H, ArH); 7.31 (d, J = 9.0 Hz, 1H, H4); 7.81 (d, J = 9.0 Hz, 1H, H3); 8.01 (s, 1H, H5); 8.63 (d, J = 5.1 Hz, 1H, H1); 13C NMR (CDCl3, 75 MHz): δ ppm = 21.1, 29.4, 45.7, 51.2, 52.0, 78.2, 79.6, 108.7, 111.2, 113.5, 118.0, 121.7, 124.2, 124.6, 125.8, 126.5, 128.9, 134.9, 136.3, 147.1, 149.6, 151.0, 157.7, 181.9, HRMS Calculated for C26H23ClN4O2 [M]+458.1510found 458.1518; Analysis calculated for C26H23ClN4O2: C, 68.04; H, 5.05; N, 12.10, found: C, 68.14; H, 5.11; N, 12.15.
3.5.5. 1-{4-[4-(7-Chloro-quinolin-4-yl)-piperazin-1-yl]-but-2-ynyl}-5-nitro-1H-indole-2,3-dione (7e)
Red solid; yield: 82%; m.p. 116–118 °C. 1H NMR (CDCl3, 300 MHz): δ2.75 (s, 4H, 2x-CH2–); 3.18 (s, 4H, 2x-CH2–); 3.36 (s, 2H, –N–CH2–); 4.55 (s, 2H, –N–CH2–); 6.67 (d, J = 5.1 Hz, 1H, H2); 6.82–6.85 (m, 1H, ArH); 7.16–7.25 (m, 2H, ArH); 7.31 (d, J = 9.0 Hz, 1H, H4); 7.81 (d, J = 9.0 Hz, 1H, H3); 8.01 (s, 1H, H5); 8.63 (d, J = 5.1 Hz, 1H, H1); 13C NMR (CDCl3, 75 MHz): δ 29.4, 45.7, 51.2, 52.2, 78.2, 79.6, 108.7, 111.2, 113.5, 118.0, 121.7, 124.3, 124.6, 125.9, 126.5, 128.9, 134.9, 136.3, 147.1, 149.6, 151.1, 157.7, 181.9, HRMS Calculated for C25H20ClN5O4 [M]+489.9104 found 489.9117; Analysis calculated for C25H20ClN5O4: C, 61.29; H, 4.11; N, 14.30, found: C, 61.38; H, 4.03; N, 14.42.
3.6. In vitro protozoal parasite susceptibility assay
To perform the initial susceptibility screens on T. foetus strain D1, compounds were dissolved in DMSO to obtain concentrations of 100 μM; 5 μL aliquots of these suspensions were diluted in 5 mL of TYM Diamond's media to obtain a final concentration of 100 μM. After 24 h, cells were counted using a hemacytometer. The IC50 value for compounds 7a, 7c and 7d was determined by running assays of increasing drug concentrations, 5 μM–40 μM, and performing a regression analysis using Prism software, from GraphPad.
3.7. In vitro cytotoxic studies
PC-3 cells were cultured in DMEM supplemented with 10% FBS. Cells were plated in 96 well tissue culture plates and treated with compounds in the concentration range 20 μM–0.001 μM for 72 h 10 μl MTT (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide) 5 mg/ml in PBS was added to wells. Plates were incubated for 4 h. Plates were centrifuged at 1000 rpm for 5 min. Supernatant was removed; 100 μl DMSO was added and mixed on shaker for 30 min. Absorbance was measured at 550 nm in Envision Multilabel Plate Reader (Perkin Elmer). Percentage inhibition was calculated and dose response curve was plotted using Graph pad Prism.
Acknowledgements
Financial assistance from University Grants Commission (UGC) under Rajiv Gandhi fellowship for SC/ST students (Nisha) is gratefully acknowledged. KL would like to acknowledge 2012 Pacific Fund Summer Undergraduate Research Fellowship at the University of Pacific. We would also like to thank Dr. Lydia K. Fox, Director of Undergraduate Research for her support.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2014.01.015.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Skirrow S.Z., BonDurant R.H. Vet. Bull. 1988;58:591–603. [Google Scholar]
- 2.Yule A., Skirrow S.Z., BonDurant R.H. Parasitol. Today. 1989;5:373–377. doi: 10.1016/0169-4758(89)90298-6. [DOI] [PubMed] [Google Scholar]
- 3.Clark B.L. Aust. Vet. J. 1971;47:103–107. doi: 10.1111/j.1751-0813.1971.tb14749.x. [DOI] [PubMed] [Google Scholar]
- 4.Stewart R.J., Campbell J.R., Janzen E.D., McKinnon J. Can. Vet. J. 1998;39:638–641. [PMC free article] [PubMed] [Google Scholar]
- 5.Parker S., Campbell J., Ribble C., Gajadhar A. J. Am. Vet. Med. Assoc. 1999;215:231–235. [PubMed] [Google Scholar]
- 6.Cobo E.R., Morsella C., Cano D., Cipolla A., Campero C.M. Theriogenology. 2004;62:1367–1382. doi: 10.1016/j.theriogenology.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Rae D.O., Crews J.E. Vet. Clin. North Am. Food Anim. Pract. 2006;22:595–611. doi: 10.1016/j.cvfa.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Midlej V., Vilela R., Dias A.B., Benchimol M. Vet. Parasitol. 2009;165:216–230. doi: 10.1016/j.vetpar.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro C.M., Falleiros M.B., Bicudo S.D., Junior J.P., Golim M.A., Filho F.C. Theriogenology. 2010;73:64–70. doi: 10.1016/j.theriogenology.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Ratan B.T., Anand B., Yogeeswari P., Sriram D. Bioorg. Med. Chem. Lett. 2005;15:4451–4455. doi: 10.1016/j.bmcl.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Jiang T., Kuhen K.L., Wolff K., Yin H., Bieza K., Caldwell J., Bursulaya B., Tuntland T., Zhang K., Karanewsky D., He Y. Bioorg. Med. Chem. Lett. 2006;16:2109–2112. doi: 10.1016/j.bmcl.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 12.Tripathy R., Reiboldt A., Messina P.A., Iqbal M., Singh J., Bacon E.R., Angeles Th. S., Yang Sh. X., Albom M.S., Robinson C., Chang H., Ruggeri B.A., Mallamo J.P. Bioorg. Med. Chem. Lett. 2006;16:2158–2162. doi: 10.1016/j.bmcl.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 13.Cane A., Tournaire M.C., Barritault D., Crumeyrolle-Arias M. Biochem. Biophys. Res. Commun. 2000;276:379–384. doi: 10.1006/bbrc.2000.3477. [DOI] [PubMed] [Google Scholar]
- 14.Silveira V. Ch., Luz J.S., Oliveira C.C., Graziani I., Ciriolo M.R., Costa-Ferreira A.M. J. Inorg. Biochem. 2008;102:1090–1103. doi: 10.1016/j.jinorgbio.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Amal R.A., Raghunathan R., Sridevikumaria M.R., Raman N. Bioorg. Med. Chem. 2003;11:407–419. doi: 10.1016/s0968-0896(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Arguelles M.C., Mosquera-Vazaquez S., Touron-Touceda P., Sanmartin-Matalobos J., Garcia-Deibe A.M., Belicchi-Ferraris M., Pelosi G., Pelizzi C., Zani F. J. Inorg. Biochem. 2007;101:138–147. doi: 10.1016/j.jinorgbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Maskell L., A Blanche E., Colucci M.A., Whatmore J.L., Moody Ch. J. Bioorg. Med. Chem. Lett. 2007;17:1575–1578. doi: 10.1016/j.bmcl.2006.12.108. [DOI] [PubMed] [Google Scholar]
- 18.Verma M., Nath P.S., Nand S.K., Stables J.P. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 19.Igosheva N., Lorz C., O'Conner E., Glover V., Mehmet H. Neurochem. Int. 2005;47:216–224. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Chen L.R., Wang Y. Ch., Lin Y.W., Chou Sh. Y., Chen Sh. F., Liu L.T., Wu Y.T., Kuo Ch. J., Chen T. Sh. Sh., Juang Sh. H. Bioorg. Med. Chem. Lett. 2005;15:3058–3062. doi: 10.1016/j.bmcl.2005.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Da Silva J.F.M., Garden S.J., Pinto A.C.J. Braz. Chem. Soc. 2001;12:273–324. and references therein. [Google Scholar]
- 22.(a) Vine K.L., Locke J.M., Ranson M., Benkendorff K., Pyne S.G., Bremner J.B. Bioorg. Med. Chem. 2007;15:931–938. doi: 10.1016/j.bmc.2006.10.035. [DOI] [PubMed] [Google Scholar]; (b) Vine K.L., Locke J.M., Ranson M., Pyne S.G., Bremner J.B. J. Med. Chem. 2007;50:5109–5117. doi: 10.1021/jm0704189. [DOI] [PubMed] [Google Scholar]; (c) Matesic L., Locke J.M., Bremner J.B., Pyne S.G., Skropeta D., Ranson M., Vine K.L. Bioorg. Med.Chem. 2008;16:3118–3124. doi: 10.1016/j.bmc.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Singh P., Kaur S., Kumar V., Bedi P.M.S., Mahajan M.P., Sehar I., Pal H.C., Saxena A.K. Bioorg. Med. Chem. Lett. 2011;21:3017–3020. doi: 10.1016/j.bmcl.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 24.Singh P., Sharma P., Anand A., Bedi P.M.S., Kaur T., Saxena A.K. Eur. J. Med. Chem. 2012;55:455–461. doi: 10.1016/j.ejmech.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 25.(a) Kumar K., Carrère-Kremer S., Kremer L., Guérardel Y., Biot C., Kumar V. Organometallics. 2013 doi: 10.1039/c2dt32148c. [DOI] [PubMed] [Google Scholar]; (b) Kumar K., Sagar S., Esau L., Kaur M., Kumar V. Eur. J. Med. Chem. 2012;58:153–159. doi: 10.1016/j.ejmech.2012.10.008. [DOI] [PubMed] [Google Scholar]; (c) Kumar K., Kremer S.C., Kremer L., Guérardel Y., Biot C., Kumar V. Dalton Trans. 2013;42:1492–1500. doi: 10.1039/c2dt32148c. [DOI] [PubMed] [Google Scholar]; (d) Nisha, Singh P., Hendricks D.T., Bisetty K., Kumar V. Synlett. 2013;24:1865–1869. [Google Scholar]
- 26.Raj R., Singh P., Haberkern N.T., Faucher R.M., Patel N., Land K.M., Kumar V. Eur. J. Med. Chem. 2013;63:897–906. doi: 10.1016/j.ejmech.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Nisha, Mehra V., Hopper M., Patel N., Hall D., Wrischnik L.A., Land K.M., Kumar V. Med. Chem. Comm. 2013;4:1018–1024. [Google Scholar]
- 28.Pandey R.R., Srivastava A., Malasoni R., Naqvi A., Jain A., Maikhuri J.P., Paliwal S., Gupta G., Dwivedi A.K. Bioorg. Med. Chem. Lett. 2012;22:5735–5738. doi: 10.1016/j.bmcl.2012.06.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


